Abstract
Background
We aimed to investigate the disruptions of functional connectivity of amygdala-based networks in adolescents with untreated generalized anxiety disorder (GAD).
Material/Methods
A total of 26 adolescents with first-episode GAD and 20 normal age-matched volunteers underwent resting-state and T1 functional magnetic resonance imaging (fMRI). We analyzed the correlation of fMRI signal fluctuation between the amygdala and other brain regions. The variation of amygdala-based functional connectivity and its correlation with anxiety severity were investigated.
Results
Decreased functional connectivity was found between the left amygdala and left dorsolateral prefrontal cortex. An increased right amygdala functional connectivity with right posterior and anterior lobes of the cerebellum, insula, superior temporal gyrus, putamen, and right amygdala were found in our study. Negative correlations between GAD scores and functional connectivity of the right amygdala with the cerebellum were also observed in the GAD adolescents.
Conclusions
Adolescents with GAD have abnormalities in brain regions associated with the emotional processing pathways.
MeSH Keywords: Adolescent, Amygdala, Anxiety Disorders
Background
Anxiety disorder is the most common mental disease in adolescents [1,2], with a high incidence of suicide [3,4]. The pathophysiological mechanisms underlying generalized anxiety disorder (GAD) are still unclear. It has been reported that GAD patients exhibited abnormal amygdala subregion-based networks. GAD, with a prevalence of 2.7–4.6%, is a common anxiety disorder in adolescence, characterized by chronic, excessive worry and fear that seem unmanageable [5]. Many studies have reported that adolescents with GAD often worried excessively about things such as future events, daily life, and work stress, with symptoms of muscular tension and sleep disorder, which seriously affect the patient’s family, school work, and social interactions [6]. Compared with other types of anxiety disorders, GAD, with less effective treatment, has a lower disease recognition rate and higher degree of differentiation [7]. Studies on the pathogenesis of GAD are relatively rare [8–10]. Currently, the emotional dysregulation model is used to describe the psychological mechanism of GAD [11]. GAD patients are more sensitive to the threat which may lead to more pain, anxiety, and discomfort since they are considered to have a lower threshold for emotional experience than healthy people.
Recently, some studies have reported on the brain functions underlying the emotional disorders in GAD patients [12,13]. Abnormal brain regions and networks associated with emotion processing were shown in GAD patients, such as anterior limbic network that included the amygdala, prefrontal cortex and anterior cingulate cortex, functional connectivity between the amygdala and ventromedial prefrontal cortex (VMPLC), ventrolateral prefrontal cortex (VLPFC), anterior insula, and anterior cingulate cortex. Researchers have found that intra-regional damage or disruption of functional connectivity in the above regions could lead to anxiety. Because the amygdala plays significant roles in emotion process, researches use the amygdala as a seed region of interest (ROI) to analyze functional connectivity of anxiety patients. As reported in many studies, abnormal functional connectivity between the amygdala and prefrontal lobe may be an important neurophysiological pathway of GAD [14–16]. However, few studies have found that abnormal functional connectivity existed between the amygdala or its sub-regions and the prefrontal lobe both under the circumstance of resting state and task state. McClure reported that GAD adolescents presented increased functional connectivity between the amygdala and VLPFC under the angry facial stimuli compared with normal controls [17]. Etkin found that adult GAD patients exhibited decreased amygdala sub-regions (including basolateral amygdala and centromedial amygdala) functional connectivity with the cingulate gyrus insula. However, there was increased functional connectivity between amygdala subregions and the frontotemporal networks responsible for executive control [18]. Another study has shown that there was a disruption in the functional connectivity of amygdala sub-regions with the prefrontal cortex, insula, and cerebellum under the circumstance of resting state in GAD adolescents [19]. Recently, an increasing number of studies have explored the relationship between abnormal functional connectivity and anxiety severity. Anxiety severity was found to be positively correlated with the functional connectivity of the centromedial amygdala with insula and superior temporal gyrus, which further indicated the difference between the functional connectivity between GAD patients and normal controls [19].
In summary, although abnormal amygdala functional connectivity with the prefrontal lobe and other brain regions has been discovered in GAD patients, there are still very few reports of this. In addition, some of the results in different studies were controversial. Most studies were focused on social anxiety of adults, and few studied children or adolescents. Therefore, in our study we aimed to study the brain functional connectivity in adolescents with GAD and to more clearly understand the regions involved in the mechanism of GAD brain dysfunction. Bilateral amygdala was defined as the ROI and then we compared the amygdala functional connectivity of GAD adolescents with normal controls, and further analyzed the correlation between abnormal functional connectivity and disease severity.
