Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is common in patients with coronary artery disease (CAD). Enhanced vascular inflammation is implicated as a pathophysiologic mechanism but obesity is confounding. We aimed to address the association of OSA with inflammatory biomarkers in a nonobese cohort of revascularized patients with CAD and preserved left ventricular ejection fraction.
Design:
Cross-sectional analysis of baseline investigations of a randomized controlled trial.
Setting:
Clinic-based.
Participants:
There were 329 nonobese patients with CAD, of whom 234 with OSA (apnea-hypopnea index [AHI] ≥ 15 events/h) and 95 without OSA (AHI < 5 events/h). Obese patients with CAD and OSA (N = 105) were chosen as an additional control group.
Interventions:
None.
Measurements:
Circulating levels of high-sensitivity C-reactive protein (hs-CRP), interleukin (IL)-6, IL-8, and tumor necrosis factor-α were assessed in relation to OSA diagnosis based on AHI ≥ 15 events/h as well as oxygen desaturation index (ODI) ≥ 5 events/h.
Results:
Nonobese patients with OSA had significantly higher levels of hs-CRP and IL-6 than those without OSA. The values did not differ significantly between obese and nonobese patients with OSA. In bivariate regression analysis, AHI ≥ 15 events/h was associated with all four biomarkers but not so in the multivariate model after adjustment for confounders. ODI ≥ 5 events/h was associated with hs-CRP (odds ratio [OR] 1.49, 95% confidence interval [CI] 1.13–1.99) and IL-6 (OR 1.30; 95% CI 1.05–1.60) in multivariate analysis.
Conclusions:
Obstructive sleep apnea with oxygen desaturation index ≥ 5 was independently associated with increased inflammatory activity in this nonobese coronary artery disease cohort. The intermittent hypoxemia, rather than the number of apneas and hypopneas, appears to be primarily associated with enhanced inflammation.
Citation:
Thunström E, Glantz H, Fu M, Yucel-Lindberg T, Petzold M, Lindberg K, Peker Y. Increased inflammatory activity in nonobese patients with coronary artery disease and obstructive sleep apnea. SLEEP 2015;38(3):463–471.
Keywords: cardiovascular disease risk factors, inflammation, obstructive sleep apnea
INTRODUCTION
Inflammation plays a crucial role in the development of plaque in coronary artery disease (CAD).1 Among the traditionally recognized cardiovascular risk factors, obesity is shown to have the closest relationship with increased inflammatory activity.2 Obstructive sleep apnea (OSA) is a common condition in patients with CAD3; it is coexisting in individuals with obesity, as well as in those with hypertension and diabetes.4,5 Moreover, it is associated with increased inflammatory activity.6 However, whether inflammation is further upregulated in CAD with concomitant OSA is currently unknown.
Several biomarkers have been investigated in the context of inflammation and CAD. High-sensitivity C-reactive protein (hs-CRP) has been studied intensively and found to be associated with CAD.7 Moreover, data suggest that interleukin-(IL) 6 may also be associated with CAD.8 Furthermore, some cross-sectional observational studies in sleep clinic cohorts9,10 as well as in a community-based population,11 have suggested an association between OSA and inflammation regarding hs-CRP,10 IL-6,10,11 and tumor necrosis factor (TNF)-α.9 There are, however, conflicting studies that do not show independent associations of OSA and inflammation12,13 or no change following continuous positive airway pressure (CPAP) treatment.14,15 Little is known regarding the effect of OSA on inflammatory activity in patients with CAD. Because obesity is a common condition in OSA,16 and is also related to low-grade inflammation,4,17 it should be considered as a major confounding factor when studying the influence of OSA on inflammatory activity in patients with CAD. Heart failure, which is known to increase inflammatory activity,18 may also confound a possible relationship between OSA and inflammatory markers in a population with CAD.
The Randomized Intervention with CPAP in Coronary Artery Disease and Obstructive Sleep Apnea (RICCADSA) study is a randomized controlled trial (RCT) with 511 participants, designed to investigate whether CPAP treatment of OSA in patients with CAD decreases the risk of new cardiovascular events.19,20 In the current study, we performed a cross-sectional analysis of inflammatory biomarkers (hs-CRP, IL-6, IL-8, and TNF-α) at baseline to explore the association of OSA with inflamma-tory activity in patients with CAD and preserved left ventricular ejection fraction (LVEF) independent of obesity. Moreover, we addressed whether the number of apneas and hypopneas, or the degree of intermittent hypoxemia in OSA, is primarily associated with increased inflammatory activity in CAD.
METHODS
Patient Population
The study population included in this cohort has been previously described elsewhere.19,20 In brief, all consecutive patients with CAD (N = 1,291) who had recently (< 6 mo) undergone percutaneous coronary intervention or coronary artery bypass grafting (CABG) in the catchment area of the Skaraborg Hospitals (Skövde and Lidköping) between September 29, 2005, and November 7, 2010 were asked to participate in the trial (Figure 1). Anthropometrics and medical history were documented for the whole cohort (Table 1). After exclusion of 32 patients with a known OSA diagnosis and/or treatment of OSA, a total of 1,259 patients were eligible for the study. Among those, 662 agreed to undergo an ambulatory, polygraphic cardiorespiratory sleep study at home. For the main RCT, 511 patients fulfilled the inclusion criteria. After exclusion of 64 patients with LVEF < 50%, and 2 patients with no echocardiography, 445 patients remained for inclusion in the current study. Because obesity is the major confounding factor when analyzing the association between OSA and inflammatory markers in patients with CAD,4 the nonobese patients in the RICCADSA study (N = 329) were chosen as the main group for the purpose of the current analysis. Obese patients with CAD and OSA (N = 110) served as an additional control group (Figure 1).
