Abstract
Study Objectives:
Repeated exposure to a neutral conditioned stimulus (CS) in the absence of a noxious unconditioned stimulus (US) elicits fear memory extinction. The aim of the current study was to investigate the effects of mild tone exposure (CS) during slow wave sleep (SWS) on fear memory extinction in humans.
Design:
The healthy volunteers underwent an auditory fear conditioning paradigm on the experimental night, during which tones served as the CS, and a mild shock served as the US. They were then randomly assigned to four groups. Three groups were exposed to the CS for 3 or 10 min or an irrelevant tone (control stimulus, CtrS) for 10 min during SWS. The fourth group served as controls and was not subjected to any interventions. All of the subjects completed a memory test 4 h after SWS-rich stage to evaluate the effect on fear extinction. Moreover, we conducted similar experiments using an independent group of subjects during the daytime to test whether the memory extinction effect was specific to the sleep condition.
Participants:
Ninety-six healthy volunteers (44 males) aged 18–28 y.
Measurements and Results:
Participants exhibited undisturbed sleep during 2 consecutive nights, as assessed by sleep variables (all P > 0.05) from polysomnographic recordings and power spectral analysis. Participants who were re-exposed to the 10 min CS either during SWS and wakefulness exhibited attenuated fear responses (wake-10 min CS, P < 0.05; SWS-10 min CS, P < 0.01).
Conclusions:
Conditioned stimulus re-exposure during slow wave sleep promoted fear memory extinction without altering sleep profiles.
Citation:
He J, Sun HQ, Li SX, Zhang WH, Shi J, Ai SZ, Li Y, Li XJ, Tang XD, Lu L. Effect of conditioned stimulus exposure during slow wave sleep on fear memory extinction in humans. SLEEP 2015;38(3):423–431.
Keywords: conditioned stimulus, extinction, fear memory, slow wave sleep
INTRODUCTION
New memories are gradually stabilized after initial learning through a process called consolidation,1,2 during which memories are vulnerable to be regulated. Consistent with this theory, some research has shown that the formation and consolidation of emotional memory are critical to adapting to a changing environment in animals and humans.3–5 Most studies that have focused on memory consolidation have examined the effects of pharmacological treatments administered within several hours after learning tasks, demonstrating that memory consolidation is a time-dependent process,6 during which protein synthesis is susceptible to being influenced and memories can be impaired or enhanced.7–9 The use of pharmacological treatments administered shortly after training (i.e., within the time window of consolidation10) influence memory consolidation and result in the formation of long-term memories.
Numerous recent studies have suggested that sleep is beneficial for memory consolidation, including emotional memory. Sleep was shown to enhance the retention of either declarative information11,12 or procedural skills13,14 compared with staying awake after accomplishing learning tasks. Several studies have suggested that emotional memories are specifically enhanced by sleep,2,15,16 and the strengthened effect on emotional memories can last for a relatively long time.17 Additionally, the effects of consolidation on memory during slow wave sleep (SWS) and rapid eye-movement (REM) sleep have been documented in human studies.18 Increasing evidence indicates that REM sleep and SWS both contribute to nondeclarative memory15,19,20 and declarative memory consolidation,21–23 suggesting that sleep enhances the retention of newly encoded memories, depending on specific neurophysiological activity associated with particular stages, such as SWS and REM sleep. Contradictory findings have been reported concerning the association between sleep stages and emotional memories. One study indicated that REM-rich sleep enhanced aversion to previously viewed emotional pictures.24 Other studies reported that the recall of negative pictures is less impaired by sleep deprivation.25,26 In summary, how sleep affects emotional memory expression requires further investigation.
Recent work has gradually focused on the role of SWS in memory modulation. Research findings suggest that memories that are learned while awake can be enhanced through the representation of the same context during SWS.27–29 These results further indicate that consolidation is a labile state during which memory traces are prone to being changed, and SWS serves as a particular period that facilitates memory regulation. Hauner et al.30 demonstrated that reexposure to an odorant context during SWS in human subjects who were subjected to an olfactory contextual fear conditioning paradigm decreased fear responses,30 which is inconsistent with previous findings.29,31 Conditioned fear responses can be inhibited by repeated exposure to a conditioned stimulus (CS) in the absence of an unconditioned stimulus (US). This process is termed memory extinction. It is the simplest way to attenuate the expression of fear memory while awake.32 However, still unknown is whether reexposure to mild tones (CS) can elicit fear extinction during SWS. Our hypothesis is that reexposure to a CS during SWS promotes fear memory extinction in humans. We applied an auditory fear conditioning paradigm, with mild tones as the CS, to test this hypothesis.
METHODS
Participants
Ninety-six volunteers (44 male; age, 24.0 ± 2.4 y [mean ± standard deviation]) participated in the study. All of the female participants (n = 52) were reported to have regular menstrual cycles and were not using oral contraceptives prior to or during the study. They were asked to participate in the study 3–7 days before their menstrual cycle began. All of the volunteers were in good health with no history of neurological, psychiatric, or sleep disorders and no history or current use of medications known to affect cognitive functioning or sleep. Subjects were excluded from the study if they had a history of drug or alcohol addiction. The experiments were performed at the Sleep Medicine Center, West China Hospital, Sichuan University, Chengdu, China. We conducted the study in a temperature- and humidity-controlled room. All of the experimental procedures were conducted in accordance with the Declaration of Helsinki and performed with adequate understanding and written informed consent from the subjects. The study protocol was approved by the Research Ethics Board of the West China Hospital of Sichuan University. Each participant was paid 160 RMB (equivalent to $26.40 USD) upon completion of the study.
