Abstract
Study Objectives:
The goals of the study were to assess semantic priming to emotion and nonemotion cue words using a novel measure of associational breadth for participants who either took rapid eye movement (REM) or nonrapid eye movement (NREM) naps or who remained awake, and to assess the relation of priming to REM sleep consolidation and REM sleep inertia effects.
Design:
The associational breadth task was applied in both a priming condition, where cue words were signaled to be memorized prior to sleep (primed), and a nonpriming condition, where cue words were not memorized (nonprimed). Cue words were either emotional (positive, negative) or nonemotional. Participants were randomly assigned to either an awake (WAKE) or a sleep condition, which was subsequently split into NREM or REM groups depending on stage at awakening.
Setting:
Hospital-based sleep laboratory.
Participants:
Fifty-eight healthy participants (22 male) ages 18 to 35 y (mean age = 23.3 ± 4.08 y).
Measurements and Results:
The REM group scored higher than the NREM or WAKE groups on primed, but not nonprimed emotional cue words; the effect was stronger for positive than for negative cue words. However, REM time and percent correlated negatively with degree of emotional priming. Priming occurred for REM awakenings but not for NREM awakenings, even when the latter sleep episodes contained some REM sleep.
Conclusions:
Associational breadth may be selectively consolidated during REM sleep for stimuli that have been tagged as important for future memory retrieval. That priming decreased with REM time and was higher only for REM sleep awakenings is consistent with two explanatory REM sleep processes: REM sleep consolidation serving emotional downregulation and REM sleep inertia.
Citation:
Carr M, Nielsen T. Morning REM sleep naps facilitate broad access to emotional semantic networks. SLEEP 2015;38(3):433–443.
Keywords: associational processes, emotion, memory, naps, NREM sleep, REM sleep
INTRODUCTION
Different sleep stages have been linked to different forms of memory consolidation and learning. In general, nonrapid eye movement (NREM) sleep has been associated with improved episodic and declarative memory, whereas rapid eye movement (REM) sleep has been associated with gains in semantic and emotional memory.1–3 Because episodic memory is the consolidation of explicit individual memories as they were experienced in time, and semantic memory is the consolidation of generalized concepts and ideas, NREM sleep may function to consolidate the specific memories of personal experiences whereas REM sleep integrates these experiences into associative networks of more generalized knowledge and experience. Further, these purported NREM/REM differences in memory consolidation have parallels in the dreams sampled from either state: NREM dreams are emotionally and perceptually dry, drawing their content mainly from episodic memories, whereas REM dreams are more emotional, novel, and bizarre, incorporating more pseudosensory qualities and combining recent and remote memories into an ongoing narrative.4,5
Associational Structure of Semantic Memory Networks is Broadened in REM Sleep
A memory network can be conceptualized as a set of nodes that each represent unique items in memory, connected by links that represent the semantic associations between nodes. Retrieval of knowledge from such a network relies on spreading activation, a process by which activation of one node spreads to surrounding nodes by virtue of the semantic closeness of their associational links.6 The learning of associations between nodes is widely accepted to be a Hebbian process,7 such that when two items in memory are simultaneously active, the association between them is strengthened and the likelihood increases that activation of one node will lead to activation of the other.
In sleep, the breadth of spreading activation may be modulated by changes in neurophysiology of the sleep state8; for example, the activation of corticocortical connections9 or altered regional modulation of brain neurotransmitters.8 During REM sleep, as opposed to during either NREM sleep or wake, there may be a broader spread of activation through the network such that individual memory traces activate more distant, uncommon, or atypical semantic associations but fail to activate more proximal, common, or typical associations.
In the waking state, processes of spreading activation may be assessed by comparing participant performance with and without semantic priming.10 Semantic priming refers to a change in one's ability to identify or produce an item as a result of specific prior encounters with a related item.11,12 To illustrate, target words are processed more quickly or produced at a higher rate when preceded by (i.e., primed by) semantically related words than when preceded by unrelated words. Presumably, the priming words activate an underlying semantic network via spreading activation that improves access to the target words.13,14 Priming effects have been documented for both short (e.g., seconds, hours) and long (e.g., days, months) postprime delays.15 They are, furthermore, facilitated by a multiplicity of interacting factors, including those that modulate state, such as mood,16,17 neurotransmitters,18 and sleep.19
NREM and REM sleep states may differentially inhibit or facilitate underlying semantic networks and these differences may be measurable during association tasks that are completed after awakening from these states. In fact, participants awakened from REM sleep and immediately administered an associative task display a priming effect for distantly related words (e.g., bread-health), whereas participants awakened from NREM sleep or tested during a wake condition display a priming effect for closely related words (e.g., bread-butter).8 These findings are thought to reflect qualitative differences in sleep inertia or the “carry-over” of associational processes that were active during the two sleep stages, i.e., greater associativity and spread of activation during REM than during NREM sleep or wake.8 Similarly, associative processes as indexed by the quantity and originality of anagram solutions reflect such a carry-over effect from sleep into wakefulness: immediate postREM testing produces a more than 30% increase in the number of anagrams solved compared with post-NREM or wake testing.20 These findings, too, are interpreted to reflect greater fluidity and flexibility of thought in REM than in NREM sleep.20 We conceptualize these findings to indicate that access to semantic networks is broader during REM sleep than it is at other times and that this altered access, when carried over into wakefulness, may modulate priming effects in the same way as other state-modulating factors such as mood. One possibility we examine in the current study is that the nature of the preawakening sleep episode (REM versus NREM) modulates subsequent priming effects. Research on sleep inertia and carryover effects is consistent with this conceptualization in that cognitive processes are impaired or altered for variable periods of time after awakening. These effects have physiological correlates, such as reduced transcallosal inhibition after REM awakenings21 that may influence some cognitive tasks for minutes but others for hours; the effects may also be enhanced following total or partial sleep deprivation.22–24
Other types of experimental evidence bolster the notion that semantic associational networks are broadened during REM sleep and that these changes may be assessed by post-awakening testing. Semantic priming prior to sleep results in improved postsleep performance on the Remote Associates Test (RAT) for participants who undergo a REM sleep, but not a NREM sleep, nap.25 In the latter study, prime words presented presleep and then again postsleep as part of the RAT were more likely to result in solutions to task problems than were nonprime words. The presleep exposure to prime words presumably led to the priming of remote semantic associations during the broad spreading activation of REM sleep, making them easier to access during the task problems postsleep. Similarly, improvement in a second language, which is dependent on a broadening of semantic memory, correlates with an increase in %REM sleep.26 Such findings are in marked contrast to improvements in the learning of simple paired associates, whether semantically related27 or unrelated,28 which are dependent on the narrow network of associations that is presumably predominant during NREM sleep. In the current study, we introduce a new task designed to assess semantic priming under the influence of either REM or NREM sleep inertia effects; we assess the extent to which primed and nonprimed words influence an index of associational breadth in semantic networks as a function of prior sleep state.
In addition to evidence that the associational breadth of network access changes during sleep, the literature suggests that REM sleep is critical for tasks involving memory for emotional stimuli and the regulation of emotional reactivity.3,29–35 To cite but one example, retention of emotional versus neutral texts is enhanced when participants are allowed to sleep late, rather than early, in the sleep period, i.e., when sleep is much more likely to be REM, than NREM, in nature.31 Although time in REM sleep has been found to correlate with improvements in emotional tasks,29 one theory stipulates that REM sleep also involves a mechanism of emotional regulation that facilitates memory by a progressive suppression of emotional associations.36 We expected to see a positive correlation between time in REM sleep and extent of a priming effect in reaction to emotion (but not to nonemotion) cue words. Further, because distinct brain networks govern the processing of emotional words—left inferior frontal cortex is more active for emotional words than for concrete words37—similarly distinct networks might be active during REM sleep. For these reasons, our new task investigates the influence of sleep on both emotional and nonemotional, concrete cue words.