Figure 1.
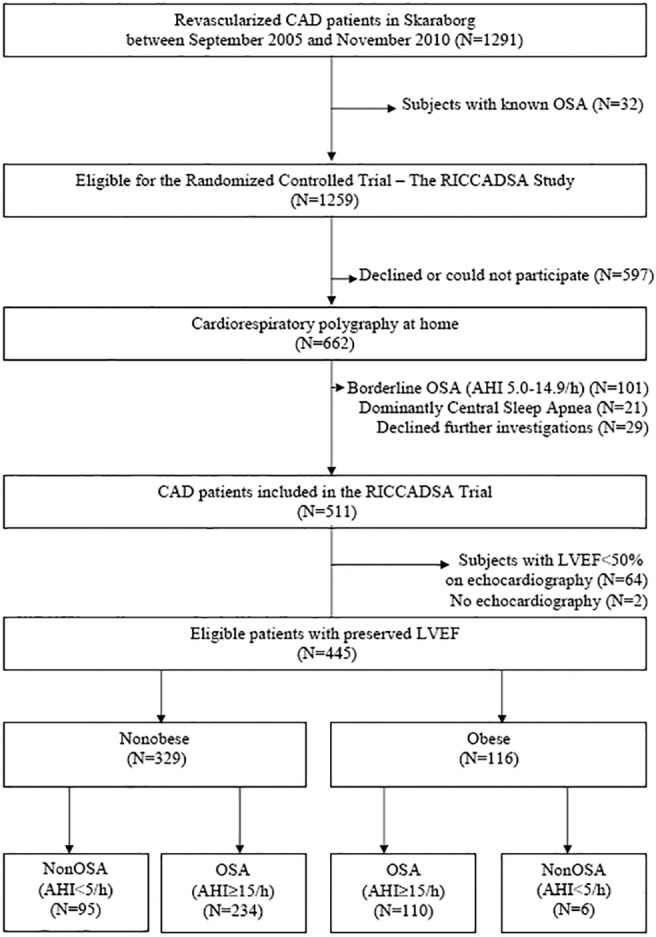
Ascertainment of the study sample. AHI, apnea-hypopnea index; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnea; RICCADSA, randomized intervention with CPAP in coronary artery disease and sleep apnea.
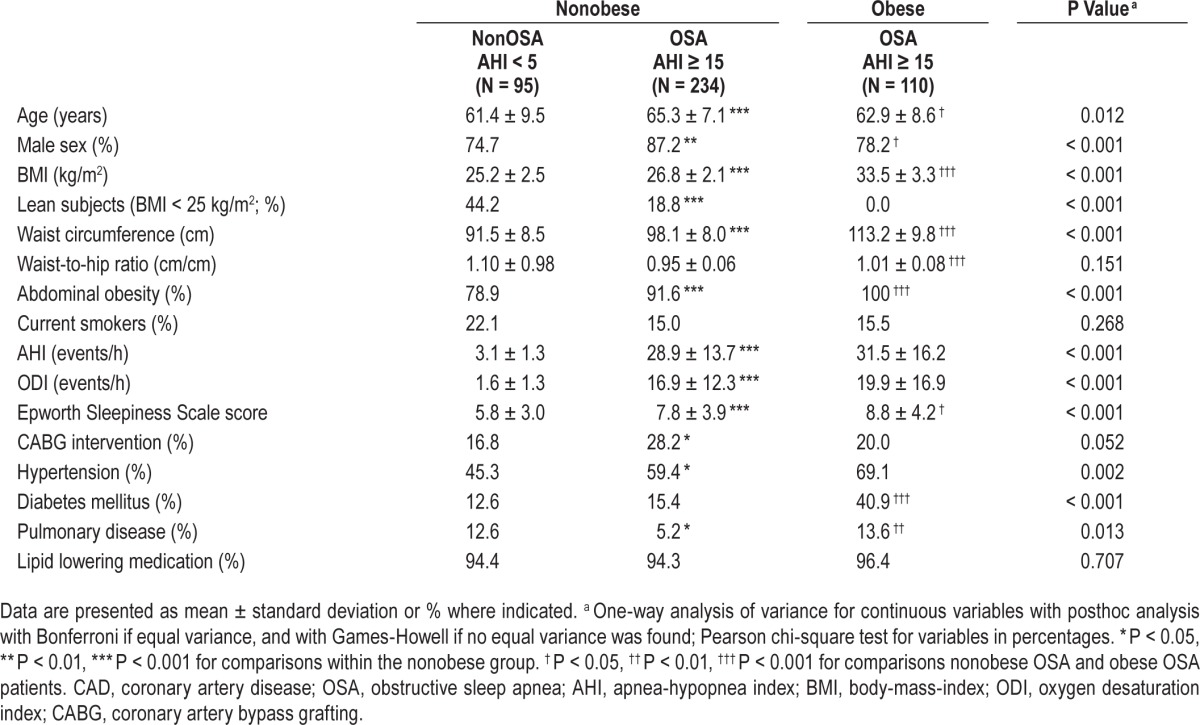
Table 1.