Behavioral Paradigm
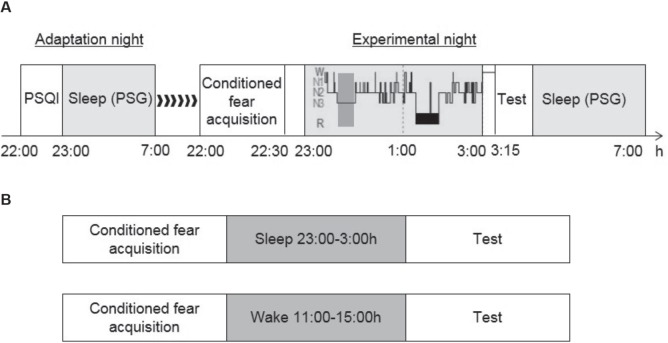
We conducted the experiments on 2 consecutive nights. The first night included adaptation, and the second night was the experimental session. On the first night, we collected the subjects' demographic data and asked them to complete the Pittsburg Sleep Quality Index (PSQI) before sleep. The PSQI33 was used to investigate sleep habits and assess the subjective sleep quality of the participants, with a cutoff score of 5. The main purpose of the first night was to exclude participants who had primary sleep disorders, such as sleep apnea or periodic limb movements. On the second night, all of the subjects (n = 66) underwent the fear conditioning paradigm approximately 30 min before sleep onset, followed by another two stages: stimulus exposure during SWS and a memory test. The timeline of the procedures is presented in Figure 1A.
Figure 1.
Experimental design and protocol. (A) Timeline of the experiment. The light gray areas represent the sleep period, and the dark gray area represents the first period of SWS when delivering the auditory stimuli. (B) A 4-h period of nocturnal sleep or daytime wakefulness separated an initial fear conditioning training phase (10 trials) from later retesting (three trials). The experimental protocol was completed in two independent experiments separated by 12 h.
The participants were randomly assigned to four groups: control group (SWS-Ctr, n = 18), 3 min CS exposure group (SWS-3 min CS, n = 17), 10 min CS exposure group (SWS-10 min CS, n = 16), and 10 min control stimulus exposure group (SWS-10 min CtrS, n = 15). All of the participants were subjected to a Pavlovian fear conditioning paradigm with partial reinforcement. The CSs were auditory tones, and the US was a mild shock to the wrist. Two auditory tones (CS+ and CS−) were used. One tone (520 Hz, 65 dB; CS+) was paired with a mild shock to the wrist (US) on a 40% partial reinforcement schedule, and another tone stimulus (250 Hz, 65 dB; CS−) was never paired with the shock. We presented the CS+ with shock in four trials and the CS+ without shock in six trials. Thus, the CS+ was presented 10 times in the conditioning phase. The CS− was also presented 10 times. The CS+ reinforced trials were conducted in random order during 10 trials. The CtrS was irrelevant to the fear conditioning training and only presented during SWS in the 10 min CtrS group. The participants were instructed to pay attention to the tone and try to determine the relationship between the stimulus and shock during the training session.
We used a “night-half paradigm,” originally developed by Fowler et al.34 and Laroush et al.35 to avoid possible confounding effects that result from rapid eye movement (REM) sleep. The subjects were allowed to sleep from 23:00 to 03:00, which was deemed SWS-rich sleep. During that time, all of the groups, with the exception of the control group, were re-exposed to the tone stimuli. A detailed description of the tone stimuli is presented in the Auditory Stimuli section. The subjects were awakened during the first sleep stage 1 or stage 2, which occurred after 4 h of sleep, because arousal from other sleep stages may influence subsequent test performance.36 The subjects were tested 15 min later to avoid sleep inertia.19 After completing the test, the participants were allowed to return to sleep and woken up at 07:00.
During the fear conditioning training and test phases, the participants were attached to skin conductance recording and shock electrodes. The skin conductance recording electrode was used to measure the skin conductance response (SCR). Shocks were delivered through a stimulating square electrode attached to the right inner wrist via a STM 200 stimulator (BioPac Systems, Goleta, CA, USA). The level of the shock was based on the criterion “uncomfortable, but not painful,” which was determined for each participant. The shock level began at mild (10 V) and gradually increased until the shock reached the maximum level (50 V).37,38 The auditory tones were presented for 3 sec, and the shocks were administered for 250 ms, which coterminated with the tones. The intertrial interval (ITI) was 10 sec. Stimulus presentation was controlled by a computer using E-Prime 2.0 professional (Psychology Software Tools, Inc.; http://www.pstnet.com). For all of the groups, the conditioning training phase consisted of 10 nonreinforced presentations of the CS− and four reinforced presentations of the CS+ (four CS+ stimuli presented with shock and six CS+ stimuli presented without shock). The test phase was similar to the conditioning training phase but only consisted of three presentations of the CS− and three presentations of the CS+ without shock.
Generally, we conducted the experiment with no instructions or information given to the participants about tone presentation during sleep. At the debriefing, two of the subjects reported to have noticed the tones while sleeping. These two subjects were then excluded from the final analysis.
Moreover, to investigate whether the extinction effect was specific to the sleep condition, we repeated a similar procedures in another experiment, using an independent group of awake subjects (n = 30) who were divided into two subgroups. One group of subjects (Wake-10 min CS, n = 15) stayed awake during the 10 min CS reexposure, and the other group served as controls (Wake-Ctr, n = 15) who were not subjected to any interventions but remained awake until the end of the experiment. Conditioning was performed at 10:00 in the morning, followed by a 4-h interval of daytime wakefulness similar to the nocturnal sleep period. Subsequently, the subjects were re-tested in three trials for conditioned responses to the CS+ and CS−39,40 (Figure 1B).
