Abstract
Introduction
As a result of drug sequestration and increased volume of distribution, the extracorporeal membrane oxygenation (ECMO) procedure might lead to a decrease in drug concentrations during a patient’s treatment. The aim of this study was to evaluate sedative, antibiotic and immunosuppressive drug loss in ECMO circuit using ex-vivo and in-vitro experiments.
Methods
Blood concentrations of propofol, midazolam, cyclosporine and vancomycin were measured in an ex-vivo ECMO circuit primed with whole human blood, and compared to controls stored in polypropylene tubes. In vitro experiments were also conducted to further explore the role of temperature, oxygen exposure and polyvinylchloride surfaces on propofol loss in the ECMO circuit.
Results
Propofol concentration decreased rapidly; 70% of its baseline concentration was lost after only 30 minutes, and only 11% remained after five hours (P <0.001 for the comparison with control polypropylene tube propofol concentration). Further experiments demonstrated that oxygen exposure and contact with polyvinylchloride tubing were respectively responsible for 70% and 85% of propofol loss after 45 minutes. Midazolam concentration also rapidly decreased in the ECMO circuit, with only 54% and 11% of baseline concentration being detected at 30 minutes and 24 hours respectively (P = 0.01 versus control). Alternatively, cyclosporine concentration remained stable for the five first hours, then decreased to 78% and 73% of the baseline value after 24 hours and 48 hours, (P = 0.35 versus control). Lastly, vancomycin concentration remained stable in the ECMO circuit for the 48-hour experimental protocol.
Conclusions
We observed important losses of propofol and midazolam, while cyclosporine concentration decreased slowly and moderately, and vancomycin concentration remained unchanged in the ex-vivo ECMO circuit primed with whole human blood. These data might help intensive care unit physicians planning clinical trials with a final objective to better adapt doses of these drugs while treating critically ill ECMO patients.
Introduction
Extracorporeal membrane oxygenation (ECMO) is a complex life-support technique used to rescue critically ill patients with severe respiratory or cardiac failure [1]. Modern ECMO circuits consist of polyvinyl chloride (PVC) tubing, a polymethylpentene membrane oxygenator and a centrifugal pump [2]. Patients on ECMO require multiple medications including sedatives, analgesics, antibiotics and sometimes immunosuppressive drugs [3,4]. Pharmacokinetics of drugs administered during ECMO is complex notably due to a larger volume of distribution in ECMO-treated patients and also to the adsorption of drugs on the PVC tubing and/or the membrane oxygenator leading to an increase in drug clearance [2,5]. The degree of drug uptake by the circuit depends on the physicochemical characteristics of the drugs [6]. For example, compounds with a high octanol/water partition (log P) will be very soluble in organic materials and may exhibit considerable loss in the ECMO circuit [6]. Conversely, the pharmacokinetics of hydrophilic drugs have been reported to be unaffected by the ECMO procedure [7,8]. Drug adsorption on the circuit may also depend on circuit duration of use, since binding sites may become saturated after a few hours of operation [3,9].
To date, very limited data on the pharmacokinetics of drugs in patients supported by ECMO are available. The objective of the present study was, therefore, to determine, in an ex vivo ECMO circuit primed with human whole blood, changes in the concentrations of propofol, midazolam, vancomycin and cyclosporine, which are frequently prescribed to ECMO patients.
Methods
Drug sequestration in an ex vivo ECMO circuit primed with human whole blood
This study was an experimental study and did not include any patients. Thus, it needed no ethical approval and no patient consent was needed. Whole ECMO circuits (Maquet®, Orleans, France) comprising a Quadrox® membrane oxygenator, a centrifugal pump, a heat exchanger and PVC tubing were used for ex vivo tests. All components of the circuit were treated with heparin (Bioline coating®, Maquet). Drug-free human whole blood (800 mL) was used to prime the circuit. In order to replicate the in vivo operating conditions, the temperature of the circulating blood was set at 37°C and circuit flow rate at 4.5 L/minute. Propofol (Diprivan®10 mg/mL, Fresenius, France), midazolam (Hypnovel 1 mg/mL, Roche, France), vancomycin (Vancomycine® 50 mg/mL, Sandoz, France), and cyclosporine (Sandimmun® 50 mg/mL, Novartis, France) were introduced into the circuit to achieve final concentrations of 2 μg/mL, 30 μg/mL, 500 ng/mL and 1.2 μg/mL, respectively. Octanol/water partition coefficients of the drugs are provided in Table 1. A high partition coefficient is associated with a high solubility in organic materials. Human blood was provided by the Etablissement Français du Sang (EFS) (Rungis, France).