To summarize, REM sleep may promote a hyperassociative spread of activation in semantic networks, which may be important for the integration of recent memory traces within broad networks of associated memories. Such activation is revealed by tests that assess the semantic priming effects after awakenings from REM and NREM sleep or that assess changes in performance on associational tasks following pre-sleep priming. In the current study, a napping protocol is used to assess associational breadth during REM and NREM sleep using emotional and nonemotional stimuli.
Objectives and Hypotheses
Our objectives were to assess semantic priming to emotion and nonemotion cue words using a novel measure of associational breadth (a presumed index of semantic spread of activation) for participants who either napped or remained awake and, for those who napped, who underwent primarily REM or NREM sleep; and to assess the involvement in priming of REM sleep consolidation and REM sleep inertia effects. Our hypotheses were that (1) priming would be positively correlated with time elapsed in REM sleep (REM sleep consolidation), a correlation that would be higher for emotion than for nonemotion cue words, and (2) priming for both emotion and nonemotion cue words would be higher after awakenings from REM than from NREM sleep or after an equivalent period of wakefulness (REM sleep inertia).
METHODS
Participants
Fifty-eight healthy male (n = 22) and female (n = 36) participants between the ages of 18 and 35 y (mean age = 23.3 ± 4.08 y) were recruited for a nap study using advertisements and posters. There were 25 participants in the NREM group (mean age = 22.9 ± 4.15 y, 9 men, 16 women), 22 in the REM group (mean age = 24.1 ± 4.13 y, 7 men, 15 women), and 10 in the WAKE group (mean age = 22.6 ± 4.12 y, 5 men, 5 women). There were no differences in ratios of males to females over the REM, NREM, and WAKE groups (χ2(2) = 0.76, P = 0.69) and there were no age differences by sex for any of the three groups (all P > 0.16). Potential participants were directed to call the laboratory for initial screening by phone. Exclusion criteria were self-reported sleep disorders, neurological, psychological, or other chronic illnesses, psychiatric disorders, addictions, inability to nap, use of certain medications, or other conditions that interfere with sleep. Participants were informed that they should not consume alcohol or caffeine for 24 h before, as well as during, the experiment day. They completed an informed consent form that had been approved by the ethics committee of the Hôpital du Sacré-Coeur de Montréal.
Procedures
Participants arrived at 08:00, filled out the informed consent form, completed a questionnaire packet approximately 30 min in duration (not reported here) and then were prepared for the experimental task and sleep recordings.
At 09:00 participants were given task instructions and a practice trial before completing the presleep associational breadth task (AB0) and memorizing the six-item prime list (Figure 1). A sleep technician then attached an electrode montage for polysomnography, collected impedance measures, and performed biocalibration. At 10:00, participants were randomly assigned to either a group that was given a 2-h window of opportunity for sleep or a group that was kept awake for the same period of time (WAKE group). Participants allowed to sleep were awakened approximately 80 min after sleep onset. A technician trained in sleep stage scoring determined stage of awakening and sleep stage measures. Participants who were awakened from NREM sleep were assigned to the NREM group and those who were awakened from REM sleep to the REM group. Some participants in the NREM group nonetheless had brief intervals of REM sleep before returning to NREM sleep. WAKE participants were allowed to read or watch a movie and were constantly monitored to ensure they did not fall asleep.
Figure 1.
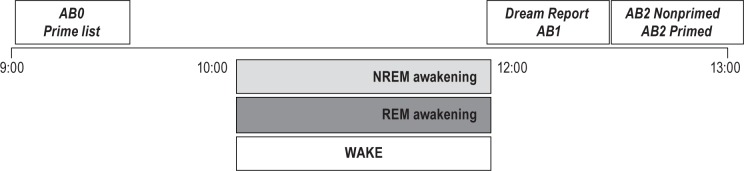
Study design: associational breadth (AB) task. Presleep, participants completed the AB task (AB0), consisting of three emotion and three nonemotion cue words, and memorized another six-item list of words that later served as primed cue words. Postnap, participants briefly summarized their dream report and repeated the AB task with new emotion and nonemotion cue words (AB1). Thirty min postsleep they repeated the AB task two more times (AB2). AB2 contained both new (nonprimed: three emotion, three nonemotion) cue words and familiar (primed: three emotion, three nonemotion) cue words. NREM awakening: participants were awakened from NREM sleep; REM awakening: participants were awakened from REM sleep; WAKE: participants were kept awake but completed, on the same schedule, a waking imagery recall task (in lieu of a dream recall task) as well as all AB tasks.
Around 12:00, sleep group participants were awakened with a nonstressful, 80 dB, 500 Hz tone and asked to follow instructions on a computer screen that swiveled out from beside the bed. They were asked to report their dream and then to complete an AB task with all new cue words (AB1). WAKE participants were also signaled by a tone and asked to report on any ongoing waking mental imagery before completing the AB1 task. The AB1 task did not include a priming condition, the focus of the current study, so responses from this task along with the imagery reports were not further assessed. The electrode montage was then removed. Approximately 30 min postawakening, participants completed two versions of the task, one that contained six new (nonprimed: three emotion, three nonemotion) cue words (AB2-NP) and a second that contained six familiar (primed: three emotion, three nonemotion) cue words (AB2-P). Participants were paid $40 for their participation.
Polysomnography
Participants slept (or stayed awake) in bedrooms with continuous audio-visual surveillance and a two-way intercom. They were recorded with an electrode montage of six standard 10–20 electroencephalography (EEG) channels (F3, F4, C3, C4, O1, O2) referenced to A1, four electrooculography (EOG) (vertical and horizontal channels) and one bipolar electromyography (EMG) channel (chin). Biosignals were recorded using Grass M12 and Grass M15 Neurodata Acquisition Systems (−6 dB filters with cut-offs at 0.30 and 100 Hz) and archived under the control of Harmonie 5.4 software (Stellate Systems, Montreal, Canada). Polysomnography (PSG) tracings were scored according to current American Academy of Sleep Medicine (AASM) standards38 by an experienced PSG technician and standard sleep variables (REM min, %REM, NREM min, %NREM, total sleep time [TST]) were calculated using in-house software.
Task Development and Administration
We developed a new task to assess levels of, and changes in, breadth of associational activation, i.e., the extent to which a cue word leads to more remote associations in a semantic network. The task is based on theories of associative processing described earlier and draws upon empirically determined norms for the typicality of associations that are given by participants in response to emotion and nonemotion (or concrete) cue words.39,40 The association norms39 contain 98 emotion and 100 nonemotion cue words, imageability and concreteness ratings for each, and the words that are most frequently associated to each cue word.39 For this study, a total of 18 emotion and 18 nonemotion cue words were chosen from these norms to be matched on ratings of imageability (> 6/7 for nonemotion words, > 4/7 for emotion words) and number of word associates (exactly three word associates given by > 70% of participants); cue words differed on concreteness ratings (> 6/7 for nonemotion words, < 4/7 for emotion words).
On each administration of the task (AB0, AB1, AB2-NP, and AB2-P), three emotion and three nonemotion cue words, each randomly selected without replacement from the sets of 18 emotion and 18 nonemotion cue words, were presented. For each trial, one cue word was presented and participants were required to respond with the first three words that came to mind as being meaningfully associated. A maximum of 30 sec was allowed to type out the three words; a countdown clock on the screen displayed the time remaining for each cue word. The presentation software41 recorded all words typed by participants. The next trial was administered 500 ms after the end of the previous for a total of six trials and a maximum task time of 3 min.