Material and Methods
Subjects
A total of 26 adolescent patients with GAD admitted for consultations at the department of the Shanghai Mental Health Center, China, from October 2010 to March 2013 were enrolled as the case group. Another control group including 20 adolescents who were matched in age, gender, and years of education with the case group was enrolled, recruited voluntarily from middle schools in Shanghai. This study was approved by the Ethics Committee of the Shanghai Mental Health Center (number: 2013-02). All participants and guardians were informed of the purpose and significance of this study, and signed a written informed consent before enrollment.
Before enrollment, patients who met the GAD diagnostic criteria of the Diagnostic and Statistical Manual (DSM-IV) were unmedicated or underwent psychotherapy. The diagnosis was made by 2 independent chief psychiatrists. No systemic medication criteria were: GAD patients did not undergo any course of treatment or take any anxiolytic and antidepressants with sufficient dosage and did not take any medication within 2 weeks before fMRI scan. Other inclusive criteria were: 13–18 years of age, right-handed, and normal intelligence. Exclusion criteria were: current or history of manic episodes, depression disorder, dysthymic disorder, obsessive-compulsive disorder, post-traumatic stress disorder, substance dependence, tic disorder, attention deficit and hyperactivity disorder, conduct disorder, psychotic disorder, anorexia nervosa, bulimia nervosa, adjustment disorder and pervasive developmental disorder, current or previous history of severe brain, and physical illness. Except for no current and/or previous history of anxiety disorder, inclusive and exclusive criteria for control group were the same as in the case group.
Research tools
Ages, height, weight, years of education, handedness, and duration were included in the demographic data questionnaire.
The Screen for Child Anxiety-Related Emotional Disorders (SCARED) was used as a self-rating anxiety scale for children and adolescents aged 9 to 18 years to evaluate the severity of anxiety symptoms, which involved 5 factors: somatization/panic, generalized anxiety, separation anxiety, social phobia, and school phobia. Each item is scored as 0–2 points: 0=without, 1=sometimes, 2=often. A higher score was associated with higher anxiety level. There was a possibility of anxiety symptoms if a total score is higher than 23 points.
Clinical Global Impression (CGI), developed by the World Health Organization (WHO), was used for psychiatric treatment and study subject. Severity of illness (SI), global improvement (GI), and efficacy index (EI) were the 3 factors in CGI. The CGI-SI is commonly used to measure the symptom severity of patients with mental disorder. It is a 7-point scale on which 1=normal, not ill at all; 2=borderline mentally ill; 3=mildly ill; 4=moderately ill; 5=markedly ill; 6=severely ill; and 7=extremely ill. This rating was measured according to the observed and reported symptoms, behaviors, and functions in the past 7 days.
The Mini International Neuro-psychiatric Interview for Children and Adolescents (MINI-Kids) is a short structured diagnostic interview developed jointly by Sheehan and Lecrubier for DSM-IV and ICD-10 psychiatric disorders. The reliability and validity of the Chinese version in children and adolescents aged 6–17 years is determined to be 0.90 for test-retest reliability, and the sensitivity and specificity range from 30.0% to 93.6% and from 66.8% to 98.8%, respectively [20].
Data acquisition
Behavioral data were collected at the outpatient department of psychological counseling for children and adolescents. Research subjects and normal controls were required to carry out the self-made general scale and SCARED scale independently. The participants were screened using the MINI-Kids scale and the CGI scale was used for evaluating disease severity by a researcher.
All MRI data were collected at the Shanghai Key Laboratory of Magnetic Resonance, East China Normal University, using a Siemens Trio 3.0 Tesla MRI scanner (Siemens, Erlangen, Germany) and 32-channel matrix coil receiver. The head of each participant was snugly fixed by using foam pads to reduce head movements and scanner noise. For data collection we performed positioning scanning, and then used T1- magnetization prepared by rapid gradient echo (MPRAGE) sequence. Scan parameters were set as follows: time of repetition=2530 ms, echo time=2.34 ms, inversion-recovery=1100 ms, slice thickness=1 mm, total 192 layers, poured angle=7°, field of view=256×224 mm, and acquisition matrix was 256×224. All subjects were required to be relaxed with their eyes closed during the resting state data collection. Then T1 served as the positioning phase, and echo planar imaging (EPI) sequence was used to collect resting state data. Scan parameters are set as follows: time of repetition=2000 ms, echo time=20 ms, flip angle=90°, field of view=224×224 mm, acquisition matrix=64×64, slice thickness=3.5 mm, interlayer spacing=0 mm, 40 layers. Interleave scanning was used at 240 time points.
