Abstract
Study Objective:
To investigate the associations between sleep stage distributions and subsequent decline in cognitive function in older men over time.
Design:
A population-based prospective substudy of the Osteoporotic Fractures in Men Study.
Settings:
Six sites in the United States.
Participants:
Community-dwelling men aged 67 y or older (n = 2,601), who were free of probable dementia at sleep visit. Follow-up averaged 3.4 y.
Measurement and Results:
Sleep stages were identified by in-home polysomnography at the initial sleep visit (2003–2005). Cognitive outcomes were assessed with the Trail Making Test Part B and Modified Mini-Mental State Examination (3MS) at sleep visit and two follow-up visits. After adjusting for multiple confounders compared with men in the lowest quartile of percent of sleep time spent in Stage N1, those in the highest quartile had a twofold increase in cognitive decline for both cognitive tests (adjusted annualized percent change/y: Trail Making Test Part B Q1 = 1.06, Q4 = 2.45, P = 0.01; 3MS Q1 = −0.27, Q4 = −0.48, P = 0.03). In addition, compared with men in the highest quartile, men in the lowest quartile of percent of sleep time in Stage R revealed more cognitive decline on the 3MS (adjusted annualized percent change/y: Q1 = −0.49, Q4 = −0.22, P = 0.003). These findings were consistent even after further adjustment of total sleep time and sleep disordered breathing. No significant relationships between other sleep stages (N2, N3) and cognitive change were found.
Conclusion:
Increased time in Stage N1 sleep and less time in Stage REM sleep are associated with worsening cognitive performance in older men over time.
Citation:
Song Y, Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Stone KL, Osteoporotic Fractures in Men Study Group. Relationships between sleep stages and changes in cognitive function in older men: the MrOS Sleep Study. SLEEP 2015;38(3):411–421.
Keywords: aging, cognitive function, cohort study, sleep stages
INTRODUCTION
Sleep patterns change as people age, and sleep disorders become more common. As many as 57% of older adults have reported complaints of poor sleep.1–5 Changes in sleep architecture also occur with aging. In particular, sleep becomes less restorative as older adults spend an increasing percentage of sleep in Stages 1 (N1) and 2 (N2) and a decreasing percentage of time in Stages 3 and 4, slow wave sleep (N3), and rapid eye movement sleep (Stage R).6–8
Poor sleep in older adults has been significantly associated with decreased cognitive function in several epidemiologic studies.9–13 Only a few studies, however, have investigated the longitudinal association between different sleep stages and cognitive function. Spiegel and colleagues14–16 conducted a series of longitudinal studies on a small number of healthy older adults. They found no significant association between Stage N3 and changes in cognitive function in both older women and men over a 5-y period. In addition, no significant relationships were observed in a follow-up study between sleep stages at baseline and cognitive function at the 14-y follow-up, although other polysomnographic (PSG) sleep parameters (i.e., Stage R latency and density and number of Stage NR shifts) were significantly associated with cognitive function.15 In contrast, a recent study found that the duration of Stage R was associated with better verbal memory tasks the next morning.17
Evidence of an association between sleep stages and cognitive function over time is sparse, and sample sizes have been small. Moreover, most of the epidemiologic studies were cross-sectional and used subjective measures of sleep.9–13 A few epidemiologic studies examined sleep complaints and decline in cognitive function over time, but their results were inconsistent and they lacked objective measures of sleep data.18–23 The epidemiologic research linking measures of sleep architecture with cognitive dysfunction or decline is scant.
Our group recently reported cross-sectional results from the Outcomes of Sleep Disorders in Older Men (MrOS Sleep Study), indicating a significant association between sleep stages and cognitive function in community-dwelling older men.24 Spending less percent time in Stage R and more percent time in Stage N1 was associated with lower levels of cognitive function. Using data from the MrOS Sleep Study, we designed this study to investigate the effect of sleep stages on decline in cognitive function in older men over time. We hypothesized that increased percent time in Stages N1 and N2 and decreased percent time in Stages N3 or R would increase the risk of cognitive decline in community-dwelling older men.
METHODS
Participants
The MrOS Sleep Study is an ancillary study of the Osteoporotic Fractures in Men (MrOS) Study, which was initially designed to examine the risk factors related to osteoporosis and osteoporotic fractures in older men and to determine the effect of fractures on their quality of life.25 In the MrOS study, baseline examinations were conducted from 2000 to 2002. A total of 5,994 community-dwelling men aged 65 y or older were enrolled at six clinical centers in the United States: Birmingham, AL; Minneapolis, MN; the Monongahela Valley near Pittsburgh, PA; Palo Alto, CA; Portland, OR; and San Diego, CA.25,26 Older men were eligible for the parent study if they could walk without assistance, had no history of bilateral hip replacement, resided near a clinical site for the duration of the study, and had no medical condition that would result in imminent death.
From December 2003 through March 2005, MrOS participants were invited to participate in the ancillary study, the MrOS Sleep Study. Its purpose was to investigate the relationships between sleep, incident cardiovascular disease, and other age-related outcomes in older men.27 Men were screened for nightly use of mechanical devices during sleep including pressure mask for sleep apnea (e.g., continuous positive airway pressure or bilevel positive airway pressure), mouthpiece for snoring or sleep apnea, or oxygen therapy and were excluded if they could not forgo use of these devises during PSG recording. A total of 3,135 participants, which exceeded the goal of 3,000 participants, were recruited for sleep measures. Further details about the MrOS Sleep Study can be found in the literature.27
The focus of our prospective analysis was data gathered during three periods: MrOS Sleep Visit 1 (2003–2005), MrOS Visit 2 (2005–2006), and MrOS Visit 3 (2007–2009). To be included in this prospective analysis, men had to have data on sleep architecture at Sleep Visit 1, which is considered baseline for this analysis, and at least one additional measure of cognitive functioning during follow-up. In addition, men had to be free of probable dementia at Sleep Visit 1, based on a Modified Mini-Mental State Examination (3MS) score ≥ 80 and no use of medication for dementia. Baseline (Sleep Visit 1) cognitive function data for either (or both) the Trail Making Test Part B (Trails B) or the 3MS were available for 3,130 men, but 163 were excluded because of probable dementia. Of the 2,967 men remaining, 2,724 had in-home, overnight PSG recordings with data on sleep architecture.
Of the 2,724 cognitively intact men with PSG data, 2,601 provided cognitive function data at either Visit 2 or Visit 3. Of the 123 men without cognitive data by Visit 3, 61 had died, 49 had questionnaire data only (no clinic visit), 7 had withdrawn from the study, 5 had refused to participate in the Visit 2 or 3, and 1 participated in the clinic visit but had no cognitive testing (Figure 1).
Figure 1.