The norms39 were used to score associational breadth of responses to each cue word; any word associate not in the norms was considered to be uncommon and scored as 1, otherwise as 0. Associational breadth scores for emotion and nonemotion cue words on a single task administration therefore varied between 0 (all common associates) and 9 (all uncommon associates). For some analyses, associational breadth scores were assessed separately for positive and negative valence emotion cue words; because emotion cue words were randomly selected for presentation, proportions of positive and negative valence cue words were not equally represented in all cells of the experimental design.
Priming Task
Following administration of the prenap AB0 task, participants were instructed to memorize a list of three emotion and three nonemotion cue words, each randomly selected without replacement from the aforementioned sets of 18 emotion and 18 nonemotion cue words. These six words were presented for memorization in randomized order, 4 sec/word, on each of three sequential presentations. After the sleep (or wake) interval, all six memorized words were subsequently presented as primed cue words in administration of the postnap AB2-P task, whereas new cue words were presented in the AB2-NP task. Participants were never actually tested on their recall of these words, but their earlier presentation as to-be-remembered stimuli endowed them with the properties of semantic primes that were either emotional or nonemotional in nature. The primary dependent measure, relative priming, was calculated by subtracting nonprimed AB2-NP from primed AB2-P scores for emotion and nonemotion cue words separately. This measure controls for within-subject factors not specific to change-from-baseline effects, e.g., multiple task administrations, and other state- and context-related confounds. Positive scores indicated that primed cue words produced more uncommon associates than did nonprimed cue words; negative scores indicated the opposite.
Statistical Analyses
The principal set of analyses assessed priming and the implication of a possible REM sleep consolidation effect. We conducted a 2 × 3 analysis of variance (ANOVA) with cue word type (emotion, nonemotion) as a repeated measures factor, sleep state (REM, NREM, WAKE) as a between-groups factor, and extent of relative priming as the dependent measure. Oneway ANOVAs and post hoc t-tests then isolated the interaction effect and compared positive, negative and nonemotion priming cue words. Spearman correlations between positive, negative, and nonemotion cue word priming scores and min/% time in sleep stage (REM and N3) tested a possible REM sleep consolidation effect. To assess REM sleep inertia effects, sleep groups were split into subgroups containing different compositions of REM and NREM sleep (see later). Relative priming scores for five awakening subgroups (WAKE, N, N-R, N-R-N, N-R-N-R) were compared using one-way ANOVAs and post hoc t-tests comparing specifically N-R-N versus N-R and N-R-N-R subgroups.
RESULTS
Sleep Architecture
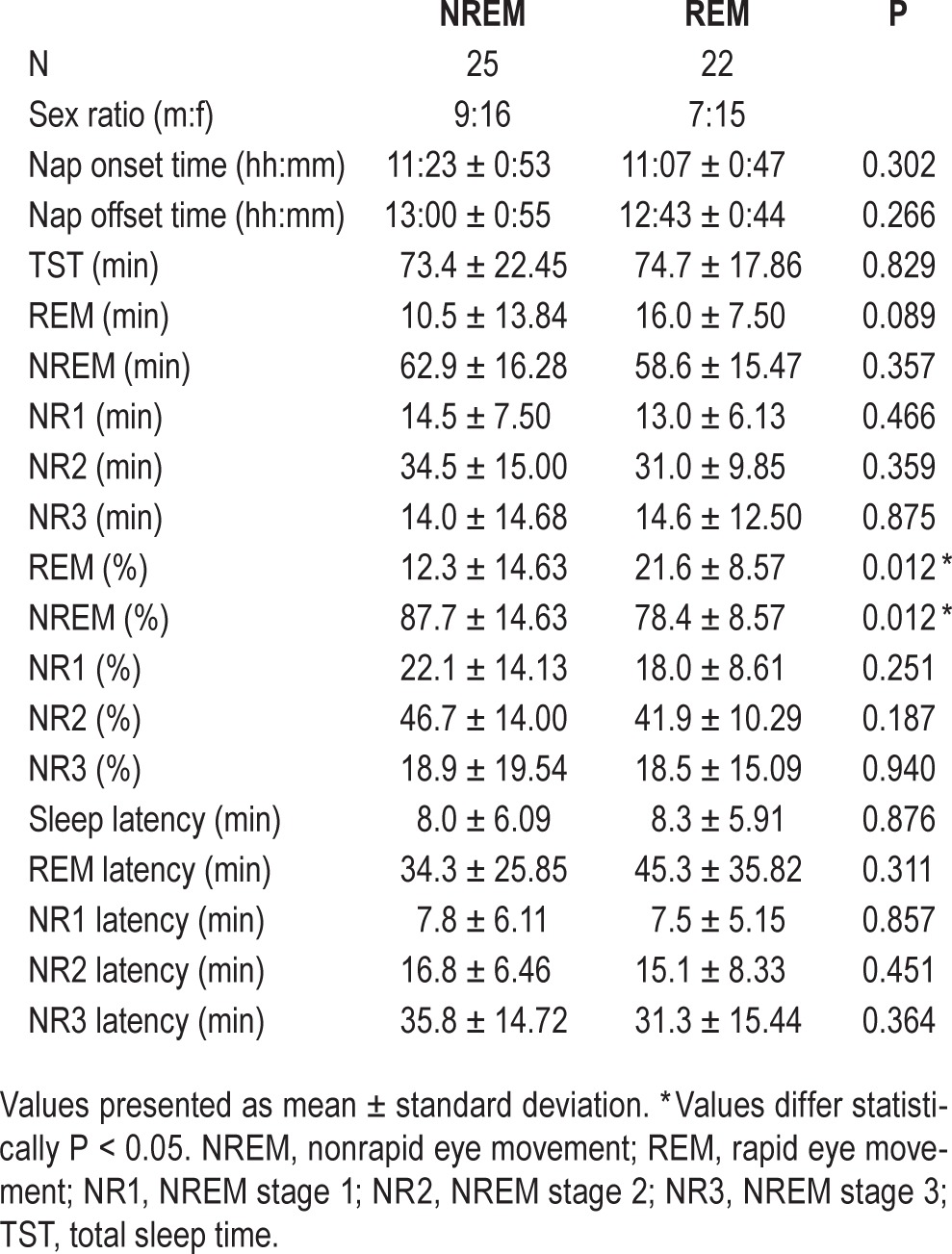
The sleep recording for one participant in the REM group was removed from these analyses because of technical difficulties. Sleep measures for the NREM and REM sleep groups are reported in Table 1; groups differed in %REM sleep (t45 = −2.60, P = 0.012, two-tailed) and marginally in REM min (t45 = −1.75, P = 0.089). They also differed in %NREM sleep (t45 = 2.60, P = 0.012), as this measure is inversely proportional with %REM, but they did not differ in TST or NREM sleep min.
Table 1.
Sleep stage measures for naps of the rapid eye movements and nonrapid eye movement groups.
Task Performance as a Function of Sleep Stage
Mean associational breadth scores and standard deviations for each task session for each of the three groups are reported in Table 2. Groups did not differ on prenap AB0 scores for either emotion or nonemotion cue words (all P > 0.65).
Table 2.
Mean (± standard deviation) associational breadth scores for all conditions and groups.