Table 1.
Octanol/water partition coefficients of propofol, midazolam, cyclosporine and vancomycin
| Drug | Partition coefficient (log P) |
|---|---|
| Vancomycin | - 3.1 |
| Cyclosporine | 2.9 |
| Midazolam | 3.9 |
| Propofol | 4.0 |
During ex vivo ECMO runs, serial post-membrane blood samples were obtained at 30 minutes, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 24 hours and 48 hours after drug introduction into the circuit. Until analysis, samples were kept at 4°C and plasma separated and frozen at −20°C. To determine spontaneous drug degradation, blood containing identical concentrations of the drugs studied was kept in polypropylene tubes under agitation at 25°C for the same time before measurements.
Propofol and midazolam concentrations were measured using high performance liquid chromatography with an ultra-violet detector (Waters, Milford, MA, USA). Vancomycin and cyclosporine concentrations were measured using an immuno-enzymatic method on a Dimension® system (Siemens, Munich, Germany).
The operating conditions (oxygen flow, blood flow, circuit temperature) remained stable during the 48 hours of each experiment which were repeated three times with three different ECMO circuits.
Impact of temperature, oxygen and polyvinylchloride surfaces on propofol concentrations in vitro
To further explore the role of temperature, oxygen exposure and PVC surfaces on propofol loss in the ECMO circuit, the following experiments were conducted. A polypropylene tube containing human whole blood was spiked with the propofol stock solution to obtain a concentration of 2 μg/L. The effect of the temperature, oxygen exposure and contact with PVC surfaces were assessed by incubating the tube at 37°C, applying oxygen (1 L/minute) for 10 seconds to the preparation and by distributing the preparation into 5-cm PVC tubes, respectively. Polypropylene tubes containing blood and propofol at the same concentration were stored at room temperature and were used as controls. Propofol concentrations were measured at 0 minutes, 5 minutes, 10 minutes, 15 minutes, 20 minutes, 25 minutes, 30 minutes and 45 minutes from each preparation. These experiments were conducted in triplicate.
Statistical analyses
Changes of mean drug concentrations over time were compared between control and experimental conditions by modeling using a linear mixed effect model. This model accounts for the repeated responses from the same experiments by using a random effect on intercept solely (propofol and vancomycin) and using a random effects on intercept and time (midazolam and cyclosporine). Model choice was guided by the lowest Akaike criteria value. The mixed effect model was then fitted with R software version 3.0.2 and the ‘nlme’ library [10].
Results
Changes in drug concentrations in ex vivo circuits primed with whole human blood
Propofol, midazolam, cyclosporine and vancomycin concentrations remained stable over time in control polypropylene tubes. Alternatively, propofol concentration decreased rapidly in the ECMO circuit, 70% of its baseline concentration being lost only 30 minutes after introduction of the drug into the circuit (Figure 1). After 5 hours, only 11% of the initial propofol concentration remained and the fraction remaining beyond 24 hours was negligible. Differences between experiment and control polypropylene tube propofol concentrations were statistically significant (P = 0.0006). Midazolam concentration also decreased rapidly in ECMO circuits (Figure 2), with only 54% and 11% of baseline concentrations being detected at 30 minutes and 24 hours, respectively (P = 0.01 versus polypropylene control). The cyclosporine concentration remained stable in the circuit for the first 5 hours, then decreased to 78% and 73% of the baseline value after 24 hours and 48 hours, respectively (P = 0.35 versus polypropylene control) (Figure 3). In contrast, vancomycin concentration remained stable in the ECMO circuit during the 48 hours of the experimental protocol (P = 0.86) (Figure 4).
Figure 1.
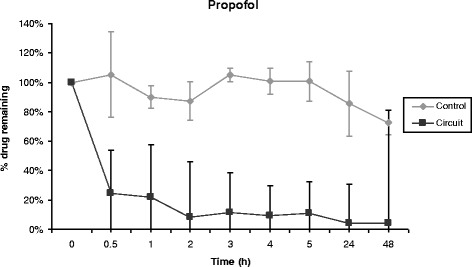
Percentage of propofol remaining in the ECMO circuit according to time (mean ± standard error of the mean; n = 3). ECMO, extracorporeal membrane oxygenation.
Figure 2.