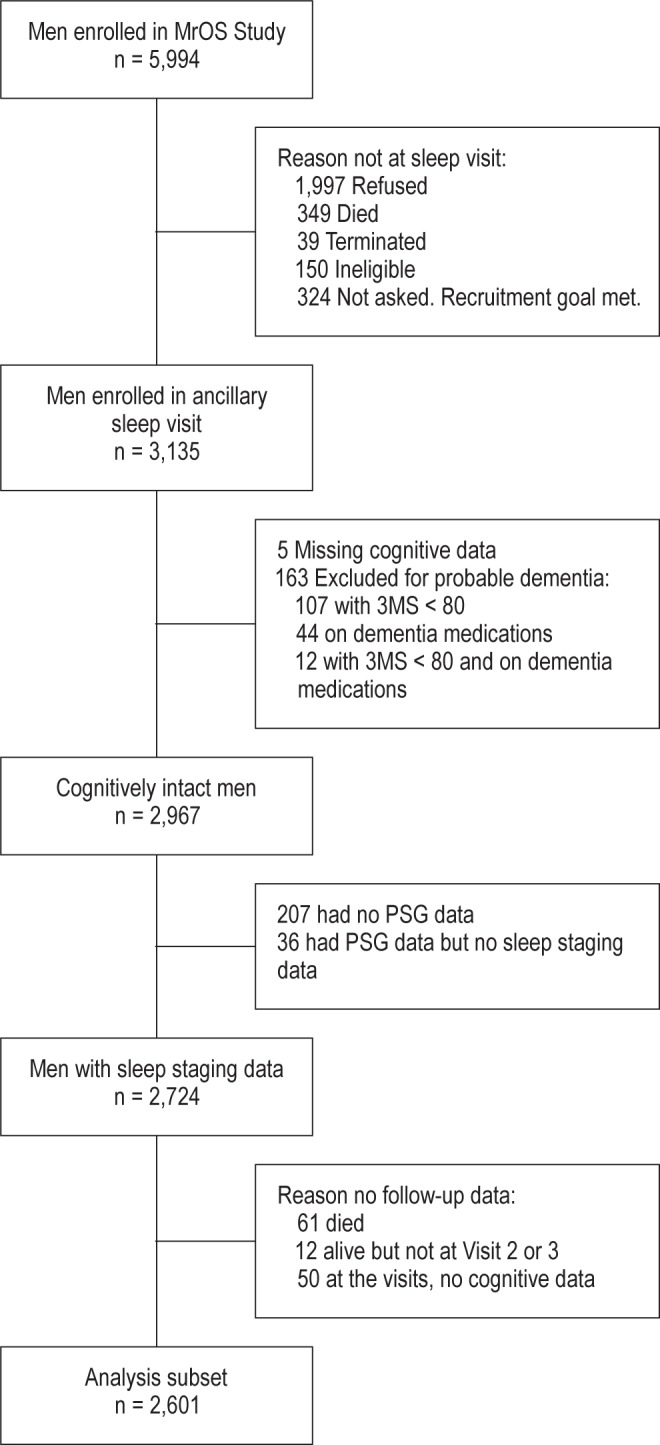
Progression of participants through the MrOS and MrOS sleep studies. MrOS, Osteoporotic Fractures in Men (MrOS) Study; 3MS, Modified Mini-Mental State Examination; PSG, polysomnography.
All men provided written informed consent, and the study was approved by the Institutional Review Board at each site.
PSG Predictors
Sleep measures were obtained on one night using unattended, portable, in-home PSG (Compumedics, Safiro, Inc., Melbourne, Australia). Measures were obtained by C3/A2 and C4/A1 electroencephalograms, a bilateral electrooculogram, a bipolar submental electromyogram, thoracic and abdominal respiratory inductance plethysmography, airflow (by nasal-oral thermocouple and nasal pressure cannula), finger pulse oximetry, electrocardiogram (EKG) lead I, body position by mercury switch sensor, and bilateral tibialis leg movements by piezoelectric sensors. Centrally trained and certified staff visited participants at their home to set up the PSG, to verify the values of the impedances for each channel, to confirm calibration of position sensors, to note problems during setup, and to collect data by the next morning. Certified research polysomnologists scored the sleep data, which were downloaded to the Central Sleep Reading Center (Case Western Reserve University, Cleveland, OH) using standard criteria.28,29 The PSG data showed excellent quality: The failure rate was less than 4%, and more than 70% of the studies were graded as “excellent” or of “outstanding quality.” Thirty-nine PSG recordings were omitted from the analysis because information on sleep architecture was not available. If data on any of the sleep stages was thought to be unreliable, the PSG scorer considered it to be missing information (195 for N1, 118 for N2, 89 for N3, and 60 for R). The reliability of these measures was determined by rescoring studies over time. High interscorer reliability was calculated with 0.60, 0.91, 0.96, and 0.94 for the intraclass correlation coefficients for percent of sleep time spent in sleep Stages N1, N2, N3, and R, respectively. Similarly, high levels of intrascorer reliability were also calculated.
The apnea-hypopnea index (AHI) was calculated as the number of apneic and hypopneic episodes per hour of sleep. Apnea was defined as the complete or near-complete cessation of airflow for more than 10 sec, and hypopneas were scored if clear reductions in breathing amplitude (at least 30% below baseline breathing) occurred and lasted more than 10 sec.30 Only apneic and hypopneic episodes that were associated with a 3% or greater desaturation were included in the AHI. Total sleep time (TST) and minutes of wake after sleep onset (WASO), a measure of sleep fragmentation, were also obtained from PSG.
Measurement of Cognitive Function
Two cognitive function tests were administered at the clinic visit by trained staff: the Trails B and the 3MS.
The Trails B31 is a timed test that measures attention, sequencing, visual scanning, speed of process, and executive function. It requires that a participant connect, by drawn line, 25 encircled letters and numbers as quickly as possible, alternating between numbers and letters in the appropriate order (i.e., 1, A, 2, B, 3, C, etc.). The shorter the time for completion (in sec) the better cognitive functioning is presumed.
The 3MS32 is a modified, expanded version of the Mini-Mental State Examination, which is a global measurement of cognitive function. The 3MS's components include orientation, concentration, language, praxis, and immediate and delayed memory. It retains the brevity, ease of administration, and objective scoring of the original instrument but broadens the range of scores from 0–30 to 0–100.32 Higher scores represent better cognitive functioning. Previous studies have shown high sensitivity (range from 87% to 92%) and specificity (range from 89% to 97%) when screening for dementia using cutoff scores of ≥ 83,33 ≥ 80,32,34 or ≥ 78.35 However, the evidence of sensitivity/specificity to detect mild cognitive impairment is limited.