Priming Effect
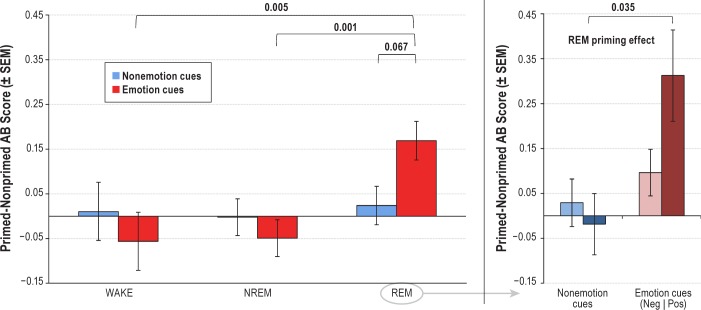
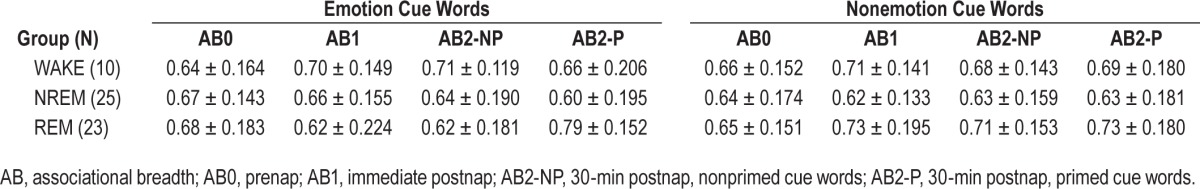
The 2 × 3 ANOVA on the relative priming measure produced a main effect for Group (F2,55 = 5.02, P = 0.010), no main effect for CueType (F1,55 = 0.06, P = 0.805), and a nearly-significant CueType × Group interaction (F2,55 = 3.07, P = 0.054). This pattern indicated that the three groups differed on the emotion but not the nonemotion cue words. Specifically, as shown in Figure 2, left panel, emotion cue words produced a priming effect that was higher for the REM group (mean = 0.169 ± 0.204) than for either the WAKE (mean = −0.056 ± 0.176; t55 = 2.90, P = 0.005) or NREM (mean = −0.049 ± 0.215; t 55 = 3.68, P = 0.001) groups. Priming for the REM emotion cue word condition was also marginally greater than for the REM non-emotion (mean = 0.024 ± 0.255) condition (t22 = 1.93, P = 0.067).
Figure 2.
Left panel: relative priming, calculated as primed minus nonprimed associational breadth scores, was elevated only for emotion cue words and only for the rapid eye movement (REM) sleep nap group. Right panel: detail of REM priming effect: post hoc tests show that only cue words with a positive emotional valence produced a priming effect at P < 0.05; two bars are shown for nonemotion cues due to slightly different size groups of participants receiving at least one negative (n = 19) and at least one positive (n = 12) cue word. P values are shown for pertinent within- and between-group contrasts.
Post hoc analyses of cue word differences by emotional valence revealed a significant one-way ANOVA on priming for positive cue words (F2,31 = 6.86, P = 0.004) such that the REM group (mean = 0.313 ± 0.352) differed from the NREM group (mean = −0.150 ± 0.363; t23 = 3.23, P = 0.004, not shown) and from the WAKE group (mean = −0.048 ± 0.081; t17 = 2.64, P = 0.017) whereas the NREM and WAKE groups did not differ (t18 = 0.72, P = 0.479). A similar one-way ANOVA on priming for negative cue words was not significant (F2,47 = 1.88, P = 0.165) with no differences between the REM (mean = 0.096 ± 0.227) and NREM (mean = −0.008 ± 0.258) groups (t37 = 1.35, P = 0.187, not shown), the REM and WAKE (mean = −0.092 ± 0.294) groups (t26 = 1.87, P = 0.072), or the NREM and WAKE groups (t27 = −0.78, P = 0.443). Within the REM condi -tion alone, post hoc tests showed that priming for positive (mean = 0.313 ± 0.352) vs. nonemotion (mean = −0.018 ± 0.236) cue words differed (t11 = 2.40, P = 0.035, Figure 2, right panel) but not priming for negative (mean = 0.096 ± 0.227) vs. non-emotion (mean = 0.029 ± 0.231) cue words (t18 = 0.81, P = 0.428; Figure 2, right panel). No other differences between or within conditions were found.
In summary, relative priming comparisons revealed substantial evidence for a REM sleep emotional priming effect that was more apparent for positive than for negative emotion cue words.
Correlational Analyses
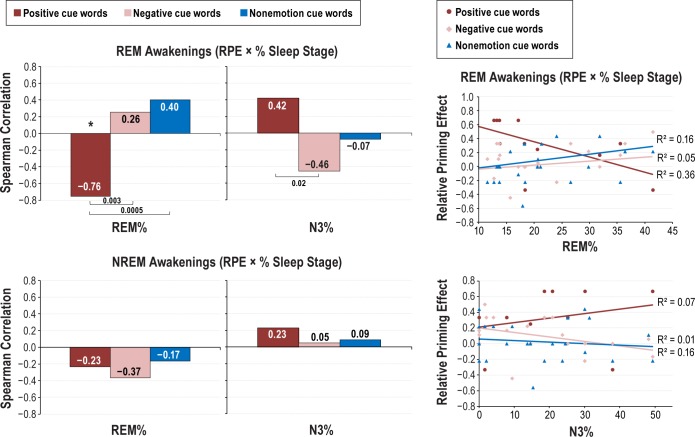
As shown in Table 3 and Figure 3, Spearman correlations between relative priming measures (positive, negative, non-emotion) and measures of time and % in sleep stage revealed that, for REM awakenings, priming for positive cue words correlated negatively with REM min (r12 = −0.649, P = 0.022) and REM% (r12 = −0.757, P = 0.004) and positively, albeit nonsig -nificantly, with NR3 min (r12 = 0.413, P = 0.182) and NR3% (r12 = 0.420, P = 0.174). Priming for negative cue words showed an opposite pattern of correlations: nonsignificantly positive with REM min and REM% (both P > 0.280) and negative with NR3 min (P = 0.100) and NR3% (P = 0.050). For NREM awakenings alone, only nonsignificant or marginal correlations between priming and times in sleep stage were found. Although only one of the previous correlations would survive a family-wise error correction for multiple comparisons (0.05/7 sleep measures per CueType family = 0.007), Fisher r-to-z analyses nonetheless indicate that REM × positive cue word correlations differ substantially from those for REM × nonemotional cue words, i.e., REM minutes (z = −2.81, P = 0.005) and REM% (z = −3.50, P = 0.0005; see Table 3).
Table 3.
Spearman correlations between sleep stage measures and relative priming scores for positive, negative, and nonemotion cue words.
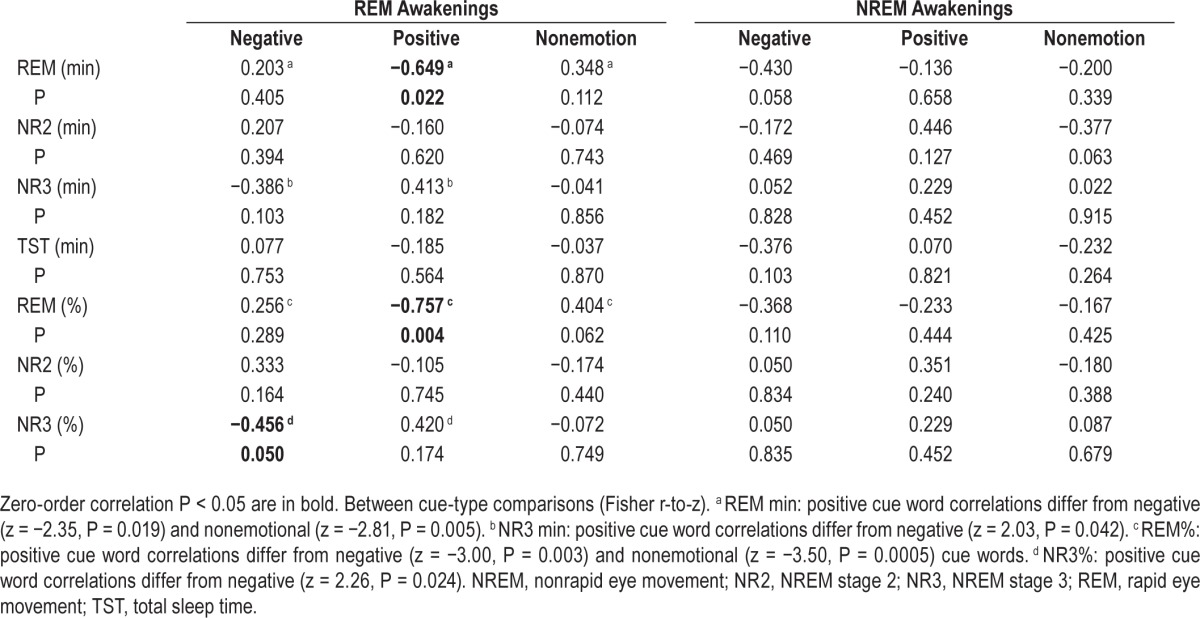
Figure 3.