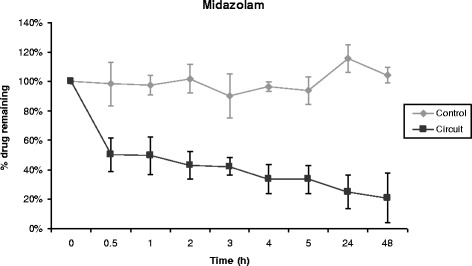
Percentage of midazolam remaining in the ECMO circuit according to time (mean ± standard error of the mean; n = 3). ECMO, extracorporeal membrane oxygenation.
Figure 3.
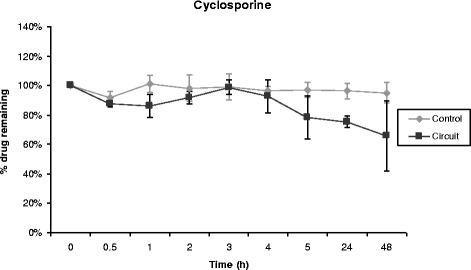
Percentage of cyclosporine remaining in the ECMO circuit according to time (mean ± standard error of the mean; n = 3). ECMO, extracorporeal membrane oxygenation.
Figure 4.
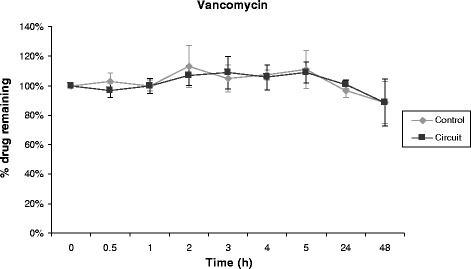
Percentage of vancomycin remaining in the ECMO circuit according to time (mean ± standard error of the mean; n = 3). ECMO, extracorporeal membrane oxygenation.
Impact of temperature, oxygen and PVC surfaces on propofol concentration in vitro
Oxygen exposure and contact with PVC tubing resulted in 70% and 85% loss of propofol after 45 minutes, respectively. Alternatively, heating the polypropylene tube to 37°C had no effect on propofol concentration (Figure 5).
Figure 5.
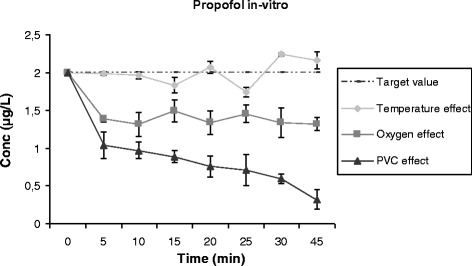
Effect of temperature, oxygen and PVC contact on propofol concentrations (mean ± standard error of the mean; n = 3). PVC, polyvinyl chloride.
Discussion
This study showed important losses of propofol and midazolam in ex vivo ECMO circuits primed with whole human blood, while cyclosporine concentration decreased slowly and moderately and vancomycin concentration remained unchanged. Furthermore, we demonstrated that oxygen exposure and contact with PVC tubing were major factors leading to propofol degradation in ECMO circuits.
Patients on ECMO frequently receive many drugs, such as sedatives and analgesics, antibiotics or immunosuppressants. Inadequate blood concentration of these drugs might lead to treatment failure or toxicity, underlining the need for better knowledge of the complex pharmacokinetics and pharmacodynamics in this particular setting [11,12]. Indeed, drug concentrations in critically ill ECMO patients might be altered by an increase in drug volume of distribution or alteration in drug elimination. Drugs might also be adsorbed in the ECMO circuit, with greater loss being reported for more lipophilic and higher octanol/water partition coefficient drugs (Table 1) [6,13-16].
Consistent with previous reports [1,17], we observed a rapid and major decrease in propofol concentration in our experiments. Although its lipophilic properties might be associated with substantial loss due to adsorption on circuit components, our results suggested that oxidation might also be an important determinant of propofol degradation. Indeed, propofol is a reducing agent which can be oxidized in the presence of an electron acceptor [18].
The decrease in midazolam concentration was rapid and only 25% of the initial concentration remained in the circuit after 24 hours. This observation is also consistent with previously reported in vitro and ex vivo data on midazolam concentration evolution in ECMO circuits, with the decrease reaching 68% to 87% after 24 hours [1,6,19].