The progression of clinically relevant cognitive decline from the sleep visit to Visit 3 was defined, for each test, by either a decline in five points on the 3MS36 or a change in score over 1.5 standard deviations from the mean for Trails B37 (sleep visit-Visit 3 change score ≥ 82.09). A five-point change on the 3MS has been used as the cutoff for clinically relevant cognitive decline in longitudinal studies of large sample size.38–41 In a large longitudinal sample of older adults, a five-point decline in the 3MS score was more likely to be clinically meaningful in consideration of biological gradient (i.e., odds of cognitive decline increase with age), although a one-point decline was clinically detectable, with a medium effect size (Cohen d = 0.5).42 Using cutoff of 1.5 standard deviations below the normative mean in a single cognitive domain has also been commonly used.37,43–45
Other Measurements
During their sleep clinic visit, all participants completed self-administered questionnaires, which included items about demographics, medical history, self-reported health status, physical activity, smoking, caffeine intake, alcohol use, and depression. For this study, demographics included age, race, and education. Self-reported prior diagnoses of common chronic illnesses included angina, congestive heart failure, diabetes, hypertension, myocardial infarction, and stroke or transient ischemic attack (TIA). Subjective, categorized health status was assessed. Level of physical activity was assessed with the Physical Activity Scale for the Elderly,46 which consists of occupational, household, and leisure activities over 1 w. Higher scores represent a higher level of physical activity. Self-reported smoking status was categorized as none, past, or current smoker. Self-reported caffeine intake (mg/day) was based on intake of caffeinated coffee, tea, and soda.47 Alcohol intake (number of drinks per week) was also assessed by self-report. Depression was assessed with the Geriatric Depression Scale, with a standard cutoff point of six or more defining depression.48
Participants were asked to bring in all of the medications (prescription and nonprescription) that they had used within the past 30 days. Medication use was then entered into an electronic database, and each medication was matched to its ingredient(s) based on the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).49 Dichotomous variables for the use of antidepressants, benzodiazepines, and sleep medications (nonbenzodiazepines, nonbarbiturate sedative hypnotics) were used for the study. Body weight and height were measured to calculate body mass index (BMI: weight in kilograms divided by the square of height in meters).
Statistical Analysis
Sleep stage predictors were expressed as quartiles. The participants' characteristics were compared across quartiles of the percent of sleep time spent in Stage R using chi-square tests for categorical variables, analysis of variance for normally distributed continuous variables, and the Kruskal-Wallis test for continuous variables with skewed distributions. Similar comparisons were performed across quartiles of the percent of sleep time spent in Stages N1, N2, and N3 (data not shown).
Random-effects models were used to study the association between sleep stages and changes in cognition over time, as measured by the 3MS and Trails B. These models account for between-participant variation and within-participant correlation of repeated outcomes.50 The random effect terms included the intercept and the slope of the cognitive measurements over time, allowing for individual time trends. Variances and covariances were estimated using the restricted maximum likelihood method. Time was modeled as a continuous covariate, measured as years from the sleep visit. A quadratic term for time was considered to account for a nonlinear time trend. Because the interaction of the quadratic term for time and the predictors were not significant, time was modeled linearly in all models. All models were minimally adjusted for age and clinic. Additional covariates (fixed effects) were selected for inclusion in a multivariable model by examining the univariate association of the covariate and the four sleep stage predictors and the association with the 3MS and Trails B outcomes in unadjusted random-effects models. Age, clinic site, and those covariates associated with a predictor and an outcome at P < 0.10 were kept in all multivariable models (i.e., race; education; depression; history of angina, congestive heart failure, hypertension, myocardial infarction, and stroke/ TIA; benzodiazepine use; antidepressant use; self-reported health status; physical activity and smoking status). All continuous covariates were centered (value-mean) for use in the models. Change in cognition is presented as average percent change per year, which was calculated using the coefficients derived from the random-effects models. The mean change over time for the quartiles was compared with the reference quartile, and a P value for trends across the quartiles was determined. The continuous cognitive scores were transformed to meet model requirements (log transformation for Trails B, cube transformation for 3MS) and back-transformed for display of results.