Spearman correlations between relative priming scores and percent time in sleep stage (REM%, N3%) for REM (upper bar graphs) and NREM (lower bar graphs) awakenings. Scattergrams shown for REM awakenings (right panel). * P < 0.02. Correlations with positive cue words differed from those with negative and nonemotion cue words for REM awakenings (P values for Fisher r-to-z comparisons shown with horizontal brackets). NREM, nonrapid eye movement; REM, rapid eye movement.
Sleep Stage Inertia Effects
To test whether the REM priming effect was facilitated by REM stage awakenings (REM sleep inertia), NREM and REM awakening groups were divided into the following four subgroups: “N” (single NREM episode); “N-R” (episode consisting of NREM followed by REM); “N-R-N” (sequence of NREM, REM, NREM) and “N-R-N-R” (sequence of NREM, REM, NREM, REM). Subgroup sizes were n = 10, 13, 15, and 10, respectively. REM awakenings thus occurred for N-R and N-R-N-R whereas NREM awakenings occurred for N and N-R-N. As shown in Figure 4, upper panel, all four groups contained similar NREM sleep minutes and N-R-N did not differ from either N-R or N-R-N-R in REM sleep minutes (although N-R and N-R-N-R did differ). One-way ANOVAs and t-tests specifically contrasting N-R-N with N-R and N-R-N-R groups revealed that means for emotion cue words differed significantly (F4,53 = 3.47, P = 0.021) whereas those for nonemotion cue words did not (F4,53 = 0.48, P = 0.751). As shown in Figure 4, lower panel, priming for emotion cue words did not differ for N-R vs. N-R-N-R groups, and both of these groups had greater priming than group N-R-N (P = 0.002 and 0.005, respectively). Secondary analyses revealed that: the former two groups differed marginally from group N (P = 0.055 and 0.077, respectively); N and N-R-N did not differ from each other; N-R and N-R-N-R were greater than WAKE (P = 0.012 and 0.020); and N and N-R-N were not different from WAKE. There were no differences among groups for nonemotion cue words (all P > 0.218). Limited sample sizes precluded running similar analyses for positive and negative cue words separately.
Figure 4.
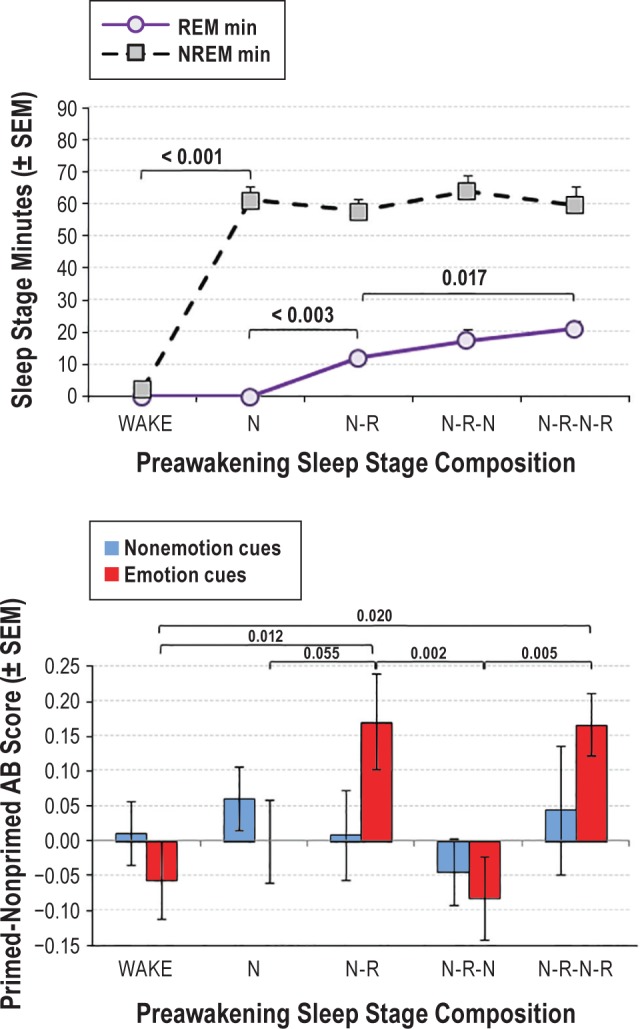
Relative priming for subgroups with differing preawakening stage compositions. Upper panel: subgroup of NREM awakenings with some prior REM (N-R-N) did not differ from subgroups of REM awakenings (N-R and N-R-N-R) in total minutes of NREM (P = 0.309 and 0.512 respectively) or REM sleep (P = 0.119 and 0.331). Lower panel: Priming is elevated for emotion cue words only after awakenings from REM sleep even though N-R-N contains substantial REM sleep, suggesting a selective carryover effect on priming for REM groups. There was no carryover effect for nonemotion cue words. N = NREM; N-R = NREM + REM; N-R-N = NREM + REM + NREM; N-R-N-R = NREM + REM + NREM + REM. NREM, nonrapid eye movement; REM, rapid eye movement.
To summarize these findings for WAKE, REM and NREM group analyses, a priming effect for emotional cue words was elevated for participants awakened from REM but not NREM sleep, whether or not the preceding NREM sleep possessed any REM sleep.
DISCUSSION
The associational task developed for this study proved successful in demonstrating a selective association between REM sleep and a priming effect for emotion cue words—especially for cue words of a positive emotional valence. As predicted, when participants were awakened from REM sleep and asked to provide word associations to emotion cue words that had been primed prior to sleep, the semantic atypicality of these words increased relative to when associations were to cue words that were either not previously primed or not emotional. This pattern of differences was not seen for participants who had been awakened from NREM sleep, nor was it clearly characteristic of participants who had not slept at all.
These results extend findings demonstrating that REM sleep is associated in a general sense with performance improvements on emotional memory tasks.3,29–34 This includes improvement in discrimination accuracy for facial emotions,3 recall of emotional texts,31 recognition of emotional pictures,42 and consolidation of complex negative scenes.43 And although much of this previous research has examined REM sleep's implication in tasks involving negative emotions, an increasing number of studies3,44 indicate that positive emotional stimuli are also REM sleep dependent. Our post hoc finding of a priming effect primarily for positive cue words is consistent with the latter studies3,44 but clearly requires further replication.