Inadequate concentrations of antibacterial agents can lead to treatment failure that might compromise the outcomes of critically ill patients. Vancomycin, which is the drug of choice for treating beta-lactam resistant Gram-positive bacteria, is frequently prescribed to ECMO patients. Data on vancomycin concentrations reported in ECMO are controversial. In agreement with our observation, Mehta et al. [20] and Shekar et al. [19], using ex vivo models, found that vancomycin levels remained unchanged in the circuit for 24 hours. Conversely, Dagan et al. observed that vancomycin concentrations decreased by 36% when the drug was introduced in a new circuit while it decreased by only 11% when introduced in a circuit which had been running for five days [3]. Saturation of adsorption sites was suggested by these authors [3]. Similarly, Wildschut et al. also reported a decrease in vancomycin concentration (up to 46%) in a study conducted with neonatal and pediatric circuits [6]. These observations suggest that more data are needed to better determine changes in drug concentrations of vancomycin in an ECMO circuit.
Immunosuppressive agents, such as cyclosporine, are defined by a narrow therapeutic window and low serum concentrations of these drugs might lead to acute graft rejection. Although a previous study suggested in vitro adsorption studies of cyclosporine on PVC infusion sets significantly higher than that in those made of polyethylene or polybutadiene [21], this study is the first to evaluate cyclosporine pharmacokinetics in ECMO patients. Our results suggested that cyclosporine concentration decreases slowly in the ECMO circuit with 66% of the initial concentration remaining in the circuit after 48 hours. This decrease might be attributed in part to adsorption on the ECMO circuit component, given its high partition coefficient (log P = 2.92).
Conclusions
We reported herein on propofol, midazolam, vancomycin and cyclosporine concentration profiles in ex vivo whole-blood primed ECMO circuits. Our observations showed important losses of propofol and midazolam, while cyclosporine concentration decreased slowly and moderately and vancomycin concentration remained unchanged. These data might help intensive care unit physicians better adapt doses of these drugs while treating critically ill ECMO patients. Further studies should focus on the in vivo pharmacokinetics of drugs during ECMO.
Key messages
This paper confirmed important loss of lipophilic drugs within ECMO circuits.
It also highlighted the role of oxidation as an influencing factor of ex vivo stability of reducing drugs.
Drug properties should be taken into account for therapeutic strategy in ECMO patients.
Acknowledgments
Maquet Getinge group graciously provided the ECMO circuits for this study.
Abbreviations
- ECMO
Extracorporeal membrane oxygenation
- EFS
Etablissement Français du Sang
- PVC
Polyvinyl chloride
Footnotes
Competing interests
Pr Combes has received honoraria for lectures from Maquet. The other authors declare that they have no competing interests.
Authors’ contributions
FL conducted experiments and drafted and revised the manuscript; NH conducted experiments and assayed drugs; PL conceived the study and gave final approval to the manuscript; GB assayed drugs; EC ensured the functionality of the ECMO circuits; PF and CL conducted the statistical analysis; CEL conceived of the study and ensured the functionality of the ECMO circuits; RF, CF and AC conceived of the study and revised and gave final approval to the manuscript. All authors read and approved the final manuscript.
Contributor Information
Florian Lemaitre, Email: florian.lemaitre@chu-rennes.fr.
Nesrine Hasni, Email: nesrinehasni@yahoo.fr.
Pascal Leprince, Email: pascal.leprince@psl.aphp.fr.
Emmanuel Corvol, Email: emmanuel.corvol@psl.aphp.fr.
Ghassen Belhabib, Email: belhabib.ghassen@yahoo.fr.
Pierre Fillâtre, Email: pierre.fillatre@chu-rennes.fr.
Charles-Edouard Luyt, Email: charles-edouard.luyt@psl.aphp.fr.
Cyril Leven, Email: cyril.leven@gmail.com.
Robert Farinotti, Email: robert.farinotti@u-psud.fr.
Christine Fernandez, Email: christine.fernandez@u-psud.fr.
Alain Combes, Email: alain.combes@psl.aphp.fr.