The association of sleep stages with clinically relevant cognitive decline was assessed using logistic regression models. These models were minimally adjusted as previously described. Results are presented as odds ratios (OR) and 95% confidence intervals (95% CI).
Sensitivity analysis was performed using the predictors as minutes of sleep time spent in the different stages of sleep rather than percent of TST spent in the different stages of sleep. The predictors were also used as continuous variables. Because sleep fragmentation and TST were shown to be associated with cognition in this cohort in cross-sectional analysis,51 the multivariable models were further adjusted by WASO and TST to determine if any associations seen between sleep staging and cognitive decline held. Similarily, models were further adjusted for AHI because of the association found between sleep disordered breathing and mild cognitive impairment/dementia.52 Tests were also run to determine if any sleep stage predictor significantly associated with cognitive change in the multivariable models persisted after controlling for other significant sleep stages and vice versa. To test the effect of medication use on our main findings, additional sensitivity analysis was done by excluding participants who were taking benzodiazepines and selective serotonin reuptake inhibitors (SSRIs). All reported significance levels were two-sided, and all analyses were conducted using SAS Version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Characteristics of the Study Population
The cohort that we analyzed comprised 2,601 men with an average age of 76.0 ± 5.3 y; 91.5% were white. The average percent of time spent in stages of sleep were as follows: N1, 6.7% ± 4.1%; N2, 62.6% ± 9.5%; N3, 11.3% ± 8.9%; and R, 19.4% ± 6.6%. On average, the men had relatively high levels of cognitive function at the initial visit: 116.4 ± 49.8 sec for completion of Trails B and 93.7 ± 4.5 points for the 3MS.
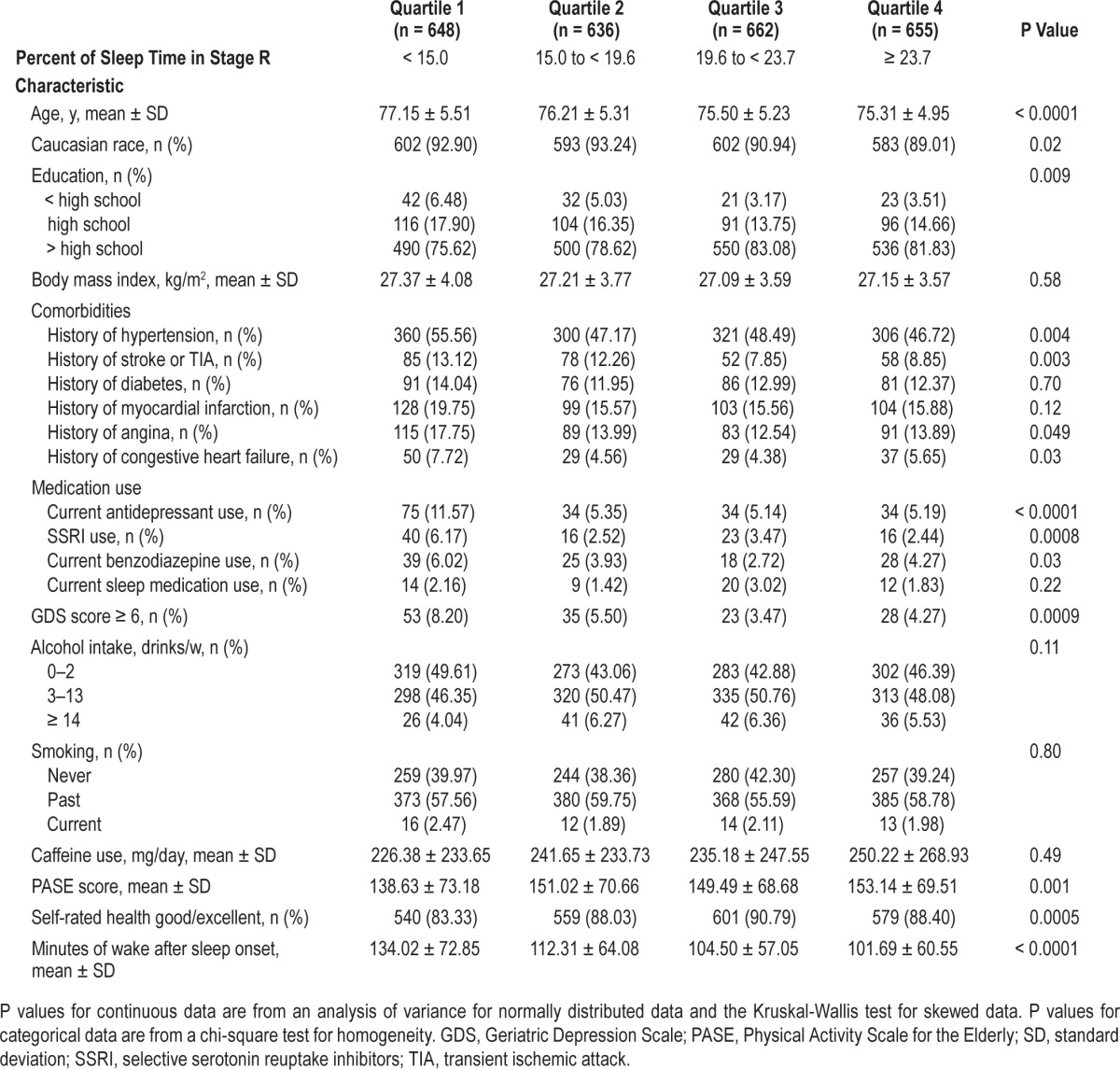
Many covariates differed significantly across quartiles of the percent of sleep stages (Table 1). On average, those in the lowest quartile of Stage R (< 15.0%) were older; had longer WASO; had higher rates of self-reported angina, congestive heart failure, hypertension, and stroke or TIA; and had higher rates of depression. Those with lower time in Stage R also had lower levels of education, had higher rates of benzodiazepines and antidepressant use (including use of SSRIs), were less physically active, and had worse self-reported health (P < 0.05). Those men who spent a higher percent of sleep time in N1 were on average older, less educated, and had longer WASO than the other groups. Those with less time spent in N1 had higher rates of benzodiazepine use (data not shown, P < 0.05). On average, those men who spent more time in N2 were older; had higher rates of angina, hypertension, and myocardial infarction; were more likely to take antidepressants or benzodiazepines; had higher rates of depression, lower levels of physical activity, and longer WASO (data not shown, P < 0.05). Those men with less time in N3 were more likely to be a minority, had higher rates of benzodiazepine use, and longer WASO (data not shown, P < 0.05).
Table 1.
Sleep visit characteristics by quartile of percent of sleep time spent in Stage R.
Association of Sleep Stages and Longitudinal Change in Cognitive Scores
On average, by Visit 3 (mean 3.4 ± 0.5 y later), the men had declined on both cognitive tests. The unadjusted average increase in time to complete the Trails B test was 8.89 ± 48.80 sec, and the 3MS score was lower by 1.22 ± 5.53 points. The annualized percent changes in Trails B completion time and the 3MS score across quartiles of sleep stage distributions are summarized in Tables 2 and 3. After multivariable adjustment, an association was seen in the percent of sleep time spent in N1 and annualized cognitive decline on both the Trails B Test and the 3MS. Greater percent of sleep time spent in N1 was associated with a greater rate of decline in Trails B Test completion time (P trend = 0.007). Men with the highest quartile of N1 had a twofold increase in time to complete the test (2.45 sec versus 1.06 sec), and the difference between the two quartiles was statistically significant (P = 0.01). Changes in Trails B Test completion time across quartiles of percent of sleep time spent in N1 are shown in Figure 2. On average, the Trails B completion time at time zero increased as the quartiles increased, but over time the change in completion time increased at an accelerated rate for those men in Quartile 4.
Table 2.
Adjusted annualized mean percent change in Trails B completion time by quartile of sleep stages.
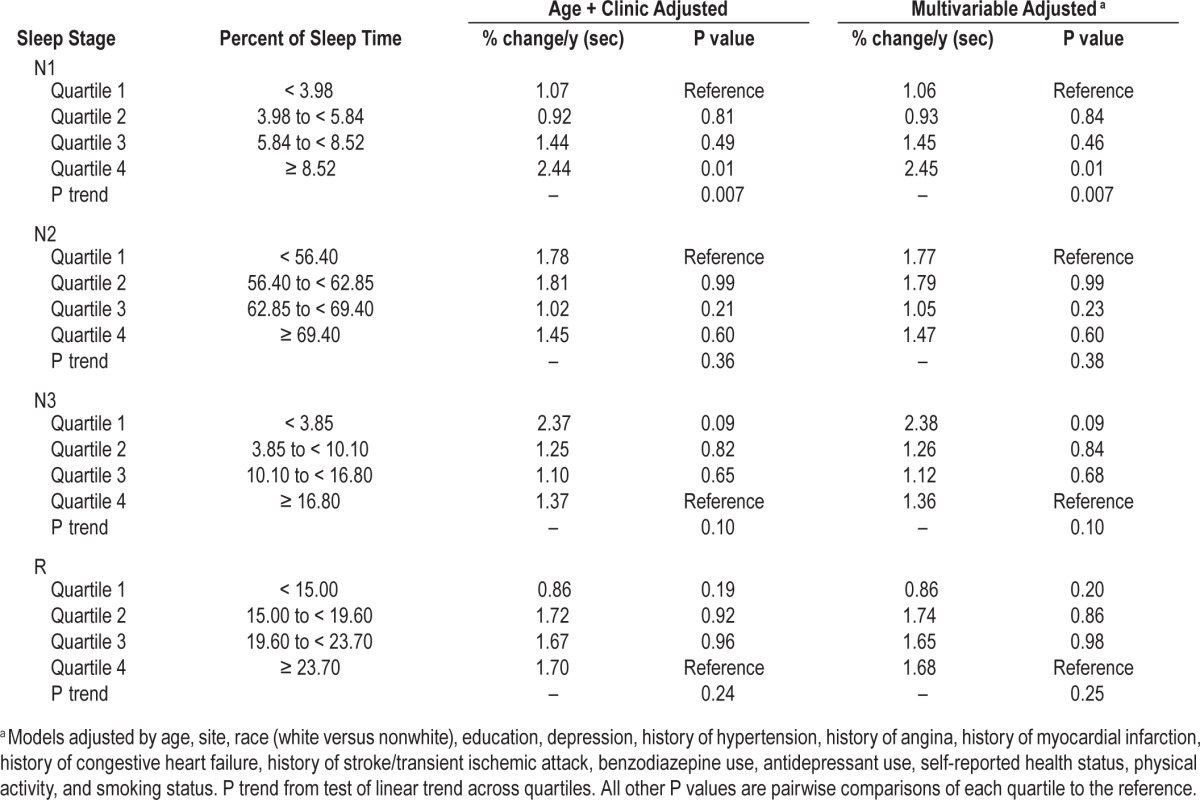
Table 3.
Adjusted annualized mean percent change in 3MS score by quartile of sleep stages.