Data processing and analysis
Data were analyzed by Statistical Package for Social Science 17.0 (SPSS, Inc., Chicago, IL, USA) after inputting and double checking with Epidata. Total and factor scores on self-made general scale, SCARED, and CGI scale were compared using independent sample test between the case group and control group.
The resting-state fMRI data were processed by using Data Processing Assistant for Resting-State fMRI 2.0 (DPARSFA2.0, http://restfmri.net/forum/) developed by the State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University [21]. DICOM images were converted into NIFTI format and the images at the first 10 time points were removed. Then, the slice timing correction and realignment were performed. Excessive movement was defined as parallel movement in any direction >2.5 mm, or rotary movement >2.5°. Second, the image space after realignment was normalized to the Montreal Neurological Institute (MNI) space, and the resampled voxel size was 3×3×3 mm. After that, the images were subjected to Detrend and Filter (0.01 Hz < f <0.08 Hz) to reduce low-frequency drift and filter high-frequency physiological noise. Finally, multiple linear regression analysis was performed to remove covariates, including 6 parameters of head motion, mean signal of the whole brain, cerebrospinal fluid signal, and white matter signal.
In the processing of brain image data, the bilateral amygdala served as seed ROI-based on ALL template, and the correlation coefficients were calculated between the average time series of the bilateral amygdala and time series of the other voxels of the whole brain. Fisher’s r-to-z transformation was used to convert the correlation coefficient (r) into z-score so that the correlation coefficient would be distributed approximately normally [22–25]. Finally, the results of the functional connectivity of the bilateral amygdala were analyzed in the case group and control group. Independent sample t test was used for intergroup comparison and P<0.005 and a minimum cluster size of 12-voxel (P<0.05, AlphaSim correction) were used as thresholds.
Spearman correlation analysis was performed in GAD patients to investigate the 5-factor score of SCARED, total score of SCARED, and CGI-SI.
Results
Behavioral results
The total SCARED scores, generalized anxiety factor scores, separation anxiety, social anxiety, school phobia, and somatization were significantly different in GAD patients compared with the normal control group (P<0.05). There was no difference in the gender ratio, age, education years, height, and weight between GAD patients and normal controls (Table 1).
Table 1.
Comparison of baseline data and scores on SCARED.
| Variables | GAD group | Control group | t or χ2 | P |
|---|---|---|---|---|
| Gender (male/female) | 10/16 | 9/11 | 0.01 | 0.92 |
| Age (mean ±SD χ̄±s, year) | 15.54±1.53 | 15.55±1.67 | 0.34 | 0.73 |
| Years of education (mean ±SD χ̄±s, year) | 9.33±1.63 | 9.65±1.53 | −0.61 | 0.55 |
| Height (mean ±SD χ̄±s, cm) | 162.19±7.68 | 165.50±9.04 | −1.19 | 0.24 |
| Weight (mean ±SD χ̄±s, kg) | 54.00±10.93 | 57.05±8.41 | −0.34 | 0.74 |
| Course of disease (mean ±SD χ̄±s, month) | 11.69±10.44 | NA | NA | NA |
| Right handedness | 26 (100%) | 20 (100%) | NA | NA |
| Comorbidity n (%) | 10 (38.5) | 0 | NA | NA |
| Oppositional defiant disorder | 1 (3.8) | 0 | NA | NA |
| Separation anxiety | 2 (7.7) | 0 | NA | NA |
| Social anxiety | 3 (11.5) | 0 | NA | NA |
| Panic disorder | 3 (11.5) | 0 | NA | NA |
| Agoraphobia | 3 (11.5) | 0 | NA | NA |
| Specific phobia | 3 (11.5) | 0 | NA | NA |
| CGI-SI | 5.96±0.72 | 0 | NA | NA |
| SCARED, Total score | 37.12±12.00 | 16.55±8.18 | 6.57 | 0.00 |
| Generalized anxiety factor | 10.65±3.33 | 3.90±2.88 | 7.22 | 0.00 |
| Separation anxiety factor | 6.35±3.19 | 2.90±2.17 | 4.35 | 0.00 |
| Social anxiety factor | 8.15±3.40 | 5.45±2.93 | 2.84 | 0.01 |
| School phobias factor | 3.15±2.19 | 1.10±0.97 | 4.98 | 0.00 |
| Somatization factor | 8.50±4.73 | 3.20±2.31 | 4.60 | 0.00 |
CGI-SI – Clinical Global Impression-Severity of Illness; SCARED – Screen for Child Anxiety Related Disorders.