Demographic and clinical characteristics of the patients with revascularized CAD and preserved ejection fraction.
This study complies with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of the Medical Faculty of the University of Gothenburg. The trial was registered with ClinicalTrials.gov (NCT 00519597).
Cardiorespiratory Polygraphy at Home
The portable, limited sleep study was performed with the Embletta Portable Digital System device (Embla, Broomfield, CO, USA), and consisted of a nasal pressure detector using a nasal cannula/pressure transducer system, thoracoabdominal movement detection through two respiratory inductance plethysmography belts, and a finger pulse oximeter detecting heart rate and oxyhemoglobin saturation (SpO2) as well as body position and movement detection. The transformed flow signal from the cannula was used as the primary signal for scoring apneas and hypopneas Apnea was defined as an almost-complete (≥ 90%) cessation of airflow, and hypopnea was defined as a reduction in nasal pressure amplitude of ≥ 50% and/ or thoracoabdominal movement ≥ 50% for ≥ 10 sec according to the recommendations of the American Academy of Sleep Medicine.21 In addition, the total number of significant oxy-hemoglobin desaturations (decrease by at least 4% from the immediately preceding baseline value) was scored, and the oxygen desaturation index (ODI) was calculated as the number of significant desaturations per hour of estimated sleep. Events with a nasal pressure amplitude of ≥ 30% and/or with a reduction in thoracoabdominal movement ≥ 30% for ≥ 10 sec were also scored as hypopneas if there was significant oxygen desaturation (≥ 4%).21 OSA was defined as an apnea-hypopnea index (AHI) ≥ 15 events/h of the total recording time. All baseline screening recordings were scored by the same observer (YP).
Blood Sampling
All fasting blood samples were collected in EDTA and serum tubes in the morning between 08:00–10:00 at the time of study inclusion following the baseline sleep recordings (median 35 days; interquartile range (IQR) 20 –45 days with no differences between OSA and non-OSA subjects), and on average 97 days after intervention (median 92 days; IQR 71–120 days, without significant group differences). The tubes were centrifuged and the plasma/serum samples were aliquoted and stored at −70°C. Serum levels of hs-CRP were measured by immunoturbidimetry using the infrared immunoassay rate method and the near-infrared particle immunoassay at the Karolinska University Laboratory (Solna, Sweden) in a routine clinical analyzer.22 The detection limit for hs-CRP was 0.20 mg/L with a measuring range of 0.20–380 mg/L. The levels of the proinflammatory biomarkers IL-6, IL-8, and TNF-α were analyzed in undiluted plasma samples using commercially available Milliplex MAP (based on Luminex technology) human serum adipokine assay kits in accordance with the manufacturer's instructions (Merck Millipore, Billerica, MA). Minimum detectable concentrations (the assay sensitivities) for IL-6, IL-8, and TNF-α were 0.6, 0.2, and 0.14 pg/mL, respectively. The concentrations of all samples (undiluted) were observed within the standard curve, ranging from 0 to 10,000 pg/mL for the biomarkers IL-6, IL-8, and TNF-α. The intra-assay and interassay variabilities (generated from the mean of the percentage coefficient of variability from multiple reportable results across two different concentrations of analytes in one experiment, or from two results each for two different concentrations of analytes across several different experiments) were 1.4–7.9% and < 21%, respectively.
Variables
Baseline anthropometrics, smoking habits, and medical histories of the entire study population were obtained from the medical records after mechanical revascularization. Body mass index (BMI) was calculated according to the formula of body weight divided by height squared. Obesity was defined as a BMI ≥ 30 kg/m2, and abdominal obesity was defined as waist-to-hip ratio ≥ 0.9 for men and ≥ 0.8 for women.23 Data regarding known concomitant diseases, type of mechanical intervention (percutaneous coronary intervention or CABG) as well as medications at baseline were based on the self-reported and/or physician-diagnosed conditions reported in the hospital records and/ or national registers. Excessive daytime sleepiness was based on the subjective Epworth Sleepiness Scale (ESS) questionnaire.24 Information regarding LVEF was obtained from the echocardio-graphic investigations at baseline as part of the study protocol for secondary endpoints of the main RICCADSA trial.19
Statistical Analysis
Statistical analysis was performed using the IBM SPSS version 20.0 for Windows software (SPSS Inc., Chicago, IL, USA). For comparison between nonobese non-OSA, nonobese OSA, and obese OSA groups, one-way analysis of variance was applied for continuous variables, and Pearson chi-square test for percentages. For comparison between patients with and without OSA within the nonobese group as well as between nonobese and obese patients with OSA, post hoc Bonferroni analysis was performed where equal variances were found, and Games Howell analysis was used where there was no equal variance. Because of skewed distribution, all inflammatory markers were transformed into their natural logarithmic values for determining the relationship between increased inflamma-tory activity and all demographics, clinical, and sleep parameters. Bivariate logistic regression was used to determine the relationship between OSA as a categorical variable based on AHI ≥ 15 events/h, ODI ≥ 5 events/h, or ODI ≥ 15 events/h, respectively, and the levels of inflammatory biomarkers, anthropomorphic variables, and other comorbidities. All variables that were significantly associated in the bivariate analyses were subsequently included in the multivariate models. All ORs are presented with their 95% confidence intervals (CI). Results are presented as mean ± standard deviation, and when appropriate, as median with interquartile range. Additional linear associations among biomarker values, obesity measures, and OSA severity were assessed using Pearson and Spearman correlations, and statistical adjustments were done in multiple linear regression models. All statistical tests were two-sided, and P < 0.05 was considered statistically significant.