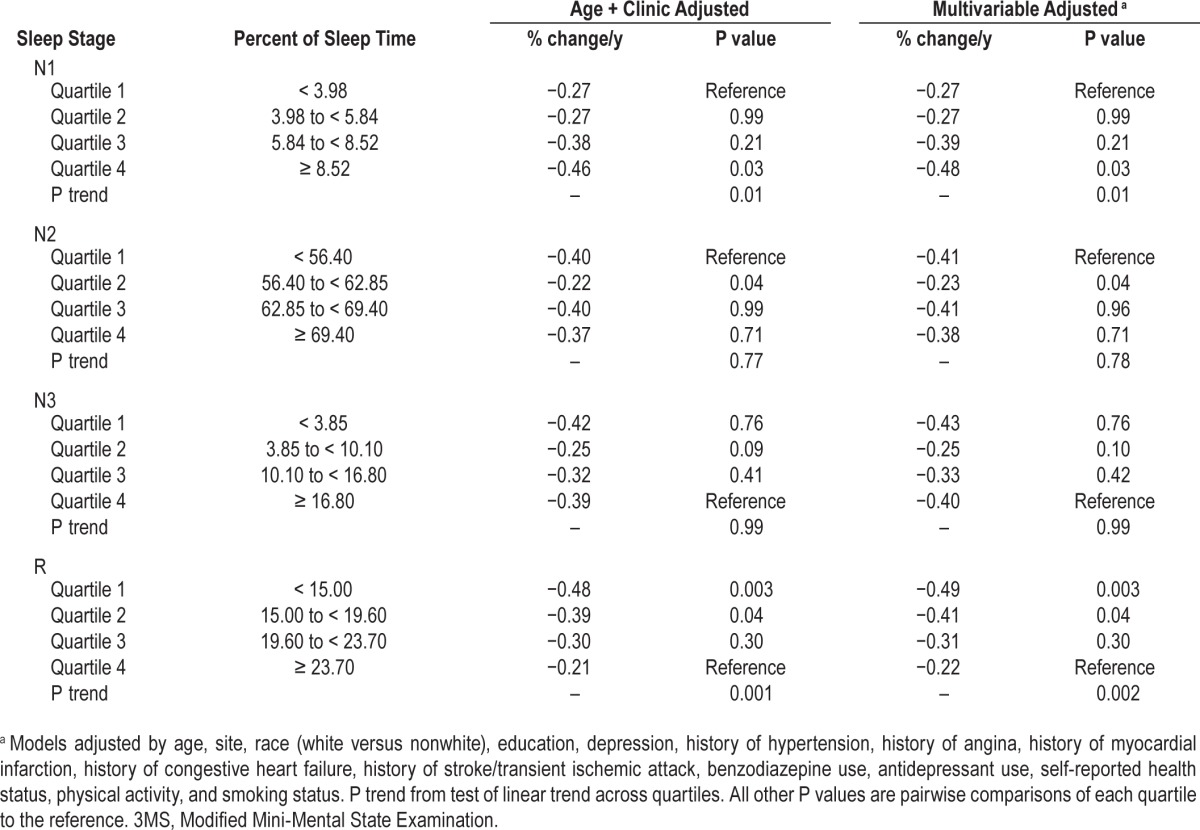
Figure 2.
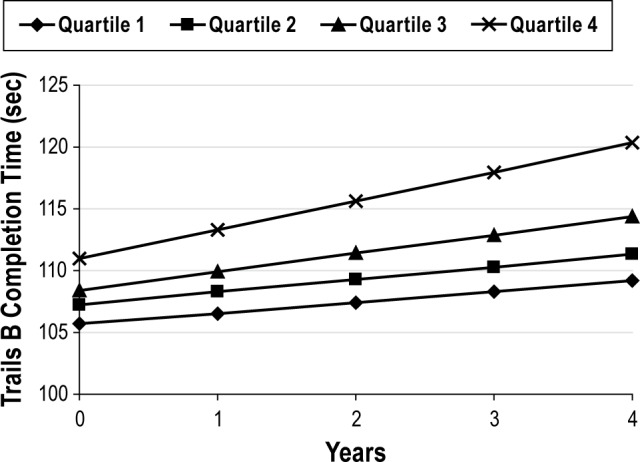
Adjusted mean change in Trails B completion time by quartile of percent of sleep time spent in Stage N1. Adjusted by age, site, race (white versus nonwhite), education, depression, history of hypertension, history of angina, history of myocardial infarction, history of congestive heart failure, history of stroke/transient ischemic attack, benzodiazepine use, antidepressant use, self-reported health status, physical activity, and smoking status.
For the 3MS (Table 3), greater percent of time spent in N1 was associated with an increased rate of cognitive decline over follow-up (P trend = 0.01). A statistically significant difference was noted between Quartile 1 and Quartile 4 (P = 0.03).
After multivariable adjustment, spending less percent of sleep time in Stage R was associated with a greater rate of decline in 3MS scores over time (P trend = 0.002). Men with the lowest quartile of Stage R experienced more than twice the rate of annual decline in 3MS scores (−0.49 versus −0.22 points, respectively), and the differences between Quartile 4 and Quartiles 1 and 2 were statistically significant (P ≤ 0.04). Changes in 3MS scores across quartiles of percent of sleep time spent in Stage R are shown in Figure 3. On average, the 3MS score at time zero was similar across quartiles, but over time the rate of change increased more rapidly for men in the lowest quartile of % time spent in Stage R. No significant associations were seen between the percent of sleep time spent in Stages N2 or N3 and cognitive change over time.
Figure 3.
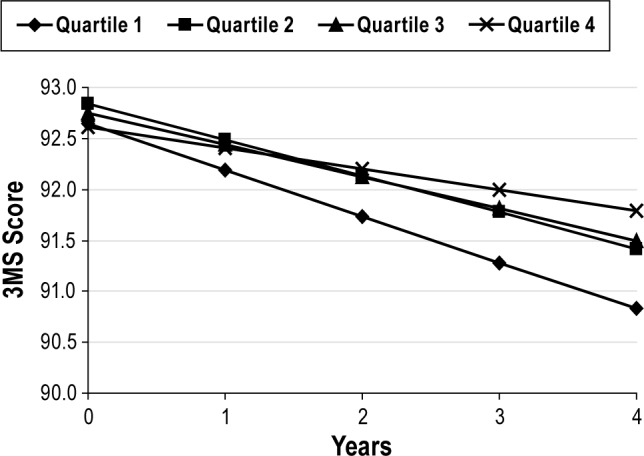
Adjusted mean change in 3MS score by quartile of percent of sleep time spent in Stage R. Adjusted by age, site, race (white versus nonwhite), education, depression, history of hypertension, history of angina, history of myocardial infarction, history of congestive heart failure, history of stroke/transient ischemic attack, benzodiazepine use, antidepressant use, self-reported health status, physical activity, and smoking status.
Association of Sleep Stages and Clinically Relevant Cognitive Decline
Of clinical significance, at Visit 3, 18.6% of men were considered to have cognitive decline based on the 3MS (five-point decline), and 6.8% of men showed evidence of cognitive decline based on Trails B (completion time increased by 82.09 sec or more). The associations between sleep stages and these outcomes are shown in Table 4.