Beyond supporting a role for REM sleep in emotional memory, our results are also consistent with sleep studies that have used specific types of semantic priming tasks. First, our results are fully consistent with the prior demonstration25 that performance on a primed RAT is enhanced when participants have intervening REM sleep but not when they have NREM sleep or quiet rest. The solving of RAT word triads (e.g., RABBIT CLOUD MILK; solution: WHITE) has been described as due to spreading activation through the weak (remote) links of semantic networks for the cue word triads.45 When one of the cue words is primed (i.e., presented in another context) prior to sleep, its semantic network is presumably activated and consolidated during subsequent REM sleep. And, as we suggest for the priming effect in our own results (discussed later), the information in this network is therefore more readily accessible later during the RAT administration. Previous work on priming effects may help explain why in the current study the REM-facilitated priming effect was observed primarily for emotionally positive cue words. Induction of a positive mood is now widely known to facilitate spread of activation and, thus, priming effects.17 Positive mood also improves success with RAT solutions46–48 and increases the unusualness and diversity of word associations.49
Second, the current results converge with the finding that there occurs less loss of priming to word-stem stimuli after an interval of late night sleep (rich in REM) than there is after an interval of early night sleep (rich in NREM).50 Third, the results are consistent with those of a study that showed more response errors in a masked visuomotor priming task after late-night than after early-night sleep.51 Errors in that study were due to increased priming of masked visuomotor stimuli, and thus the increase in errors following more time spent in REM suggests successful priming. Finally, our findings converge with those from a study in which enhanced positive repetition priming in a face identification task was found after late night, REM rich sleep.52
Two REM Sleep Mechanisms for Priming Facilitation?
Although a REM sleep related priming effect is clear in our findings, evidence points to two mechanisms that may underlie this effect: REM sleep mediated consolidation and REM sleep inertia (or carryover). REM sleep consolidation is suggested primarily by a significant correlation between magnitude of the priming effect for positive cue words and minutes and percent of REM sleep; it suggests that priming stimuli were further processed during REM sleep and may thus have influenced the postsleep retest. In contrast, a REM sleep inertia effect is suggested primarily by the specific association of priming with REM sleep awakenings (N-R, N-R-N-R subgroups) but not with NREM awakenings that nonetheless contained prior REM sleep (N-R-N subgroup). Stage of awakening (REM) was thus independent of prior REM time, a result consistent with our suggestion that both REM consolidation and REM sleep inertia together produced the REM-related priming apparent in our results.
REM Sleep Consolidation
Although other explanations are possible, we speculate that presentation of the word list for memorization prior to sleep led it to be “tagged” for memory storage,53 and thus for its semantic networks to be selectively reactivated during subsequent REM sleep. The hyperassociative nature of REM sleep presumably facilitated—and consolidated—a broader than normal spread of activation through these networks. Because REM sleep is particularly sensitive to emotional material,54 activation of the networks for primed emotion words were likely favored over those for primed nonemotion words. When participants were awakened from REM (but not from NREM) sleep and the primed emotion words were subsequently presented as cue words, the now-consolidated broad networks linked to these words were presumably more easily accessed, leading to the production of more distantly related word associates.
Although our principal findings strongly support a role for REM sleep in emotional priming, the unexpected negative correlations between the magnitude of positive cue word priming and time and percent REM sleep requires further consideration. The finding is strikingly similar to a previously reported, unexpected result8: that amount of priming of distantly related word pairs (e.g., bread-health) elicited after REM sleep awakenings decreased, rather than increased, as these awakenings took place progressively later in the night, i.e., as REM time increased. As in our study, these authors found that priming diminished as REM periods became longer and physiologically more intense. We propose an explanation that is consistent with the possibility that the associational REM sleep activity contributing to priming is also implicated in a functional mechanism of emotional regulation at this time.
Specifically, an emotion regulation function for REM sleep has been proposed by several authors.30,54–56 Among these, the SFSR (sleep to forget and sleep to remember) model of emotional memory processing54 is a particularly apt fit to the current findings. The SFSR model stipulates that connectivity of emotional memory elements depends on the progressive diminution—within and across REM periods—of the emotional charge that was associated with those elements during encoding. Accordingly, in the current study emotional words that were presented for memorization prior to sleep may have been queued for a process of emotional downregulation during the next available REM sleep episode. As that REM episode unfolded, the prime words may have become progressively more consolidated in memory yet progressively denuded of their emotional charge, resulting in REM-associated priming that was less marked for longer REM periods. Thus, although REM sleep mechanisms were necessary for emergence of the priming effect, more time elapsed in REM sleep may have successfully decreased the emotional charge of the primed cue words and thus diminished the magnitude of the subsequent priming effect.
The combination of REM-dependence and a negative correlation between priming and REM time is not unlike work demonstrating sleep stage association with a learning effect that is nonetheless independent of time-in-stage.28,57 In one such study, declarative memory performance was found to improve after a 60-min NREM sleep nap even though performance was not correlated with time in SWS.58 A follow-up study found that naps as short as 6 min nevertheless conferred memory improvement relative to a waking state control. Thus, it may be the appearance of the sleep stage, rather than the amount of time spent in that stage, that is critical for memory gains. It should be noted that the priming effect we observed was also positively, albeit not significantly, correlated with minutes of N3 sleep, so we cannot exclude the possibility that the hypothesized consolidation process was linked to NREM, rather than REM, sleep processes. NREM sleep is known to facilitate consolidation of many types of stimuli, including emotional stimuli.59
REM Sleep Inertia
That the priming effect for emotion cue words was associated selectively with REM (but not NREM) sleep awakenings in our results replicates in a general sense the findings of many previous studies that have demonstrated sleep inertia and carryover effects of various durations.24,60 Although findings are inconsistent on the exact duration of sleep inertia—estimates range from minutes to hours22–24,48—inconsistencies are likely a function of task type, outcome measure, experimental manipulation, quality of prior sleep, and other confounding factors. The appearance of our priming effect at approximately 30 min postawakening is thus well within expectations, but only for some types of cognitive tasks. Lower level processes, such as attention, are more severely affected by sleep inertia (and thus recover more slowly) than are higher level processes such as working memory60 and, presumably, spread of activation in semantic networks is a lower level cognitive process whose automaticity is prerequisite to basic language comprehension and production.
Our findings also replicate the more specific finding that REM sleep inertia affects cognitive processes differently than does NREM sleep inertia,8,61 e.g., increasing priming of remote semantic associates after only REM sleep awakenings.8 In the latter study8, a target word (e.g., “wrong”) that was only weakly related to a prior prime word (“thief”) was more quickly recognized as a word a few minutes after REM than after NREM sleep awakenings; in contrast, priming of a strongly related target word (e.g., “hot-cold”) was not facilitated. Weakly related word pairs correspond closely to the atypical responses on the associational breadth task in our results, as these consist of words that are not strongly related to cue words in a normative sample.39 In both studies, awakenings from REM sleep selectively facilitated access to these more weakly related semantic associations. In addition, our results go further in separating stage of awakening from prior time-in-stage in that our NREM awakening participants in the N-R-N subgroup showed no increase in priming, even though their time in REM sleep did not differ from that of either of the REM awakening groups (N-R, N-R-N-R). Further, whereas Stickgold and colleagues8 demonstrated increased priming in response to nonemotion cue words, we found that emotion, but not nonemotion, cue words facilitated priming. It is thus possible that REM sleep inertia for nonemotional priming dissipates well before 30 min postawakening whereas inertia for emotional priming persists for 30 min or longer. The relative durations of REM sleep inertia effects on different types of cognitive tasks remains relatively unknown; much more research is clearly warranted.