RESULTS
As shown in Table 1, the nonobese CAD group with OSA was older, consisted of more men, had higher BMI and waist circumference, and the proportion of subjects with abdominal obesity, hypertension, and CABG at baseline was higher whereas concomitant lung disease was less common compared with nonobese patients with CAD without OSA. The proportion of lean patients was also lower in the nonobese OSA group. When comparing obese patients with OSA with the nonobese OSA group, the obese subjects were slightly younger, and had higher AHI, ODI, and ESS. The proportion of women and patients with diabetes mellitus as well as lung disease were also higher in the obese group (Table 1). Differences in demographic and clinical characteristics between patients with and without OSA did not change much when applying the ODI cutoff levels 5 and 15 (data not shown).
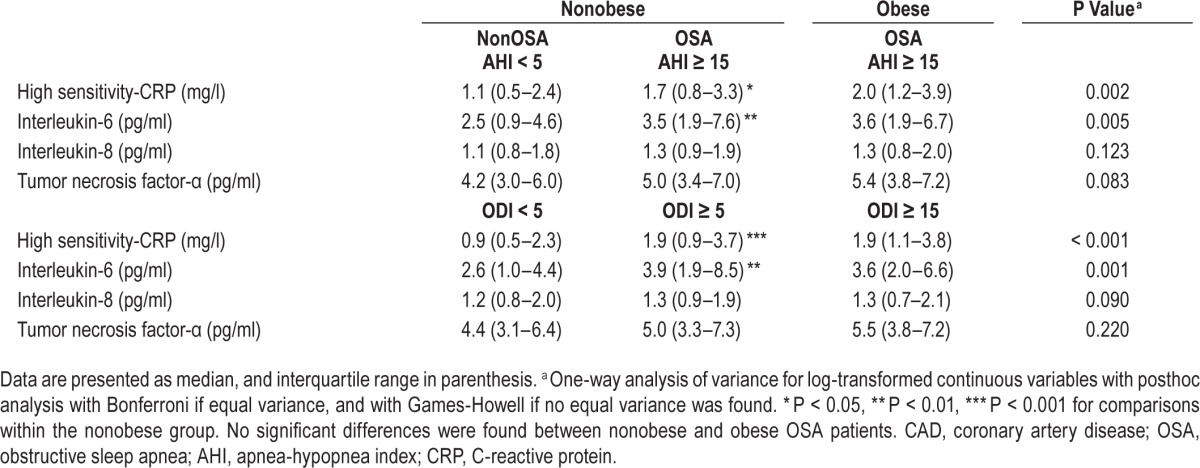
As shown in Table 2, the nonobese OSA group with AHI ≥ 15 events/h had significantly higher levels of hs-CRP and IL-6 compared with the levels in nonobese patients without OSA with AHI < 5 events/h. When comparing obese and nonobese patients with OSA, the levels of the biomarkers did not differ significantly. Similar results were observed regarding the biomarker levels within the groups based on OSA categories with ODI cut-off level of 5 events/h (Table 2). In the entire cohort, all of the biomarker levels measured were within the detection limits.
Table 2.
Levels of inflammatory biomarkers in the revascularized CAD cohort with preserved ejection fraction.
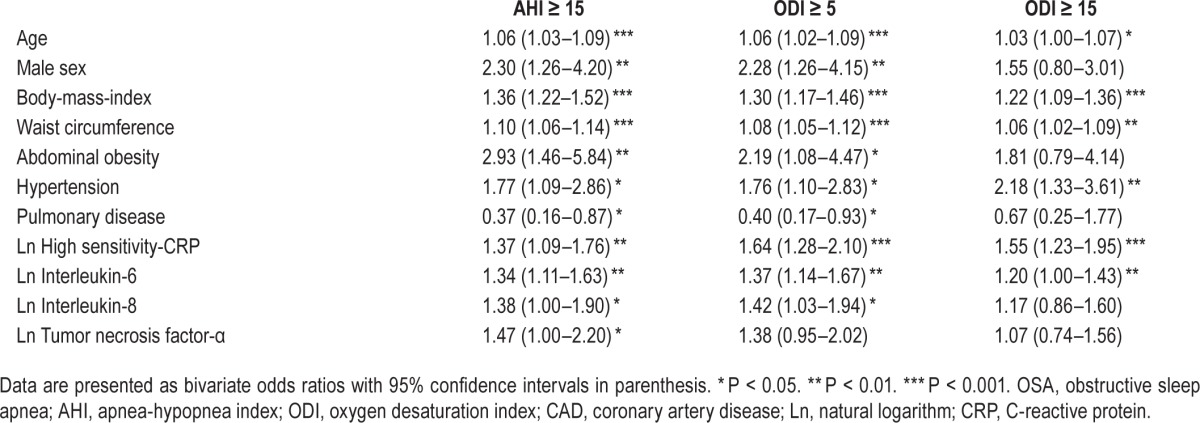
To further address the relationship between inflammatory biomarkers and OSA in the nonobese cohort, a logistic regression analysis was conducted (Table 3). Bivariate regression analysis showed that OSA based on AHI ≥ 15 was associated with all analyzed markers in addition to BMI, waist circumference, abdominal obesity, age, male sex, lung disease, and hypertension. Similar results were obtained with similar significance levels when OSA diagnosis based on ODI ≥ 5 was used, except for TNF-α. For the cutoff level of ODI ≥ 15, there was a significant relationship with BMI, waist circumference, age, and hypertension as well as with hs-CRP and IL-6 (Table 3). Lipid-lowering treatment and other comorbidities were not significant in the univariate regression models.