Polysomnographic Recordings
Overnight polysomnographic recording included electroencephalography (EEG; including F3, F4, C3, C4, O1, and O2, with reference to the contralateral mastoid; International 10–20 system), bilateral electrooculography (EOG), bilateral electromyography (EMG), and electrocardiography (ECG). Finger pulse oximetry was also recorded. High-pass filters were set at 0.3 Hz, and low-pass filters were set at 35 Hz for all of the EEGs and EOGs. Thirty-second epochs were used for manual analysis, and sleep stages were scored offline according to the criterion of the American Academy of Sleep Medicine (AASM) using the standard polysomnographic sleep recordings.
We collected polysomnographic parameters, including total time in bed (TIB), total sleep time (TST), sleep latency (SL), min and percentage of sleep stages 1, 2, 3, and REM sleep, wake after sleep onset (WASO), and sleep efficiency (SE = TST/ TIB) for 2 consecutive nights.
Auditory Stimuli
Auditory tones were broadcast through two loudspeakers. They were placed 1 m from the head of the bed. The first conditioned auditory stimulus (520 Hz sine-wave auditory tone, 65 dB) or control stimulus (1,250 Hz sine-wave auditory tone, 55 dB) was presented when we identified 2 min of continuous SWS after the participants fell asleep. We used these auditory stimuli based on a previous study, and they were adjusted individually.41 The tone stimuli were presented in a 15 sec on/15 sec off schedule, which lasted 3 or 10 min. Once a microarousal occurred, the stimulus that evoked the microarousal were not be counted into the total numbers of stimuli. In summary, the stimuli were presented 12 times in the SWS-3 min CS group, 40 times in the SWS-10 min CS group, and 40 times in the SWS-10 min CtrS group.
Psychophysiological Recording and Assessment
The SCR was acquired using two Ag-AgCl electrodes that were attached to the second and third fingers of the left hand on the middle phalanges. The SCR waveforms were measured using a BioPac MP150 system and recorded with AcqKnowl-edge 4.2 software (BioPac Systems).
AcqKnowledge 4.2 software was also used to analyze SCR waveforms. The level of the SCR was determined by taking the base-to-peak difference for each waveform in a 3-sec window following stimulus onset. At each stage, the differential fear response was calculated by subtracting responses to the CS− from responses to the CS+ in corresponding trials.38
Statistical Analysis
We used one-way analysis of variance (ANOVA) to analyze the demographic data and PSQI scores between the six groups. The polysomnographic variables for the 2 consecutive nights were analyzed using two-way repeated-measures ANOVA, with group as the between-subjects factor and time (first night and second night) as the within-subjects factor, followed by the Bonferroni/Dunn post hoc test.
EEG signals were sampled online at 512 Hz using Profusion Net Beacon software (Compumedics Sleep Study System, Melbourne, Australia). Detailed power spectral analysis was performed offline using fast Fourier transform (FFT) with Hanning window tapering, showing the frequency and power of an analyzed portion of the EEG signal (Compumedics Sleep Study System). We chose the C3 and C4 central electrodes to perform further analysis and defined the frequency bands as the following: delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (30–40 Hz). Because no difference was observed between the central C3 and C4 electrodes, the data were collapsed across these two electrodes. The mean EEG power at each frequency band across blocks was compared between tone-on and tone-off blocks using two-tailed paired t-tests. Data from eight subjects were excluded because of a lack of artifact-free epochs.
Both during the sleep and wakefulness conditions, subjects who showed successful levels of fear acquisition were included in the analysis. We assessed successful fear acquisition by skin conductance recording. The exclusion criteria were based on differential responses to the CS+ and CS− in the second half of acquisition. That is, subjects were excluded if the difference during acquisition was in the opposite direction (CS− > CS+) or smaller than 0.1 μs38; two subjects were excluded based on this criterion. For the sleep conditions, the SCR results for the fear acquisition and test phases were analyzed using two-way repeated-measures ANOVA, with group as the between-subjects factor and trial as the within-subjects factor, followed by the Bonferroni/Dunn post hoc test. The effect of CS exposure on memory extinction in each group was assessed using a two-way ANOVA that evaluated main effects of group and time (the average of the last three learning trials and average of three test trials), followed by the Bonferroni/Dunn post hoc test to assess the extinction effect between groups. Moreover, we applied a three-way ANOVA that assessed the effect of CS exposure on memory extinction in distinct groups that evaluated main effects of group (CS exposure and No CS exposure), condition (SWS and wakefulness) and time (the average of the last three learning trials and average of three test trials), followed by the Bonferroni/Dunn post hoc test as appropriate.
Differences were considered significant when P < 0.05.