References
- 1.Mulla H, Lawson G, von Anrep C, Burke MD, Upton DU, Firmin RK, et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion. 2000;15:21–6. doi: 10.1177/026765910001500104. [DOI] [PubMed] [Google Scholar]
- 2.Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet. 2003;42:403–17. doi: 10.2165/00003088-200342050-00001. [DOI] [PubMed] [Google Scholar]
- 3.Dagan O, Klein J, Gruenwald C, Bohn D, Barker G, Koren G. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit. 1993;15:263–6. doi: 10.1097/00007691-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Leprince P, Aubert S, Bonnet N, Rama A, Léger P, Bors V, et al. Peripheral extracorporeal membrane oxygenation (ECMO) in patients with posttransplant cardiac graft failure. Transplant Proc. 2005;37:2879–80. doi: 10.1016/j.transproceed.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Shekar K, Fraser JF, Smith MT, Roberts JA. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care. 2012;27(741):e9–18. doi: 10.1016/j.jcrc.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Wildschut ED, Ahsman MJ, Allegaert K, Mathot RAA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36:2109–16. doi: 10.1007/s00134-010-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemaitre F, Luyt CE, Roullet-Renoleau F, Nieszkowska A, Zahr N, Corvol E, et al. Impact of extracorporeal membrane oxygenation and continuous venovenous hemodiafiltration on the pharmacokinetics of oseltamivir carboxylate in critically ill patients with pandemic (H1N1) influenza. Ther Drug Monit. 2012;34:171–5. doi: 10.1097/FTD.0b013e318248672c. [DOI] [PubMed] [Google Scholar]
- 8.Lemaitre F, Luyt CE, Roullet-Renoleau F, Nieszkowska A, Zahr N, Fernandez C, et al. Oseltamivir carboxylate accumulation in a patient treated by haemodiafiltration and extracorporeal membrane oxygenation. Intensive Care Med. 2010;36:1273–4. doi: 10.1007/s00134-010-1882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spriet I, Annaert P, Meersseman P, Hermans G, Meersseman W, Verbesselt R, et al. Pharmacokinetics of caspofungin and voriconazole in critically ill patients during extracorporeal membrane oxygenation. J Antimicrob Chemother. 2009;63:767–70. doi: 10.1093/jac/dkp026. [DOI] [PubMed] [Google Scholar]
- 10.The R Project for Statistical Computing. http://www.r-project.org/.
- 11.Shekar K, Roberts JA, Ghassabian S, Mullany DV, Ziegenfuss M, Smith MT, et al. Sedation during extracorporeal membrane oxygenation-why more is less. Anaesth Intensive Care. 2012;40:1067–9. [PubMed] [Google Scholar]
- 12.Shekar K, Roberts JA, Ghassabian S, Mullany DV, Wallis SC, Smith MT, et al. Altered antibiotic pharmacokinetics during extracorporeal membrane oxygenation: cause for concern? J Antimicrob Chemother. 2013;68:726–7. doi: 10.1093/jac/dks435. [DOI] [PubMed] [Google Scholar]
- 13.Drug Bank: Propofol (DB00818). www.drugbank.ca/drugs/DB00818. Accessed 1 Oct 2014.
- 14.Drug Bank: Midazolam (DB00683). www.drugbank.ca/drugs/DB00683. Accessed 1 Oct 2014.
- 15.Drug Bank: Cyclosporine (DB00091). www.drugbank.ca/drugs/DB00091. Accessed 1 Oct 2014.
- 16.Drug Bank: Vancomycin (DB00512). www.drugbank.ca/drugs/DB00512. Accessed 1 Oct 2014.
- 17.Hynynen M, Hammarén E, Rosenberg PH. Propofol sequestration within the extracorporeal circuit. Can J Anaesth J Can Anesthésie. 1994;41:583–8. doi: 10.1007/BF03009997. [DOI] [PubMed] [Google Scholar]
- 18.Farinotti R. Physicochemical interactions and mode of storage of Diprivan. Ann Françaises Anesthèsie Rèanimation. 1994;13:453–6. doi: 10.1016/S0750-7658(05)80670-7. [DOI] [PubMed] [Google Scholar]
- 19.Shekar K, Roberts JA, McDonald CI, Fisquet S, Barnett AG, Mullany DV, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care. 2012;16:R194. doi: 10.1186/cc11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta NM, Halwick DR, Dodson BL, Thompson JE, Arnold JH. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med. 2007;33:1018–24. doi: 10.1007/s00134-007-0606-2. [DOI] [PubMed] [Google Scholar]
- 21.Shibata N, Ikuno Y, Tsubakimoto Y, Hoshino N, Minouchi T, Yoshio K, et al. Adsorption and pharmacokinetics of cyclosporin A in relation to mode of infusion in bone marrow transplant patients. Bone Marrow Transplant. 2000;25:633–8. doi: 10.1038/sj.bmt.1702196. [DOI] [PubMed] [Google Scholar]