Table 3.
Significant demographic, clinical and inflammatory biomarker variables associated with OSA based on AHI and ODI cut-off values in the nonobese CAD cohort.
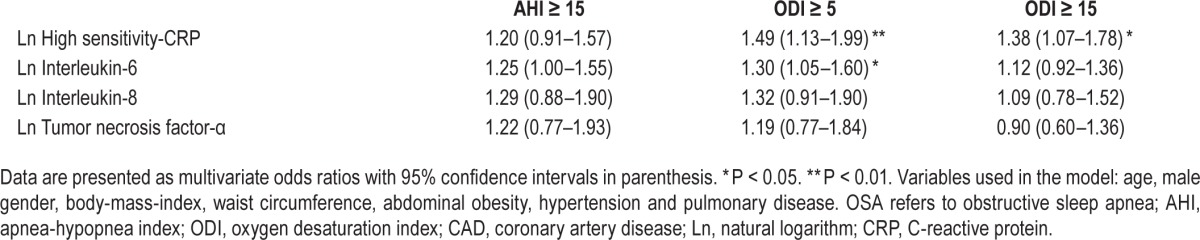
As shown in Table 4, after adjustment for all significant confounding factors, which included age, male sex, BMI, waist circumference, abdominal obesity, lung disease, and hypertension, AHI ≥ 15 events/h was not significantly associated with the biomarkers anymore, whereas ODI ≥ 5 events/h was significantly associated with both IL-6 and hs-CRP. When applying ODI cutoff level of 15, only hs-CRP remained significant.
Table 4.
Association between OSA based on AHI and ODI cut-off values and the inflammatory biomarkers adjusted for the confounding variables in the nonobese CAD cohort.
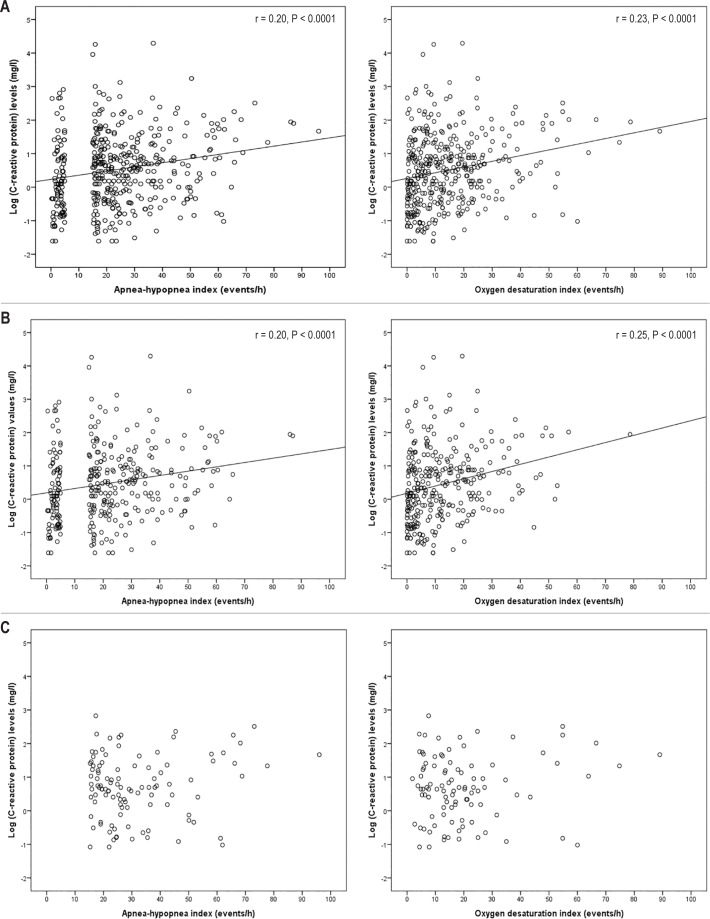
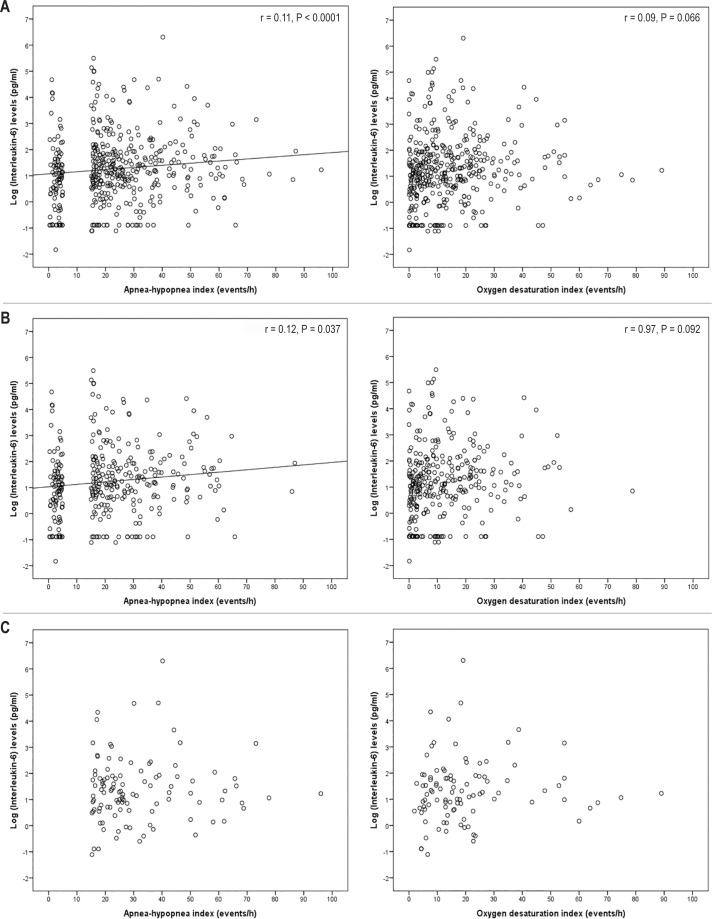
As shown in Figure 2, hs-CRP levels increased significantly with increasing AHI and ODI, respectively, in the entire cohort and in the nonobese group whereas there was no linear relationship between hs-CRP and AHI or ODI within the obese OSA group. IL-6 levels increased also with increasing AHI in the entire cohort as well as in the nonobese group, whereas there was no linear association between IL-6 and AHI in the obese patients with OSA (Figure 3). No significant associations were found between IL-6 levels and ODI values in any of the groups. In the multiple linear regression models, AHI was associated with hs-CRP levels adjusted for age, male sex, BMI, and waist circumference in the entire cohort (beta coefficient 0.114; 95% CI 0.001–0.013; P = 0.029), and in the non-obese population (beta coefficient 0.121; 95% CI 0.000–0.016; P = 0.043). When including ODI instead of AHI in the models, there was a linear relationship with hs-CRP in the entire cohort (beta coefficient 0.163; 95% CI 0.004–0.020; P = 0.002) as well as in the nonobese population (beta coefficient 0.183; 95% CI 0.006–0.027; P = 0.003).
Figure 2.
Correlations between obstructive sleep apnea measures and high-sensitivity C-reactive protein levels in serum in the entire cohort (A), the nonobese group (B), and the obese group (C).
Figure 3.
Correlations between obstructive sleep apnea measures and interleukin-6 levels in serum in the entire cohort (A), the nonobese group (B), and the obese group (C).
DISCUSSION
The main finding of the current study was that OSA, based on ODI ≥ 5, was independently associated with increased inflammatory activity in this nonobese CAD cohort, suggesting that inflammatory activity appears to be more closely associated with the intermittent hypoxemia rather than the number of apneic events.