RESULTS
Population and Demographic Data
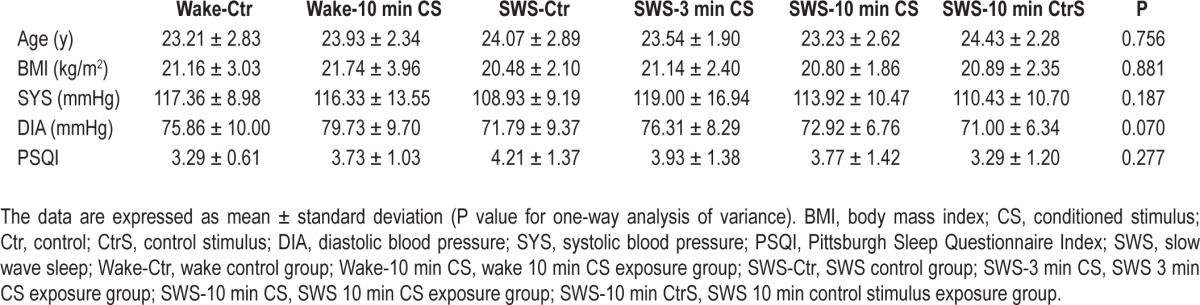
A total of 96 healthy subjects (44 males; age, 24.0 ± 2.4 y [mean ± standard deviation]) completed the study. Thirteen subjects were excluded from the final analysis because of failures in PSG recording (n = 3) and SCR extraction (n = 2), inadequate levels of fear acquisition (n = 2), maintenance of sleep during tone presentation (n = 2), and difficulty falling asleep after fear conditioning training on the experimental night (n = 4).
As shown in Table 1, no significant differences were found in age, body mass index, systolic blood pressure (SYS), diastolic blood pressure (DIA), and PSQI score among the six groups. All of the participants' PSQI scores were less than 5, indicating that they had regular sleep habits and good sleep quality.
Table 1.
Demographic data collected from the six groups (n = 83).
Effects of CS Exposure on Sleep Architecture
The participants were required to maintain a regular sleep-wake cycle (i.e., go to sleep between 23:00 and 24:00 and wake up between 07:00 and 08:00) for at least 3 days before the study began. Table 2 shows that reexposure to the CS or irrelevant tones during SWS on the experimental night did not affect sleep profiles (TST, F3,50 = 0.72, P = 0.55; SL, F3,50 = 0.89, P = 0.50; N1 [%], F3,50 = 0.74, P = 0.54; N2 [%], F3,50 = 0.57, P = 0.64; SWS [%], F3,50 = 0.36, P = 0.78; REM [%], F3,50 = 0.35, P = 0.79; WASO, F3,50 = 0.23, P = 0.86; SE, F3,50 = 0.24, P = 0.87).
Table 2.
Polysomnographic data for 2 consecutive nights (n = 54).
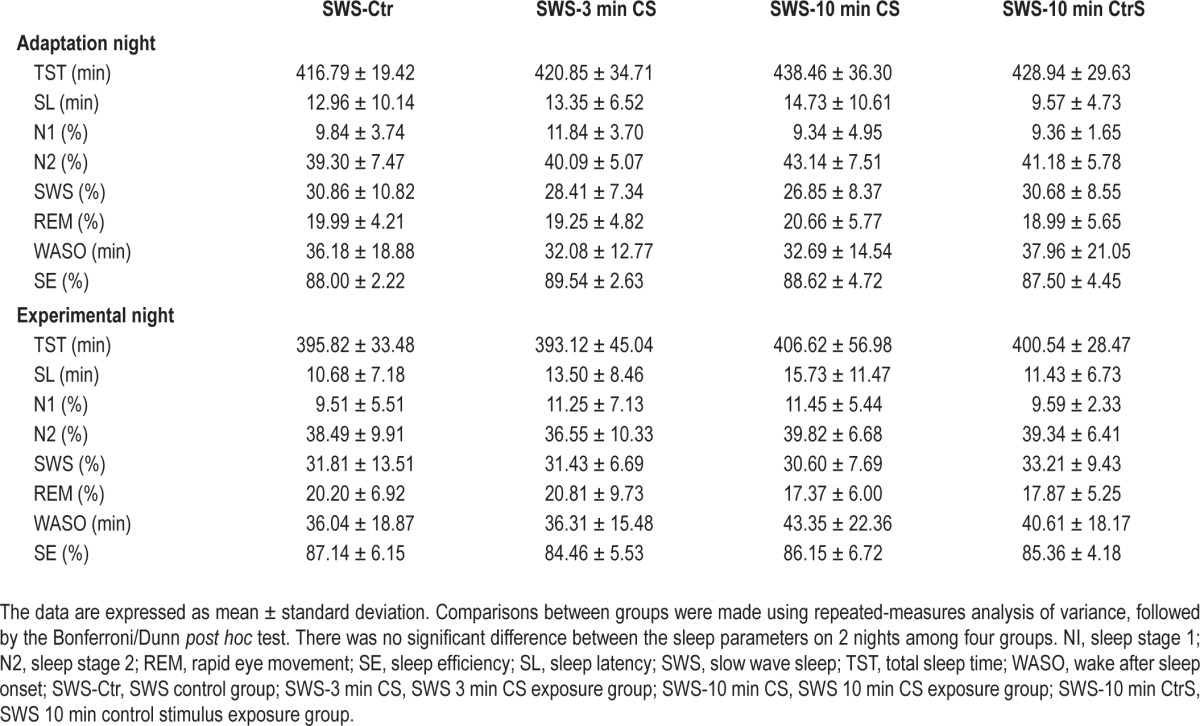
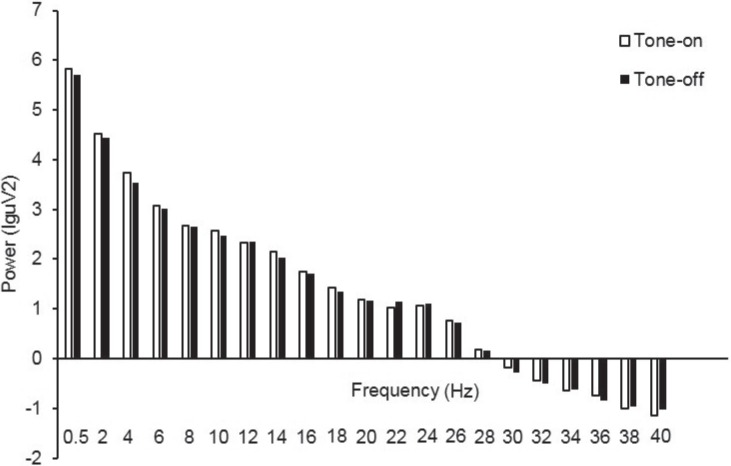
We compared PSG data between 2 days to roughly determine the sleep architecture with and without CS+ presentation, and the sleep profile appeared to be unchanged. Further power spectral analysis confirmed that the subjects remained asleep during tone stimulus presentation (Figure 3). Mean EEG power for tone-on and tone-off blocks did not significantly differ for the following frequency bands derived from the central electrodes: delta (0.5–4 Hz, t31 = 0.065, P = 0.95), theta (4–8 Hz, t31 = −1.06, P = 0.30), alpha (8–12 Hz, t 31 = 0.62, P = 0.54), beta (12–30 Hz, t31 = 0.79, P = 0.44), and gamma (30–40 Hz, t31 = 1.05, P = 0.30). Alpha and beta power were similar before compared to after tone onset, suggesting that cue presentation influenced memory extinction without briefly waking the participants. Further analysis over the full range of frequencies (1–40 Hz) showed an apparent increase in power during tone-on versus tone-off blocks in the frequency range of 10–16 Hz for central electrodes; however the increase did not reach the threshold for significance (P = 0.063).