It is noteworthy that sleep inertia may be modulated by circadian rhythmicity62 and, because our naps occurred in late morning, which is earlier than most nap studies reported in the literature and close to the zenith of REM sleep propensity, it is likely that REM sleep inertia was at a maximum for our participants. This, too, requires further study. Finally, it should be noted that we administered the unprimed associational breadth task on two other occasions before the priming administration, once presleep and once immediately postawakening. It is therefore possible that a participant's later performance on the task, the unprimed task in particular, was influenced by factors such as learning. For example, our additional post-awakening administrations may have facilitated dissipation of sleep inertia for the unprimed task selectively, thus enhancing the apparent difference between later primed and unprimed scores. However, some considerations mitigate against such an interpretation. First, multiple administrations of a task are common in sleep inertia studies60 and generally accepted as a valid procedure for assessing dissipation of sleep inertia. Some investigators reduce learning effects by giving multiple “practice” sessions of a task prior to testing, but this could also induce unwanted priming effects in semantic priming protocols such as ours. Second, each of our unprimed task administrations used different cue words; thus, the likelihood of learning, habituation, or even secondary priming of the unprimed task was reduced.
In summary, we suggest that our findings reflect the operation of two REM sleep related mechanisms that facilitate an emotional priming effect: a consolidation mechanism related to emotional downregulation and a sleep inertia effect that persists until the time of testing. This two-factor explanation closely parallels a similar explanation by Kvavilashvili and Mandler15 of how autobiographical memories appear involuntarily during wakefulness: they are produced by “the cumulative action of long term residual activation of a prime per se and relatively short term (associative) spreading of activation in response to one's current situation” (see page 82 of Kvavilashvili and Mandler).15
A Possible Role for Dreaming?
Several of the previously cited authors consider dreaming to be implicated in the observed sleep-related priming effects. For example, Stickgold et al. concluded that their findings help explain the “bizarre and hyperassociative nature of REM sleep dreaming” (page 188 in the Stickgold et al. study).8 Similarly, Mazzetti et al.63 suggest that the integrative processes of dreaming are implicated in the altered spread of semantic activation reflected by their priming effect. Although we also postulate that our findings reflect the hyperassociativity of semantic networks during REM sleep, and although additional analyses of our detailed mentation samples may yet provide support for the claim that dreaming participates in this hyper-associativity, nothing reported in the current results directly supports this more general claim. The current findings do suggest, however, that the affective content of dreams—positive affect especially—may be associated with the emotional priming effect. For example, positive dream affect may either facilitate the consolidation of emotional primes in semantic networks during REM sleep or it may form a key component of postawakening, REM sleep inertia, a type of “dream inertia.” It should be noted, however, that as our findings for positive and negative cue words were post hoc and based on smaller samples than were those for other statistical tests, more study is needed to replicate the findings and assess their possible implication in dreaming's involvement in memory consolidation.
In summary, our findings add to a growing body of research demonstrating that REM sleep is associated with emotional memory consolidation and, in particular, to a modification of semantic priming effects. They thus support the notion of a hyperactivation of semantic networks during REM sleep and of a further facilitation of such activation in the postREM sleep awakening period. Our results point especially to a role for positive emotional stimuli that other work has found to have a facilitating effect on spreading activation in semantic networks. An unexpected negative correlation between priming and time in REM sleep may reflect the activity of an underlying function regulating emotion, such as that predicted by the SFSR model of Walker,36 whereas the association of priming exclusively with REM sleep awakenings may signal the influence of REM sleep inertia on this cognitive measure.
DISCLOSURE STATEMENT
This study was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Institutes of Health Research (CIHR) and the Dream Science Foundation/International Association for the Study of Dreams (DSF/IASD). Dr. Nielsen has received research grants from NSERC, CIHR and DSF/IASD. Michelle Carr received a research grant from DSF/ISAD. The research was conducted at the Dream and Nightmare Laboratory, Center for Advanced Research in Sleep Medicine, Hôpital du Sacré-Coeur de Montréal.
REFERENCES
- 1.Rauchs G, Desgranges B, Foret J, Eustache F. The relationships between memory systems and sleep stages. J Sleep Res. 2005;14:123–40. doi: 10.1111/j.1365-2869.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 2.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 3.Gujar N, McDonald SA, Nishida M, Walker MP. A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cereb Cortex. 2011;21:115–23. doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antrobus J. REM and NREM sleep reports: comparison of word frequencies by cognitive classes. Psychophysiology. 1983;20:562–8. doi: 10.1111/j.1469-8986.1983.tb03015.x. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen T. A review of mentation in REM and NREM sleep: “covert” REM sleep as a possible reconciliation of two opposing models. Behav Brain Sci. 2000;23:851–66. doi: 10.1017/s0140525x0000399x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JR. A spreading activation theory of memory. J Verbal Learning Verbal Behav. 1983;22:261–95. [Google Scholar]
- 7.Hebb DO, editor. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- 8.Stickgold R, Scott L, Rittenhouse C, Hobson JA. Sleep-induced changes in associative memory. J Cogn Neurosci. 1999;11:182–93. doi: 10.1162/089892999563319. [DOI] [PubMed] [Google Scholar]
- 9.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–66. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 10.Collins AM, Loftus EF. A spreading-activation theory of semantic processing. Psychol Rev. 1975;82:407–28. [Google Scholar]
- 11.Overson C, Mandler G. Indirect word priming in connected semantic and phonological contexts. Bull Psychon Soc. 1987;25:229–32. [Google Scholar]
- 12.Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–6. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- 13.McDermott KB. Priming on perceptual implicit memory tests can be achieved through presentation of associates. Psychon Bull Rev. 1997;4:582–6. [Google Scholar]
- 14.Graf P, Mandler G. Activation makes words more accessible, but not necessarily more retrievable. J Verbal Learning Verbal Behav. 1984;23:553–68. [Google Scholar]
- 15.Kvavilashvili L, Mandler G. Out of one's mind: a study of involuntary semantic memories. Cogn Psychol. 2004;48:47–94. doi: 10.1016/s0010-0285(03)00115-4. [DOI] [PubMed] [Google Scholar]
- 16.Ashby F, Isen A, Turken A. A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev. 1999;106:529–50. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- 17.Topolinski S, Deutsch R. Phasic affective modulation of semantic priming. J Exp Psychol Learn Mem Cogn. 2013;39:414–36. doi: 10.1037/a0028879. [DOI] [PubMed] [Google Scholar]
- 18.Foster PS, Branch KK, Witt JC, et al. Acetylcholinesterase inhibitors reduce spreading activation in dementia. Neuropsychologia. 2012;50:2093–9. doi: 10.1016/j.neuropsychologia.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Sio UN, Monaghan P, Ormerod T. Sleep on it, but only if it is difficult: effects of sleep on problem solving. Mem Cognit. 2013;41:159–66. doi: 10.3758/s13421-012-0256-7. [DOI] [PubMed] [Google Scholar]
- 20.Walker MP, Liston C, Hobson JA, Stickgold R. Cognitive flexibility across the sleep–wake cycle: REM-sleep enhancement of anagram problem solving. Brain Res Cogn Brain Res. 2002;14:317–24. doi: 10.1016/s0926-6410(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 21.Bertini M, De Gennaro L, Ferrara M, et al. Reduction of transcallosal inhibition upon awakening from REM sleep in humans as assessed by transcranial magnetic stimulation. Sleep. 2004;27:875–82. doi: 10.1093/sleep/27.5.875. [DOI] [PubMed] [Google Scholar]