It has been shown that upregulated inflammatory activity is associated with increased risk of the development of adverse CAD events.25,26 Among inflammatory markers, hs-CRP is one of the most studied prognostic markers for new cardiovascular events in patients with CAD,7,27 whereas this relationship appears to be modest regarding the primary prevention of CAD.28,29 Moreover, IL-6 has also been related to CAD.8 However, the studies performed in the sleep clinic and population-based cohorts have demonstrated an association between OSA and inflammation, though the findings were confounded by obesity.11,30,31 Observational intervention studies are scarce, and ongoing or recently completed randomized trials in this regard are not yet published. As the main analysis of the current cohort was made among the nonobese patients with CAD, and because the results were additionally adjusted for waist circumference and BMI, our findings suggest that the influence of OSA on the increased inflammatory activity in CAD is independent of obesity. The lack of significant differences regarding the levels of inflammatory markers between obese and nonobese patients with OSA further supports this conclusion.
The biomarkers TNF-α, IL-6, and IL-8 are some of the major proinflammatory cytokines and chemokines produced by multiple cells of the body, including abdominal adipose tissue.32 hs-CRP is an acute phase reactant that is produced in the liver, partly in response to IL-6 stimulation.33 The hs-CRP levels in our cohort, both in the OSA group and the non-OSA group, were higher than hs-CRP levels in an age- and sex-stratified general population,34 which might be explained by the fact that the non-OSA subjects in our study were not healthy controls, as they already had an established CAD with other comorbidities.
To further investigate the association between the increased inflammation observed in patients with CAD and OSA, we calculated OR for both OSA diagnosis categorically based on AHI ≥ 15 events/h, and for OSA diagnosis based on ODI ≥ 5 events/h as well as ODI ≥ 15 events/h, respectively. In bivariate regression analysis, OSA based on AHI ≥ 15 events/h was significantly associated with all four biomarkers but not so in the multivariable model, while already at the ODI level of ≥ 5 events/h, there was a significant relationship between OSA and both hs-CRP and IL-6. Interestingly, the relationship between ODI ≥ 15 and hs-CRP was still significant, whereas the relationship between ODI ≥ 15 and IL-6 was not. This might indicate that IL-6 was triggered earlier than hs-CRP, already at a lower ODI cutoff level. Moreover, some patients with ODI 5–15 events/h, categorized as non-OSA, might have attenuated the significance of this relationship.