Figure 3.
Electroencephalography (EEG) power spectral analysis during slow wave sleep (SWS). Power spectrum collapsed from two central electrodes (C3 and C4) for tone-on and tone-off blocks during SWS (n = 31). Frequencies (Hz) are presented on the x-axis, and power (lgμV2) is presented on the y-axis.
Effects of CS Exposure on Memory Extinction
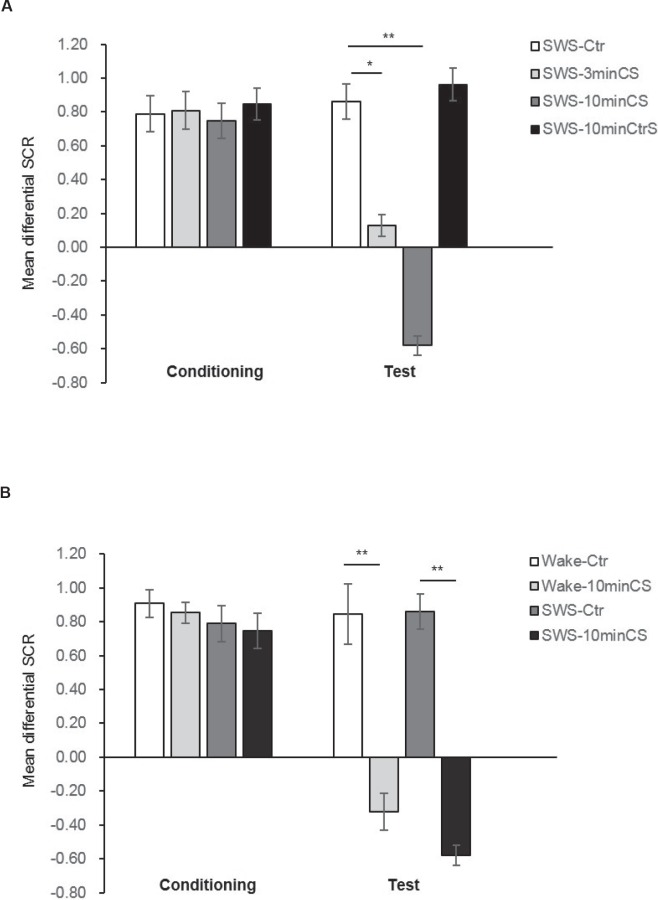
The SCR results during conditioning training and the test are presented in Figure 2. For the sleep condition, all of the participants exhibited similar levels of fear acquisition after 10 conditioning trials (F3,50 = 1.94, P = 0.14). The decline in fear responses from acquisition (the average of the last three learning trials) to testing (the average of three trials) for each group was assessed using a two-way ANOVA, with group as the between-subjects factor and time (the average of the last three learning trials and average of three test trials) as the within-subjects factor, followed by the Bonferroni/Dunn post hoc test. The analysis showed significant main effects of time (F1,50 = 17.122, P < 0.001) and group (F3,55 = 3.569, P < 0.05) and a group × time interaction (F3,55 = 10.000, P < 0.001). Concerning the change from conditioning to test, the post hoc test showed that an extinction effect occurred in the 3 min CS group and 10 min CS group (P < 0.05, P < 0.01, respectively; Figure 2A). Concerning test performance, post hoc tests showed that there were statistically significant differences between SWS-3 min CS (P < 0.05), SWS-10 min CS (P < 0.01) and the SWS-Ctr group (Figure 2A). Moreover, there also were significant differences between SWS-3 min CS (P < 0.05), SWS-10 min CS (P < 0.01) and the SWS-CtrS group (Figure 2A). These results indicate that CS exposure during SWS elicited fear memory extinction and the presentation of other nonassociated tone stimuli did not disrupt the expression of memory, which excludes the possibility that the tone stimulus itself affected the memory trace. Moreover, in an additional control experiment with 30 other participants who remained awake, tones were presented after approximately a 1.5-h interval yoked between sleep and awake subjects. All of the participants exhibited similar levels of fear acquisition after 10 conditioning trials (F3,52 = 1.52, P = 0.22). We then conducted a group (CS exposure and No CS exposure) × condition (SWS and wakefulness) × time (the average of the last three learning trials and average of three test trials) ANOVA and found significant main effects for group (F1,52 = 16.400, P < 0.01), time (F1,52 = 27.826, P < 0.01), and a significant time × group interaction (F1,52 = 28.234, P < 0.01). The post hoc tests showed that CS presented during either SWS or wakefulness altered memory expression from acquisition to test compared with No CS exposure (both comparisons, P < 0.01; Figure 2B). In addition, when we compared test performance only, we found that the decline of fear responses induced by CS exposure occurred in both the Wake-10 min CS group and SWS-10 min CS group compared with the Wake-Ctr group and SWS-Ctr group, respectively (both comparisons, P < 0.01; Figure 2B). Furthermore, the post hoc tests also found no significant differences between the Wake-10 min CS group and SWS-10 min CS group during the memory acquisition (P = 0.661) and test (P = 0.441) phases. These results revealed that CS exposure during either SWS or wakefulness promoted fear memory extinction.