Table 4.
Associations of sleep stages and clinically relevant cognitive decline, odds ratio (95% confidence interval).
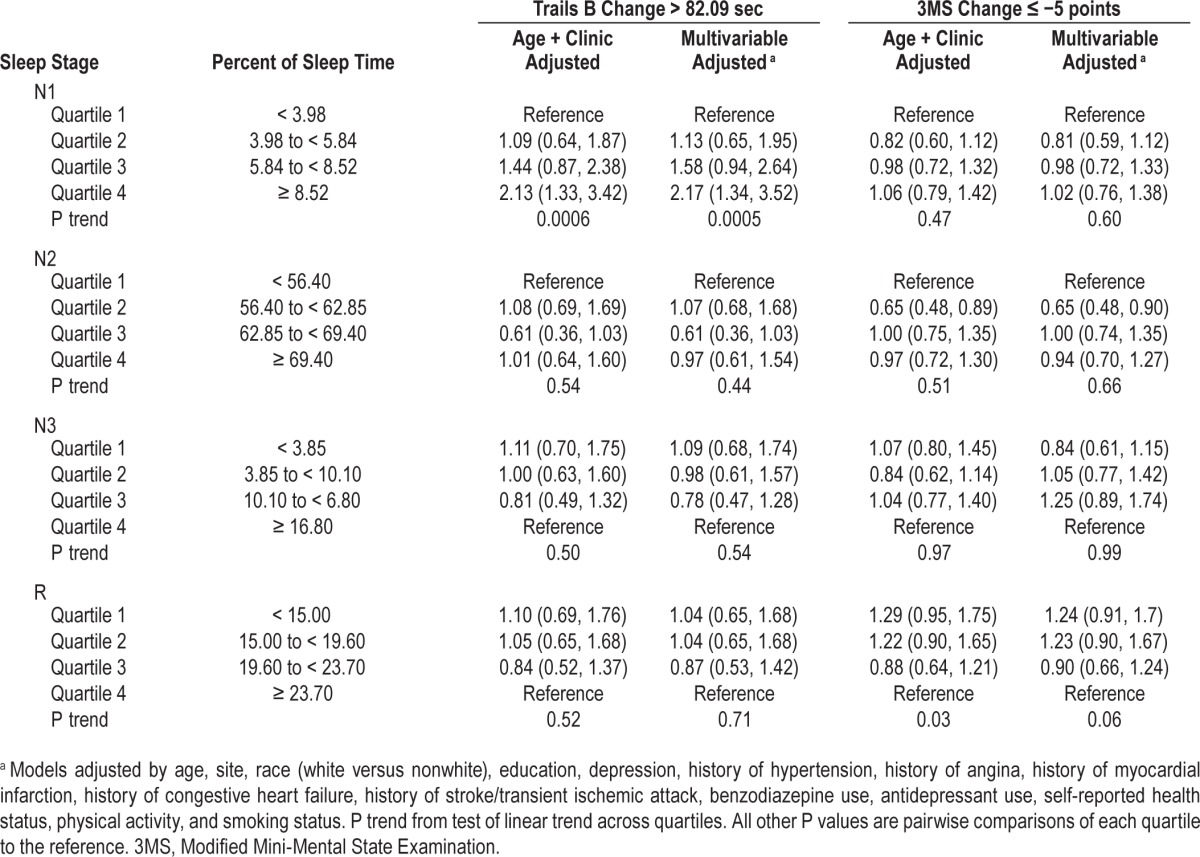
After multivariable adjustment, the highest percent of sleep time spent in Stage N1 was found to be associated with a twofold increase in the odds of clinically relevant cognitive decline, as defined by the Trails B test (Quartile 4 versus Quartile 1: OR = 2.17, 95% CI 1.34–3.52). No associations were seen with percent of sleep time spent in Stage N2, N3, or R and clinically relevant cognitive decline.
Additional Analyses
Results from analyses using minutes of time spent in the stages of sleep rather than percent time were similar. Results were also similar and remained significant after further adjustment of the multivariable models by sleep fragmentation (i.e., WASO), TST, and AHI, although effect sizes were attenuated. Significant findings of cognitive changes by quartile of Stage N1 persisted even after controlling for Stage R and vice versa. The results were also similar and remained significant even after participants who were taking benzodiazepines and SSRIs were excluded in the multivariable models.
DISCUSSION
This longitudinal analysis of 2,601, older, community-dwelling men suggests a significant association between sleep stage distribution and the risk of cognitive decline over time. A larger proportion of time spent in Stage N1 (considered “light” sleep and a marker of poorer sleep quality) and less time in Stage R (considered to play a role in memory) were each associated with more rapid declines in cognition.
Similarities and differences were found between this longitudinal study and our cross-sectional analysis of the MrOS Sleep Study. In the longitudinal analysis, we found that greater time spent in stage N1 was associated with a greater decline in ability to complete the Trails B Test and greater decline in scores on the 3MS. In the cross-sectional analysis (n = 2,909), those with the highest percent time spent in Stage N1 sleep took longer (on average of 7.4 sec) to complete the Trails B Test and had a lower 3MS score (about one point) than those with less percent time spent in N1 sleep after multivariate adjustment.51 In our longitudinal analysis, the rate of decline in 3MS scores increased as sleep time spent in Stage R decreased. These significant relationships remained even after controlling for age, race, education, comorbidities, medication use, other health status (e.g., depression), and social factors (e.g., smoking status). In our cross-sectional analysis, however, Stage R was not associated with the 3MS score but was significantly associated with Trails B time. The difference in results between the cross-sectional and longitudinal analyses could be explained by the higher sensitivity of the Trails B Test to cognitive impairment, particularly executive functioning.53 Additionally, our study participants had little cognitive impairment at baseline (sleep visit).
Our additional analysis found that these associations were robust to further adjustment for sleep fragmentation, TST, and sleep disordered breathing. Our significant findings of annual changes in Trails B and 3MS scores by Stage N1 persisted even after the multivariable models were adjusted by Stage R and vice versa. Moreover, the findings did not differ even when participants taking benzodiazepines and SSRIs were excluded in the models.
Increasing evidence indicates that sleep is important to a range of cognitive processes over a person's lifespan. Numerous studies have confirmed cognitive dysfunction following sleep deprivation in adults.54 Even children of early school age with shortened nocturnal sleep have shown worse cognitive function throughout their childhood.55,56 Sleep is also an essential function for memory consolidation; significant relationships have been reported between different sleep stages and specific cognitive function tests. For example, a visual text discrimination task (i.e., identifying the orientation of an array of diagonal bars against a background grid of horizontal bars) correlated with Stages N3 and R,57,58 whereas improvement in a motor sequence learning task (i.e., typing a simple numeric sequence)59 and motor adaption learning tasks (i.e., drawing a line from a starting point to a target against a rotating force)60 correlated with Stages N2 and N3, respectively.