Our findings regarding that hypoxemia rather than the apneas and hypopneas per se plays a major role regarding the effect of OSA on the increased inflammatory activity are in line with a previous report, suggesting a significant relationship between hypoxemia index (% sleep time with oxyhemoglobin saturation < 90%) and IL-6 in a community-based population.11 Though the applied criteria for hypopneas (combination of at least 30% decrease in nasal pressure with desaturations, and at least 50% decrease regardless of desaturations) according to scoring rules from 1999 might have affected the stronger association with ODI rather than AHI, these findings are also in accordance with the previous prospective cohort observation of the Sleep Heart Health Study suggesting that increased risk of cardiovascular mortality is primarily associated with intermittent hypoxemia following apneas, rather than apneic events themselves.35
It is difficult to compare the different results of previous studies regarding the relationship between OSA and inflamma-tory biomarkers, but as discussed intensively in a recent study by Arnardottir and colleagues,36 many of the previous studies had low numbers of patients, and few studies had nonobese patients with OSA. In the Icelandic Sleep Apnea Cohort, the interaction of OSA and obesity on the inflammatory markers hs-CRP and IL-6 was addressed, and OSA severity was found to be an independent predictor of levels of these inflammatory markers in obese patients but not in the nonobese patients with OSA.36 In contrast to those findings, our results suggest an independent relationship between OSA and inflammatory markers in a non-obese cohort. The lack of a control group without OSA in that report might be an explanation for this contrary. Moreover, our population was a CAD cohort, which means that interaction between OSA and CAD might trigger an inflammatory activity already at the lower levels of OSA severity in nonobese but overweight subjects with abdominal obesity. This interaction might be seen in otherwise healthy obese individuals with OSA but may start earlier in a cohort with concomitant CAD, and be confounded by already-established cardiovascular mechanisms and other concomitant comorbidities in obese patients with CAD. Indeed, a recent RCT in morbidly obese patients with OSA showed no effect of CPAP or weight loss, or both on hs-CRP levels after 8 to 24 weeks of treatment.37 Though the short-term and long-term mechanisms may vary in intervention studies, the interactions between OSA severity and obesity seem to be the main confounding factor regarding the causality issue of the inflammatory activity.
The current study has certain limitations. Our results were based on a cross-sectional analysis of baseline values of a revascularized CAD cohort, which means that we cannot suggest causality between OSA and increased inflammatory activity in patients with CAD. However, because the cohort of non-obese patients with CAD was relatively large, our baseline findings are reliable to suggest, at least, OSA as an important confounding factor when evaluating the secondary prevention models stratified by inflammatory biomarkers in CAD. The lack of an obese non-OSA control group is another limitation of the current report. However, finding obese control patients with CAD who do not have OSA is very challenging, as illustrated in Figure 1. As discussed previously, the main purpose of the current study was to address the relationship between OSA and inflammatory activity in a nonobese CAD cohort, and because the results were additionally adjusted for waist circumference and BMI, our findings suggest that the influence of OSA on the increased inflammatory activity in CAD is independent of obesity. However, minimal measurement of abdominal obesity and body fat in terms of waist circumference and waist-to-hip ratio is also a limitation. Although magnetic resonance imaging measurements would provide the best information regarding abdominal obesity and body fat, this was not the main focus of the RICCADSA trial. Another limitation is the lack of blood samples from subjects with AHI 5.0–14.9 events/h, based on the inclusion criteria of the main RICCADSA trial (Figure 1). Thus, the relationship between the whole spectra of AHI and inflammatory markers cannot be addressed in the current CAD cohort. Finally, one may argue that the diagnostic criteria of OSA were based on cardiorespiratory sleep studies in comparison with full polysomnography, and could therefore not reflect true sleep time and arousals. However, the cutoff value for AHI (15 events/h) chosen for OSA diagnosis, as well as the cutoff value (5 events/h) chosen for non-OSA, were both previously shown to be reliable for the Embletta system38 used in the current trial. Though we had polysomnographic recordings before the start of RCT in the protocol, these investigations were mainly planned for further evaluation of sleepy versus nonsleepy OSA phenotypes regarding the primary and secondary outcomes of the RICCADSA trial.19,20
The main strength of the study is that we have focused on the nonobese individuals with an established CAD, and also adjusted for waist circumference and BMI in the multivariable analyses, indicating that the observed associations in this CAD population was not mainly driven by obesity, which otherwise has been stated in previous reports regarding the relationship between increased inflammatory activity and CAD within the cardiovascular research field.17 Excluding CAD patients with reduced LVEF is an additional strength of the current study, minimizing the confounding effect of subclinical heart failure on inflammation.18
CONCLUSION
OSA was independently associated with increased inflammatory activity in this nonobese CAD cohort. The degree of the intermittent hypoxemia, rather than the number of apneas and hypopneas, appears to be primarily associated with enhanced inflammation. The effect of CPAP treatment on inflammatory markers in CAD patients with OSA as well as the prognostic value of these associations on the long-term outcomes in the RICCADSA trial would hopefully better contribute to the research field in this context.