Figure 2.
Auditory stimulus exposure during SWS and wakefulness both attenuated fear responses. (A) Mean differential skin conductance response (SCR) (CS+ minus CS−) during conditioning (late phase) and test phases between the four groups in the sleep condition. Error bars represent standard error of the mean (SEM). * P < 0.05, ** P < 0.01 (two-way analysis of variance [ANOVA]). (B) Mean differential SCRs (CS+ minus CS−) during conditioning (late phase) and test phases between sleep and wakefulness conditions. Error bars represent SEM. * P < 0.05 (three-way ANOVA). CS, conditioned stimulus; CtrS, control stimulus.
DISCUSSION
The main finding of the current study was that fear responses in the SWS-10 min CS and Wake-10 min CS groups both markedly declined during the test. This suggests that repeated CS exposure during SWS resulted in memory extinction that may be comparable to exposure therapy applied during wakefulness. Moreover, this manipulation only affected fear memory, which was reactivated by reexposure to the CS during sleep, and left the nonreactivated fear memory intact when the subjects were exposed to the irrelevant stimulus during sleep. The sleep macrostructure during 2 consecutive nights in each group and power spectral analysis that focused on the power difference between tone-on and tone-off blocks in distinct frequency bands during SWS suggested that reexposure to the tone stimuli during sleep did not alter sleep architecture or sleep quality. Therefore, CS reexposure during SWS appeared to promote fear memory extinction similarly to extinction during wakefulness but did not influence sleep profiles.
Sleep plays a critical role in memory consolidation. Several recent studies showed that learning-associated cues (e.g., odors or tones) presented during SWS enhanced human episodic and procedural memory retention.28,29,42 These findings suggest that targeted memory reactivation (TMR) during sleep contributes to memory consolidation and retention. We applied a similar TMR approach during sleep, but the results were inconsistent with previous research. The contradictory findings elicit a conundrum about the feature of cues and the specific parameters for cues application that are required to reactivate and modify memory traces.43 A study that used contextual sounds during sleep failed to improve memory that was learned during wakefulness because the sounds were too commonplace, such that they were not specifically and characteristically associated with the learned memory.44 In the current study, tones served as the CS that were specifically and directly associated with an intrinsically aversive stimulus (US) instead of background contextual cues. The stimulus intensity and closeness of the association between the stimuli and learned memory may be factors that contribute to this variance. Future studies of TMR are needed to characterize the specific cues parameters for memory reactivation and modification.
Our findings are consistent with a recent study. Hauner et al.30 used a human olfactory fear conditioning paradigm and showed that reexposure to an odor during SWS weakened the fear response after a brief nap. However, Rolls et al.45 studied mice and reported a longer freezing duration in response to a fear-relevant odorant after sleep, indicating that odor reexposure strengthened fear memory. It is known that the process of consolidating newly encoded memories requires gene expression and protein synthesis that begin shortly after training and last for approximately 6 h in various species and types of memory.7,10,46,47
Rolls et al. reexposed the mice to the odor to test the effect on memory 24 h after successful fear acquisition, during which the original fear memory was already well consolidated and preserved. The initial fear memory was extinguished only upon CS+ presentation after anisomycin infusion. In the current study, we reexposed the subjects to the tone stimulus during the first period of SWS, and the time interval between conditioning and tone reexposure was within the time window of memory consolidation.10 We did not apply any pharmacological intervention to directly disrupt gene expression or protein synthesis implicated in the consolidation process. Therefore, our manipulation directly targeted and influenced memory consolidation, which is a la-bile state for modulating memory.
Previous studies illustrated that three interconnected brain regions are involved in fear memory extinction: amygdala, me-dial prefrontal cortex (mPFC), and hippocampus.48 Hauner et al.30 suggested that activity in the left amygdala is involved in the biological mechanisms that underlie memory extinction. They reported that odor reexposure during sleep induced the formation of a new “safety memory” in the amygdala30,49 rather than erased the original fear memory. This is consistent with other findings, in which new “extinction memory” competes with original fear memory when the fearful object or event is reen-countered.48,50 The return of fear after extinction also indicates that extinction does not permanently erase fear memories but instead temporally inhibits the expression of fear memories. In our paradigm, we presented the CS during sleep, similar to “exposure therapy” applied during wakefulness, a process known as one of the simplest ways to attenuate the expression of fear memories.32,51 The formation of extinction memory may be attributable to the distinction between our findings and other TMR research. Moreover, humans can learn new information during sleep.31 Consequently, we propose that CSs, such as tones, that are repeatedly presented during SWS can result in memory extinction. Our results support the hypothesis that the “CS-no US” new safety memory is formed during sleep. The extinction effect may depend on activity in the amygdala, which mediates new memory formation and inhibits the expression of the original memory. Further neuroimaging studies are needed to corroborate this hypothesized biological mechanism.