Our findings demonstrate strong associations between sleep stages and decline in cognitive function over time in older men. Most of the previous longitudinal studies have focused on the relationship between sleep duration and cognitive function, suggesting sleep duration as a marker of cognitive deficits.18–20,23 A population-based cohort study of 689 older adults showed that increased sleep duration (> 9 h/night at follow-up 8.5 y later) was associated with an increased prevalence of cognitive impairment after adjusting for participants' characteristics, comorbidities, and other factors (e.g., BMI, depression, and use of sleep medication).19 Another study of more than 5,000 middle and older aged adults also reported that adverse changes in self-reported sleep duration were associated with low cognitive function, even after adjusting for covariates over 5 y.23 In another study, change in sleep efficiency measured by PSG was associated with cognitive decline over 2 y among older adults in a sleep laboratory study.61
Our findings on Stage R sleep and cognitive decline are also consistent with previous studies.17,62,63 A recent study of 57 healthy, middle-aged, older adults found a significant, positive relationship between time spent in Stage R (both in duration and percent) during the baseline night and verbal memory task the next morning, but no significant associations were found with other cognitive components such as attention, verbal fluency, and perceptual skills.17 In our study, after multivariable adjustment, we found a significant relationship between Stage R and linear change in 3MS. However, no relationship was found when the clinically relevant cutoff on the 3MS score (decline of five points or more) was used. Although verbal memory is a component of the 3MS, our study lacked a cognitive test that specifically measured verbal memory.
This study found no significant difference between Stage N3 and cognitive function, which is consistent with our cross-sectional analysis24 and previous studies.64,65 The importance of Stage N3 to cognitive function, particularly memory consolidation, has been well documented in younger adults.66–68 Moreover, the amount of hippocampal activity expressed during Stage N3 positively correlated with improved performance on episodic memory the next day in young adults. In older adults, however, experimental studies have not shown that an increase in Stage N3 is associated with improved memory, suggesting that the functional relationship between Stage N3 and cognitive function may weaken or change in older age.66 For example, sleep benefits were found with strong positive correlation between Stage N3 and retention of word pairs encoded before sleep in young adults (n = 57), whereas older adults (n = 41) showed no benefits or sleep with even a negative correlation between Stage N3 and subsequent learning.66 Among older adults, the reduced amount and/or percent of time spent in Stage N3 in older adults,8 the age-related decrease in amplitude of delta waves that composes N3, the generalized slowing of the electroencephalograph, the alteration in hippocampus, and age-related changes in some neuroendocrine and neuromodulatory systems could be part of the link.69
This study has several strengths, including a large population of community-dwelling older men who were free of probable dementia at the initial visit. We also used objective sleep measures including PSG. Additionally, the study addressed multiple potential confounding factors: demographic factors, social factors, physical and psychological states of health, and medication use. In addition, the associations between sleep and cognitive decline were robust even after adjusting for these covariates as well as sleep fragmentation and sleep disordered breathing.
Despite its numerous strengths, this study had its limitations. First, participants had relatively high levels of cognitive function at baseline (sleep visit) and follow-up (Visits 2 or 3). Moreover, the frequencies of clinically relevant cognitive impairment using the cutoffs for both Trails B and 3MS were small. Andrew and Rockwood42 have reported that a change of five or more points likely represents a clinically meaningful difference, especially when needed to define cognitive change in individuals. The failure of our study to detect a statistically significant, multivariable-adjusted association between R and the 3MS cutoff (≤ −5 points) might imply that the participants were still too healthy or that the follow-up period (3.4 y) was too short to observe significant declines in cognitive impairment. Previous longitudinal studies of cognitive function in older adults have used longer follow-up periods.52,70 Second, our findings cannot be generalized to other populations such as women or younger age groups because the study targeted only community-dwelling older men. Third, sleep stages were based on a single overnight PSG recording, which might have caused first-night effect. Moreover, interscorer reliability for Stage N1 was modest (correlation coefficient 0.6), which was smaller than other sleep stages. Thus, the relationship between Stage N1 and cognitive decline may not be weighted as much as the correlations between Stage R and cognitive decline. Finally, the study measured limited aspects of cognitive function. It did not use Trails Part A, a companion of Part B. The Trails Part B reflects working memory, mental flexibility, and sequencing, whereas Part A reflects sequencing and speed by asking individuals to connect circled numbers with a line in a numerical sequence (i.e., 1-2-3, etc.) as rapidly as possible.71 Using both Trails tests would have allowed us to determine whether the decline in cognitive function that we found reflected simply issues of speed and sequencing or a higher level of cognitive function. Although the 3MS measures a large range of cognitive function, other more specific aspects of memory (e.g., procedural memory) should be considered in future studies.
In conclusion, our prospective findings suggest that spending more time in Stage N1 sleep and less in Stage R was significantly associated with worsening cognitive performance in older men over time. Improving sleep may decrease the rate of cognitive decline in older men.
DISCLOSURE STATEMENT
This was not an industry supported study. The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute provides funding for the ancillary MrOS Sleep Study, “Outcomes of Sleep Disorders in Older Men,” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. The analysis was performed at California Pacific Medical Center Research Institute, San Francisco, CA. Dr. Ancoli-Israel is a consultant and on the scientific advisory board for Arena, Ferring Pharmaceuticals Inc., Jansen, Merck, NeuroVigil, Inc., and Purdue Pharma LP. Dr. Redline received research grants from ResMed Foundation and equipment for use in research from ResMed Inc and Philips-Respironics. Dr. Yaffe serves on data safety monitoring boards for Takeda Pharmaceuticals, Inc. and an NIH sponsored study. She is a consultant for Novartis and Pfizer and serves on the Beeson Scientific Advisory Board. The other authors have indicated no financial conflicts of interest.
Footnotes
A commentary on this article appears in this issue on page 335.