DISCLOSURE STATEMENT
This study was supported by grants from the Swedish Research Council (521-2011-537 and 521-2013-3439); the Swedish Heart-Lung Foundation (20080592, 20090708 and 20100664); the “Agreement concerning research and education of doctors” of Västra Götalandsregionen (ALFGBG-11538 and ALFGBG-150801), Research fund at Skaraborg Hospital (VGSKAS-4731, VGSKAS-5908, VGSKAS-9134, VGSKAS-14781, VGSKAS-40271 and VGSKAS-116431); the Heart Foundation of Kärnsjukhuset, ResMed Foundation and ResMed Ltd. Dr. Peker has received institutional grants from ResMed and Bioprojet, and consulting and lecture fees from ResMed. None of the funders had any direct influence on the design of the study, the analysis of the data, the data collection, drafting of the manuscript, or the decision to publish. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Greenfield JR, Samaras K, Jenkins AB, et al. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation. 2004;109:3022–8. doi: 10.1161/01.CIR.0000130640.77501.79. [DOI] [PubMed] [Google Scholar]
- 3.Lee CH, Khoo SM, Chan MY, et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med. 2011;7:616–21. doi: 10.5664/jcsm.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnea: the importance of visceral obesity and insulin resistance. J Intern Med. 2003;254:32–44. doi: 10.1046/j.1365-2796.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 6.Lui MM, Lam JC, Mak HK, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135:950–6. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 7.He LP, Tang XY, Ling WH, Chen WQ, Chen YM. Early c-reactive protein in the prediction of long-term outcomes after acute coronary syndromes: a meta-analysis of longitudinal studies. Heart. 2010;96:339–46. doi: 10.1136/hrt.2009.174912. [DOI] [PubMed] [Google Scholar]
- 8.Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minoguchi K, Tazaki T, Yokoe T, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–9. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- 10.Yokoe T, Minoguchi K, Matsuo H, et al. M. Elevated levels of c-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 11.Chami HS, Fontes JD, Vasan RS, et al. Vascular inflammation and sleep disordered breathing in a community-based cohort. Sleep. 2013;36:763–8. doi: 10.5665/sleep.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep disordered breathing. Sleep. 2004;27:1507–11. doi: 10.1093/sleep/27.8.1507. [DOI] [PubMed] [Google Scholar]
- 13.Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum C-reactive protein (CRP) – no association with sleep duration or sleep disordered breathing. Sleep. 2007;30:991–6. doi: 10.1093/sleep/30.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akashiba T, Akahoshi T, Kawahara S, Majima T, Horie T. Effects of long-term nasal continuous positive airway pressure on C-reactive protein in patients with obstructive sleep apnea syndrome. Intern Med. 2005;44:899–900. doi: 10.2169/internalmedicine.44.899. [DOI] [PubMed] [Google Scholar]
- 15.Colish J., Walker JR, Elmayergi N, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141:674–81. doi: 10.1378/chest.11-0615. [DOI] [PubMed] [Google Scholar]
- 16.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–7. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 17.Klein S, Burke LE, Bray GA, et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: Endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–67. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 18.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–59. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 19.Peker Y, Glantz H, Thunstrom E, Kallryd A, Herlitz J, Ejdeback J. Rationale and design of the randomized intervention with CPAP in coronary artery disease and sleep apnoea-RICCADSA trial. Scand Cardiovasc J. 2009;43:24–31. doi: 10.1080/14017430802276106. [DOI] [PubMed] [Google Scholar]
- 20.Glantz H, Thunström E, Herlitz J, et al. Occurrence and predictors of obstructive sleep apnea in a revascularized coronary artery disease cohort. Ann Am Thorac Soc. 2013;10:350–6. doi: 10.1513/AnnalsATS.201211-106OC. [DOI] [PubMed] [Google Scholar]
- 21.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine task force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 22.Rothkrantz-Kos S, Schmitz MP, Bekers O, Menheere PP, van Dieijen-Visser MP. High-sensitivity C-reactive protein methods examined. Clin Chem. 2002;48:359–62. [PubMed] [Google Scholar]
- 23.World Health Organization. Obesity. Preventing and managing the global epidemic. Report of a WHO consultation on obesity; 3-5 June; Geneva. Geneva: World Health Organization; 1998. [PubMed] [Google Scholar]
- 24.Johns MW. A new method of measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 25.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during instability in coronary artery disease. N Engl J Med. 2000;343:1139–47. doi: 10.1056/NEJM200010193431602. [DOI] [PubMed] [Google Scholar]
- 27.Sabatine MS, Morrow DA, Jablonski KA, et al. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–36. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 28.Helfand M, Buckley DI, Freeman M, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:496–507. doi: 10.7326/0003-4819-151-7-200910060-00010. [DOI] [PubMed] [Google Scholar]
- 29.Kaptoge S, Di Angelantonio E, Pennells L, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–20. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNicholas WT. Obstructive sleep apnea and inflammation. Prog Cardiovasc Dis. 2009;51:392–9. doi: 10.1016/j.pcad.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Sahlman J, Miettinen K, Peuhkurinen K, et al. The activation of the inflammatory cytokines in overweight patients with mild obstructive sleep apnoea. J Sleep Res. 2010;19:341–8. doi: 10.1111/j.1365-2869.2009.00787.x. [DOI] [PubMed] [Google Scholar]
- 32.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–81. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 33.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchinson WL, Koenig W, Frohlich M, Sund M, Lowe GD, Pepys MB. Immunoradiometric assay of circulating C-reactive protein: age-related values in the adult general population. Clin Chem. 2000;46:934–8. [PubMed] [Google Scholar]
- 35.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnardottir ES, Maislin G, Schwab R, et al. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic Sleep Apnea Cohort. Sleep. 2012:921–32. doi: 10.5665/sleep.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J M. 2014;370:2265–75. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dingli K, Coleman EL, Vennelle M, et al. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2003;21:253–9. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]