To investigate the effect of CS exposure during wakefulness, we applied similar procedure during the daytime based on previous research paradigm.39,40 We finally found that CS reexposure during wakefulness induced memory extinction, which was consistently with previous research findings in both rodents and humans.32,52,53 During memory consolidation when memories are unstable and susceptible to disruption, the original fear memory can be inhibited through an extinction process. However, subsequent studies had limited success, with resistance to extinction or a less durable effect of extinction,52,54,55 in which the intervention applied shortly after aversive memory encoding suppressed fear, but the effect was not maintained for a relatively long time. Moreover, patients with PTSD showed deficits in fear extinction in a CS extinction procedure compared with healthy subjects.52 CS extinction procedures applied during wakefulness are generally deficient, and further work is needed to determine the optimal parameters for reducing fear memories, such as applying this procedure during specific sleep stages (e.g., SWS and REM) and test whether this extinction effect is permanent.
One limitation of the current study is that we did not focus on other sleep stages. REM sleep is known to contribute to memory consolidation.15,19,21 We also did not address the possible influence of sleep stage 2 or spindle activity on memory. Spindle activity refers to electroencephalographic oscillations of 10–15 Hz, which are present in human sleep stage 2 and also are present at a similar level during SWS.56 Furthermore, sleep stage 2 benefits memory consolidation.57–59 Therefore, further confirmation of whether the effect of stimulus exposure on memory expression is specific to SWS is needed.
Altogether, our results demonstrated the effect of CS exposure during wakefulness and SWS on memory extinction. SWS is a brain state during which memory can be reactivated and hence regulated by sensory stimuli. Polysomnographic recordings and further power spectral analysis showed that this manipulation may have implications for developing “sleep therapies” for patients with pathological fear, such as anxiety disorders and PTSD. Because the subjects were unaware of this manipulation while they were asleep, sleep therapies may avoid certain disadvantages of traditional “extinction therapy” applied during wakefulness, such as making the original negative mood worse by making the subjects recall painful experiences again. Thus, the current study introduces an alternative approach that may safely reduce fear in patients and have potential clinical value.
DISCLOSURE STATEMENT
This was not an industry-supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported in part by the Natural Science Foundation of China (no. 81271489, 31230033, 81171251, and 81328010) and the National Basic Research Program of China (no. 2015CB856400). The authors are grateful to Fei Lei and Li-Na Du (Sleep Medicine Center, West China Hospital, Sichuan University, Chengdu, China) for polysomnographic data recording and assistance with the analysis. We also thank Dr Larry D. Sanford for his aid in revising our manuscript.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- ANOVA
analysis of variance
- BMI
body mass index
- CS
conditioned stimulus
- CtrS
control stimulus
- DIA
diastolic blood pressure
- ECG
electrocardiography
- EEG
electroencephalography
- EMG
electromyography
- EOG
electrooculography
- mPFC
medial prefrontal cortex
- PSG
polysomnography
- PSQI
Pittsburg Sleep Quality Index
- REM
rapid-eye-movement
- SCR
skin conductance response
- SE
sleep efficiency
- SL
sleep latency
- SWS
slow wave sleep
- SYS
systolic blood pressure
- TIB
total time in bed
- TST
total sleep time
- US
unconditioned stimulus
- WASO
wake after sleep onset
Footnotes
A commentary on this article appears in this issue on page 337.
REFERENCES
- 1.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–51. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 2.Holland P, Lewis PA. Emotional memory: selective enhancement by sleep. Curr Biol. 2007;17:R179–81. doi: 10.1016/j.cub.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 3.Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and Learning. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- 4.Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–45. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 6.Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–74. [PMC free article] [PubMed] [Google Scholar]
- 7.Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–59. [PubMed] [Google Scholar]
- 8.McGaugh JL. Dissociating learning and performance: drug and hormone enhancement of memory storage. Brain Res Bull. 1989;23:339–45. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblum K, Meiri N, Dudai Y. Taste memory: the role of protein synthesis in gustatory cortex. Behav Neural Biol. 1993;59:49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- 10.Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241–7. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Lahl O, Wispel C, Willigens B, Pietrowsky R. An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res. 2008;17:3–10. doi: 10.1111/j.1365-2869.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- 13.Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci. 2007;10:1206–13. doi: 10.1038/nn1959. [DOI] [PubMed] [Google Scholar]
- 14.Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proc Natl Acad Sci U S A. 2002;99:11987–91. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–9. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–48. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biol Psychiatry. 2006;60:788–90. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 18.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 19.Plihal W, Born J. Effects of early and late nocturnal sleep on priming and spatial memory. Psychophysiology. 1999;36:571–82. [PubMed] [Google Scholar]
- 20.Aeschbach D, Cutler AJ, Ronda JM. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci. 2008;28:2766–72. doi: 10.1523/JNEUROSCI.5548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rauchs G, Bertran F, Guillery-Girard B, et al. Consolidation of strictly episodic memories mainly requires rapid eye movement sleep. Sleep. 2004;27:395–401. doi: 10.1093/sleep/27.3.395. [DOI] [PubMed] [Google Scholar]