REFERENCES
- 1.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991;14:496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- 2.Ferri R, Manconi M, Lanuzza B, et al. Age-related changes in periodic leg movements during sleep in patients with restless legs syndrome. Sleep Med. 2008;9:790–8. doi: 10.1016/j.sleep.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Claman DM, Redline S, Blackwell T, et al. Prevalence and correlates of periodic limb movements in older women. J Clin Sleep Med. 2006;2:438–45. [PubMed] [Google Scholar]
- 4.Schubert CR, Cruickshanks KJ, Dalton DS, Klein BE, Klein R, Nondahl DM. Prevalence of sleep problems and quality of life in an older population. Sleep. 2002;25:889–93. [PubMed] [Google Scholar]
- 5.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 6.Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia, PA: Elsevier Saunders; 2005. pp. 24–38. [Google Scholar]
- 7.Feinsilver SH. Sleep in the elderly. What is normal? Clin. Geriatr Med. 2003;19:177–88. viii. doi: 10.1016/s0749-0690(02)00064-2. [DOI] [PubMed] [Google Scholar]
- 8.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 9.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 10.Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18:436–46. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 11.Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–7. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–8. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 13.Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behav Sleep Med. 2007;5:39–56. doi: 10.1207/s15402010bsm0501_3. [DOI] [PubMed] [Google Scholar]
- 14.Spiegel R. Sleep and sleeplessness in advanced age. New York: Spectrum; 1981. [Google Scholar]
- 15.Spiegel R, Herzog A, Koberle S. Polygraphic sleep criteria as predictors of successful aging: an exploratory longitudinal study. Biol Psychiatry. 1999;45:435–42. doi: 10.1016/s0006-3223(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel R, Koberle S, Allen SR. Significance of slow wave sleep: considerations from a clinical viewpoint. Sleep. 1986;9:66–79. doi: 10.1093/sleep/9.1.66. [DOI] [PubMed] [Google Scholar]
- 17.Lafortune M, Gagnon JF, Martin N, et al. Sleep spindles and rapid eye movement sleep as predictors of next morning cognitive performance in healthy middle-aged and older participants. J Sleep Res. 2014;23:159–67. doi: 10.1111/jsr.12108. [DOI] [PubMed] [Google Scholar]
- 18.Benito-Leon J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol. 2009;16:990–7. doi: 10.1111/j.1468-1331.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 19.Loerbroks A, Debling D, Amelang M, Sturmer T. Nocturnal sleep duration and cognitive impairment in a population-based study of older adults. Int J Geriatr Psychiatry. 2010;25:100–9. doi: 10.1002/gps.2305. [DOI] [PubMed] [Google Scholar]
- 20.Potvin O, Lorrain D, Forget H, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–9. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–9. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 22.Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS) Int J Geriatr Psychiatry. 2002;17:73–7. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- 23.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34:565–73. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2011;59:2217–25. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Mehra R, Stone KL, Blackwell T, et al. Prevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55:1356–64. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Kales A, editors. Bethesda, MD: National Institute of Neurologicla Disease and Blindness, Neurological Information Network; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 29.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 30.Quan SF, Griswold ME, Iber C, et al. Short-term variability of respiration and sleep during unattended nonlaboratory polysomnography--the Sleep Heart Health Study. [corrected] Sleep. 2002;25:843–9. [PubMed] [Google Scholar]
- 31.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 32.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 33.Hayden KM, Khachaturian AS, Tschanz JT, Corcoran C, Nortond M, Breitner JC. Characteristics of a two-stage screen for incident dementia. Journal of clinical epidemiology. 2003;56:1038–45. doi: 10.1016/s0895-4356(03)00247-6. [DOI] [PubMed] [Google Scholar]
- 34.Teng EL, Chui HC, Hubbard D, Corgiat MD. The Modified Mini-Mental State (the 3MS) test. Clin Neuropsychology. 1987;1:293. [Google Scholar]
- 35.McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50:377–83. doi: 10.1016/s0895-4356(97)00060-7. [DOI] [PubMed] [Google Scholar]
- 36.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 37.Yaffe K, Blackwell T, Barnes DE, Ancoli-Israel S, Stone KL. Preclinical cognitive decline and subsequent sleep disturbance in older women. Neurology. 2007;69:237–42. doi: 10.1212/01.wnl.0000265814.69163.da. [DOI] [PubMed] [Google Scholar]
- 38.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 39.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–42. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 40.Koster A, Penninx BW, Bosma H, et al. Socioeconomic differences in cognitive decline and the role of biomedical factors. Ann Epidemiol. 2005;15:564–71. doi: 10.1016/j.annepidem.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 42.Andrew MK, Rockwood K. A five-point change in Modified Mini-Mental State Examination was clinically meaningful in community-dwelling elderly people. J Clin Epidemiol. 2008;61:827–31. doi: 10.1016/j.jclinepi.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Schinka JA, Loewenstein DA, Raj A, et al. Defining mild cognitive impairment: impact of varying decision criteria on neuropsychological diagnostic frequencies and correlates. Am J Geriatr Psychiatry. 2010;18:684–91. doi: 10.1097/JGP.0b013e3181e56d5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachman DL, Wolf PA, Linn RT, et al. Incidence of dementia and probable Alzheimer's disease in a general population: the Framingham Study. Neurology. 1993;43:515–9. doi: 10.1212/wnl.43.3_part_1.515. [DOI] [PubMed] [Google Scholar]
- 45.Hong CH, Falvey C, Harris TB, et al. Anemia and risk of dementia in older adults: findings from the Health ABC study. Neurology. 2013;81:528–33. doi: 10.1212/WNL.0b013e31829e701d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 47.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–29. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 48.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 49.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 50.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 51.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS Sleep Study. Sleep. 2011;34:1347–56. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamez E, Myerson J, Morris L, White DA, Baum C, Connor LT. Assessing executive abilities following acute stroke with the trail making test and digit span. Behav Neurol. 2011;24:177–85. doi: 10.3233/BEN-2011-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3:553–67. [PMC free article] [PubMed] [Google Scholar]
- 55.Touchette E, Petit D, Seguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/ cognitive functioning at school entry. Sleep. 2007;30:1213–9. doi: 10.1093/sleep/30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buckhalt JA, El-Sheikh M, Keller PS, Kelly RJ. Concurrent and longitudinal relations between children's sleep and cognitive functioning: the moderating role of parent education. Child Dev. 2009;80:875–92. doi: 10.1111/j.1467-8624.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 57.Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: a multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246–54. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki H, Uchiyama M, Aritake S, et al. Alpha activity during rem sleep contributes to overnight improvement in performance on a visual discrimination task. Percept Mot Skills. 2012;115:337–48. doi: 10.2466/22.24.29.PMS.115.5.337-348. [DOI] [PubMed] [Google Scholar]
- 59.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 60.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 61.Hoch CC, Dew MA, Reynolds CF, 3rd, et al. A longitudinal study of laboratory- and diary-based sleep measures in healthy “old old” and “young old” volunteers. Sleep. 1994;17:489–96. doi: 10.1093/sleep/17.6.489. [DOI] [PubMed] [Google Scholar]
- 62.Feinberg I, Koresko RL, Heller N. EEG sleep patterns as a function of normal and pathological aging in man. J Psychiatr Res. 1967;5:107–44. doi: 10.1016/0022-3956(67)90027-1. [DOI] [PubMed] [Google Scholar]
- 63.Kahn E, Fisher C. Some correlates of rapid eye movement sleep in the normal aged male. J Nerv Ment Dis. 1969;148:495–505. doi: 10.1097/00005053-196905000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Rauchs G, Schabus M, Parapatics S, et al. Is there a link between sleep changes and memory in Alzheimer's disease? Neuroreport. 2008;19:1159–62. doi: 10.1097/WNR.0b013e32830867c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crenshaw MC, Edinger JD. Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiol Behav. 1999;66:485–92. doi: 10.1016/s0031-9384(98)00316-3. [DOI] [PubMed] [Google Scholar]
- 66.Scullin MK. Sleep, memory, and aging: the link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol Aging. 2013;28:105–14. doi: 10.1037/a0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–9. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 68.Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13:309–21. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 69.Harand C, Bertran F, Doidy F, et al. How aging affects sleep-dependent memory consolidation? Front Neurol. 2012;3:8. doi: 10.3389/fneur.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yaffe K, Falvey C, Hamilton N, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol. 2012;69:1170–5. doi: 10.1001/archneurol.2012.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salthouse TA. What cognitive abilities are involved in trail-making performance? Intelligence. 2011;39:222–32. doi: 10.1016/j.intell.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