- 22.Hofer-Tinguely G, Achermann P, Landolt H-P, et al. Sleep inertia: performance changes after sleep, rest and active waking. Brain Res Cogn Brain Res. 2005;22:323–31. doi: 10.1016/j.cogbrainres.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Jewett ME, Wyatt JK, Ritz-De Cecco A, Khalsa SB, Dijk D-J, Czeisler CA. Time course of sleep inertia dissipation in human performance and alertness. J Sleep Res. 1999;8:1–8. doi: 10.1111/j.1365-2869.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- 24.Tassi P, Muzet A. Sleep inertia. Sleep Med Rev. 2000;4:341–53. doi: 10.1053/smrv.2000.0098. [DOI] [PubMed] [Google Scholar]
- 25.Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci U S A. 2009;106:10130–4. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Koninck J, Lorrain D, Christ G, Proulx G, Coulombe D. Intensive language learning and increases in rapid eye movement sleep: evidence of a performance factor. Int J Psychophysiol. 1989;8:43–7. doi: 10.1016/0167-8760(89)90018-4. [DOI] [PubMed] [Google Scholar]
- 27.Goerke M, Cohrs S, Rodenbeck A, Grittner U, Sommer W, Kunz D. Declarative memory consolidation during the first night in a sleep lab: the role of REM sleep and cortisol. Psychoneuroendocrinology. 2012;38:1102–11. doi: 10.1016/j.psyneuen.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Tucker MA, Fishbein W. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008;31:197–203. doi: 10.1093/sleep/31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–66. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lara-Carrasco J, Nielsen TA, Solomonova E, Levrier K, Popova A. Overnight emotional adaptation to negative stimuli is altered by REM sleep deprivation and is correlated with intervening dream emotions. J Sleep Res. 2009;18:178–87. doi: 10.1111/j.1365-2869.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- 31.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–9. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosales-Lagarde A, Armony JL, del Río-Portilla Y, Trejo-Martínez D, Conde R, Corsi-Cabrera M. Enhanced emotional reactivity after selective REM sleep deprivation in humans: an fMRI study. Front Behav Neurosci. 2012;6:1–13. doi: 10.3389/fnbeh.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar T, Jha SK. Sleep deprivation impairs consolidation of cued fear memory in rats. PloS One. 2012;7:e47042. doi: 10.1371/journal.pone.0047042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Helm E, Yao J, Dutt S, Rao V, Saletin JM, Walker MP. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol. 2011;21:2029–32. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hobson JA, Goldfrank F, Snyder F. Respiration and mental activity in sleep. J Psychiatr Res. 1965;3:79–90. doi: 10.1016/0022-3956(65)90017-8. [DOI] [PubMed] [Google Scholar]
- 36.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–97. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 37.Shallice T, Cooper RP. Is there a semantic system for abstract words? Front Hum Neurosci. 2013;7:1–10. doi: 10.3389/fnhum.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV, editors. Darien, IL: American Academy of Sleep Medicine; 2012. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.0. www.aasmnet.org. [Google Scholar]
- 39.Altarriba J, Bauer LM, Benvenuto C. Concreteness, context availability, and imageability ratings and word associations for abstract, concrete, and emotion words. Behav Res Methods Instrum Comput. 1999;31:578–602. doi: 10.3758/bf03200738. [DOI] [PubMed] [Google Scholar]
- 40.Mednick S. The associative basis of the creative process. Psychol Rev. 1962;69:220–32. doi: 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- 41.Inquisit 4.0.0.1 [Computer Software] Seattle, WA: Millisecond Software LLC; 2012. [Google Scholar]
- 42.Groch S, Wilhelm I, Diekelmann S, Born J. The role of REM sleep in the processing of emotional memories: evidence from behavior and event-related potentials. Neurobiol Learn Mem. 2013;99:1–9. doi: 10.1016/j.nlm.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Payne J, Chambers AM, Kensinger EA. Sleep promotes lasting changes in selective memory for emotional scenes. Front Integr Neurosci. 2012;6:108. doi: 10.3389/fnint.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambers AM, Payne JD. Laugh yourself to sleep: memory consolidation for humorous information. Exp Brain Res. 2013:1–13. doi: 10.1007/s00221-013-3779-7. [DOI] [PubMed] [Google Scholar]
- 45.Topolinski S, Strack F. The architecture of intuition: fluency and affect determine intuitive judgments of semantic and visual coherence and judgments of grammaticality in artificial grammar learning. J Exp Psychol Gen. 2009;138:39–63. doi: 10.1037/a0014678. [DOI] [PubMed] [Google Scholar]
- 46.Isen AM, Daubman KA, Nowicki GP. Positive affect facilitates creative problem solving. J Pers Soc Psychol. 1987;52:1122–31. doi: 10.1037//0022-3514.52.6.1122. [DOI] [PubMed] [Google Scholar]
- 47.Corson Y. Effects of positive, negative, and neutral moods on associative and semantic priming. Curr Psychol Cogn. 2002;21:33–62. [Google Scholar]
- 48.Haänze M, Hesse FW. Emotional influences on semantic priming. Cogn Emot. 1993;7:195–205. doi: 10.1080/02699939308409184. [DOI] [PubMed] [Google Scholar]
- 49.Isen AM, Johnson MM, Mertz E, Robinson GF. The influence of positive affect on the unusualness of word associations. J Pers Soc Psychol. 1985;48:1413–26. doi: 10.1037//0022-3514.48.6.1413. [DOI] [PubMed] [Google Scholar]
- 50.Plihal W, Born J. Effects of early and late nocturnal sleep on priming and spatial memory. Psychophysiology. 1999;36:571–82. [PubMed] [Google Scholar]
- 51.Verleger R, Schuknecht S-V, Jaśkowski P, Wagner U. Changes in processing of masked stimuli across early-and late-night sleep: a study on behavior and brain potentials. Brain Cogn. 2008;68:180–92. doi: 10.1016/j.bandc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Wagner U, Hallschmid M, Verleger R, Born J. Signs of REM sleep dependent enhancement of implicit face memory: a repetition priming study. Biol Psychol. 2003;62:197–210. doi: 10.1016/s0301-0511(02)00125-4. [DOI] [PubMed] [Google Scholar]
- 53.Redondo RL, Morris RG. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- 54.Walker MP, van Der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–48. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cartwright R, Agargun MY, Kirkby J, Friedman JK. Relation of dreams to waking concerns. Psychiatry Res. 2006;141:261–70. doi: 10.1016/j.psychres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Greenberg R, Pillard R, Pearlman C. The effect of dream (stage REM) deprivation on adaptation to stress. Psychosom Med. 1972;34:257–62. doi: 10.1097/00006842-197205000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Schönauer M, Geisler T, Gais S. Strengthening procedural memories by reactivation in sleep. J Cogn Neurosci. 2013;26:143–53. doi: 10.1162/jocn_a_00471. [DOI] [PubMed] [Google Scholar]
- 58.Lahl O, Wispel C, Willigens B, Pietrowsky R. An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res. 2008;17:3–10. doi: 10.1111/j.1365-2869.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- 59.Cairney SA, Durrant SJ, Hulleman J, Lewis PA. Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation. Sleep. 2014;37:701–7. doi: 10.5665/sleep.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santhi N, Groeger JA, Archer SN, Gimenez M, Schlangen LJ, Dijk D-J. Morning sleep inertia in alertness and performance: Effect of cognitive domain and white light conditions. PloS one. 2013;8:e79688. doi: 10.1371/journal.pone.0079688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bertini M, Violani C, Zoccolotti P, Antonelli A, Di Stefano L. Right cerebral activation in REM sleep: evidence from a unilateral tactile recognition test. Psychophysiology. 1984;21:418–23. doi: 10.1111/j.1469-8986.1984.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 62.Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J Biol Rhythms. 2008;23:353–61. doi: 10.1177/0748730408318081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazzetti M, Campi C, Mattarozzi K, et al. Semantic priming effect during REM-sleep inertia in patients with narcolepsy. Brain Res Bull. 2006;71:270–8. doi: 10.1016/j.brainresbull.2006.09.011. [DOI] [PubMed] [Google Scholar]