- 22.Fogel SM, Smith CT, Cote KA. Dissociable learning-dependent changes in REM and non-REM sleep in declarative and procedural memory systems. Behav Brain Res. 2007;180:48–61. doi: 10.1016/j.bbr.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 23.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 24.Wagner U. Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep. Psychosom Med. 2002;64:627–34. doi: 10.1097/01.psy.0000021940.35402.51. [DOI] [PubMed] [Google Scholar]
- 25.Tamaki M, Matsuoka T, Nittono H, Hori T. Activation of fast sleep spindles at the premotor cortex and parietal areas contributes to motor learning: a study using sLORETA. Clin Neurophysiol. 2009;120:878–86. doi: 10.1016/j.clinph.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Atienza M, Cantero JL. Modulatory effects of emotion and sleep on recollection and familiarity. J Sleep Res. 2008;17:285–94. doi: 10.1111/j.1365-2869.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 27.Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nat Neurosci. 2012;15:1439–44. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antony JW, Gobel EW, O'Hare JK, Reber PJ, Paller KA. Cued memory reactivation during sleep influences skill learning. Nat Neurosci. 2012;15:1114–6. doi: 10.1038/nn.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–9. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 30.Hauner KK, Howard JD, Zelano C, Gottfried JA. Stimulus-specific enhancement of fear extinction during slow-wave sleep. Nat Neurosci. 2013;16:1553–5. doi: 10.1038/nn.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arzi A, Shedlesky L, Ben-Shaul M, et al. Humans can learn new information during sleep. Nat Neurosci. 2012;15:1460–5. doi: 10.1038/nn.3193. [DOI] [PubMed] [Google Scholar]
- 32.Quirk GJ, Pare D, Richardson R, et al. Erasing fear memories with extinction training. J Neurosci. 2010;30:14993–7. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 34.Fowler MJ, Sullivan MJ, Ekstrand BR. Sleep and memory. Science. 1973;179:302–4. doi: 10.1126/science.179.4070.302. [DOI] [PubMed] [Google Scholar]
- 35.Yaroush R, Sullivan MJ, Ekstrand BR. Effect of sleep on memory. II. Differential effect of the first and second half of the night. J Exp Psychol. 1971;88:361–6. doi: 10.1037/h0030914. [DOI] [PubMed] [Google Scholar]
- 36.Stones MJ. Memory performance after arousal from different sleep stages. Br J Psychol. 1977;68:177–81. doi: 10.1111/j.2044-8295.1977.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 37.Soeter M, Kindt M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learn Mem. 2011;18:357–66. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- 38.Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–5. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 40.Pace-Schott EF, Milad MR, Orr SP, Rauch SL, Stickgold R, Pitman RK. Sleep promotes generalization of extinction of conditioned fear. Sleep. 2009;32:19–26. [PMC free article] [PubMed] [Google Scholar]
- 41.Bruck D, Ball M, Thomas I, Rouillard V. How does the pitch and pattern of a signal affect auditory arousal thresholds? J Sleep Res. 2009;18:196–203. doi: 10.1111/j.1365-2869.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 42.Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009;326:1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oudiette D, Paller KA. Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn Sci. 2013;17:142–9. doi: 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Donohue KC, Spencer R. Continuous re-exposure to environmental sound cues during sleep does not improve memory for semantically unrelated word pairs. J Cogn Educ Psychol. 2011;10:167–77. doi: 10.1891/1945-8959.10.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rolls A, Makam M, Kroeger D, Colas D, de Lecea L, Heller HC. Sleep to forget: interference of fear memories during sleep. Mol Psychiatry. 2013;18:1166–70. doi: 10.1038/mp.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alberini CM. Genes to remember. J Exp Biol. 1999;202:2887–91. doi: 10.1242/jeb.202.21.2887. [DOI] [PubMed] [Google Scholar]
- 47.Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cell Mol Life Sci. 1999;55:564–74. doi: 10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–63. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welberg L. Learning and memory: to sleep, perchance to forget. Nat Rev Neurosci. 2013;14:737. doi: 10.1038/nrn3626. [DOI] [PubMed] [Google Scholar]
- 50.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Storsve AB, McNally GP, Richardson R. US habituation, like CS extinction, produces a decrement in conditioned fear responding that is NMDA dependent and subject to renewal and reinstatement. Neurobiol Learn Mem. 2010;93:463–71. doi: 10.1016/j.nlm.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry. 2007;164:1684–92. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- 53.Archbold GE, Bouton ME, Nader K. Evidence for the persistence of contextual fear memories following immediate extinction. Eur J Neurosci. 2010;31:1303–11. doi: 10.1111/j.1460-9568.2010.07161.x. [DOI] [PubMed] [Google Scholar]
- 54.Maren S, Chang CH. Recent fear is resistant to extinction. Proc Natl Acad Sci U S A. 2006;103:18020–5. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norrholm SD, Vervliet B, Jovanovic T, et al. Timing of extinction relative to acquisition: a parametric analysis of fear extinction in humans. Behav Neurosci. 2008;122:1016–30. doi: 10.1037/a0012604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–40. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 57.Fogel SM, Smith CT. Learning-dependent changes in sleep spindles and Stage 2 sleep. J Sleep Res. 2006;15:250–5. doi: 10.1111/j.1365-2869.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 58.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruch S, Markes O, Duss SB, et al. Sleep stage II contributes to the consolidation of declarative memories. Neuropsychologia. 2012;50:2389–96. doi: 10.1016/j.neuropsychologia.2012.06.008. [DOI] [PubMed] [Google Scholar]