MRI data results
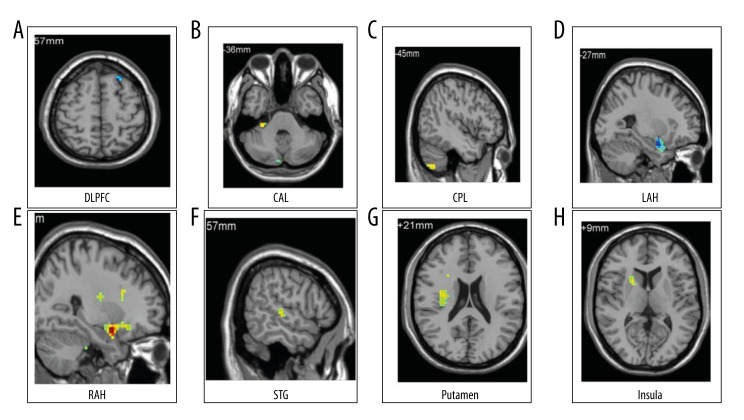
There was a decreased connectivity between the left amygdala and left DLPFC in GAD patients compared with normal controls. We found increased right amygdala functional connectivity with cerebellum, superior temporal gyrus, insula, putamen, and ipsilateral amygdala extended to the parahippocampus. A decreasing functional connectivity between right and contralateral amygdala extending to the parahippocampus was noticed in case and control groups (Table 2, Figures 1 and 2).
Table 2.
Comparison of functional connectivity of the bilateral amygdala between GAD patients and normal controls.
| Regions | BA region | MNI coordinates | Peak t-score | Number of voxels | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Left amygdala health controls >GAD | ||||||
| Dorsolateral prefrontal cortex | BA6/8 | −21 | 27 | 57 | −3.69 | 14 |
| Right amygdalaGAD>health controls | ||||||
| Cerebellum posterior lobe | NA | −45 | −63 | −51 | 4.24 | 14 |
| Cerebellum anterior lobe | NA | 33 | −30 | −36 | 4.43 | 18 |
| Amygdala/parahippocampus gyrus | NA | 27 | 0 | −15 | 7.48 | 122 |
| Putaman/caudata | NA | 21 | 9 | 9 | 4.30 | 46 |
| Insula | BA13 | 30 | −12 | 21 | 4.69 | 39 |
| Superior temporal gyrus | BA41 | 57 | −24 | 3 | 4.06 | 17 |
| Right amygdala health controls >GAD | ||||||
| Amygdala/parahippocampa gyrus | BA28/34 | −27 | 0 | −21 | −7.21 | 105 |
Figure 1.
Independent sample t test was applied for intergroup comparison, and the threshold value was set to P<0.05. The minimum number of voxels was 12 (324 mm3), the correction value was P<0.05. (A) The functional connectivity of the left amygdala in GAD patients and normal controls. (B–H) The functional connectivity of the right amygdala in GAD patients and normal controls.
Figure 2.
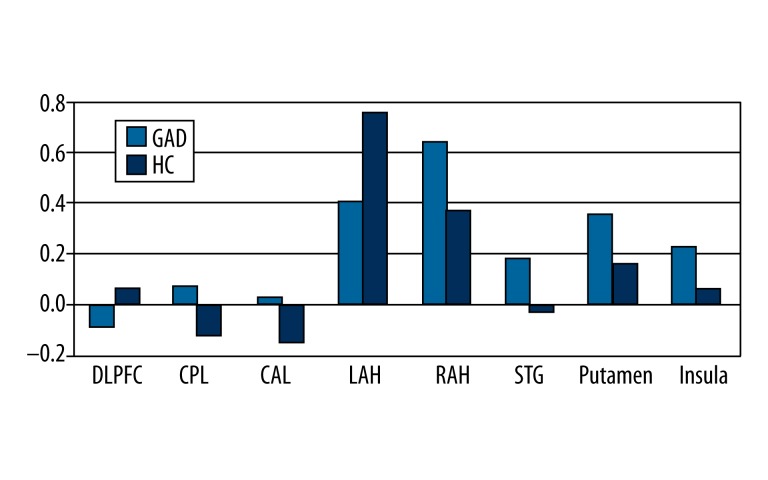
Mean intensity of the functional connectivity of the bilateral amygdala with different brain regions. Bar diagram and error bars represent the mean value and standard deviation of the functional connectivity in GAD patients and normal controls. GAD: generalized anxiety disorder, HC: health control, DLPFC: dorsolateral prefrontal gyrus, CAL: anterior cerebellum lobe, APH: right amygdala extending to parahippocampus, LPH: left amygdala extending to parahippocampus, STG: superior temporal gyrus, ACL: anterior cerebellum gyrus.
Correlation analysis of amygdala functional connectivity and GAD severity
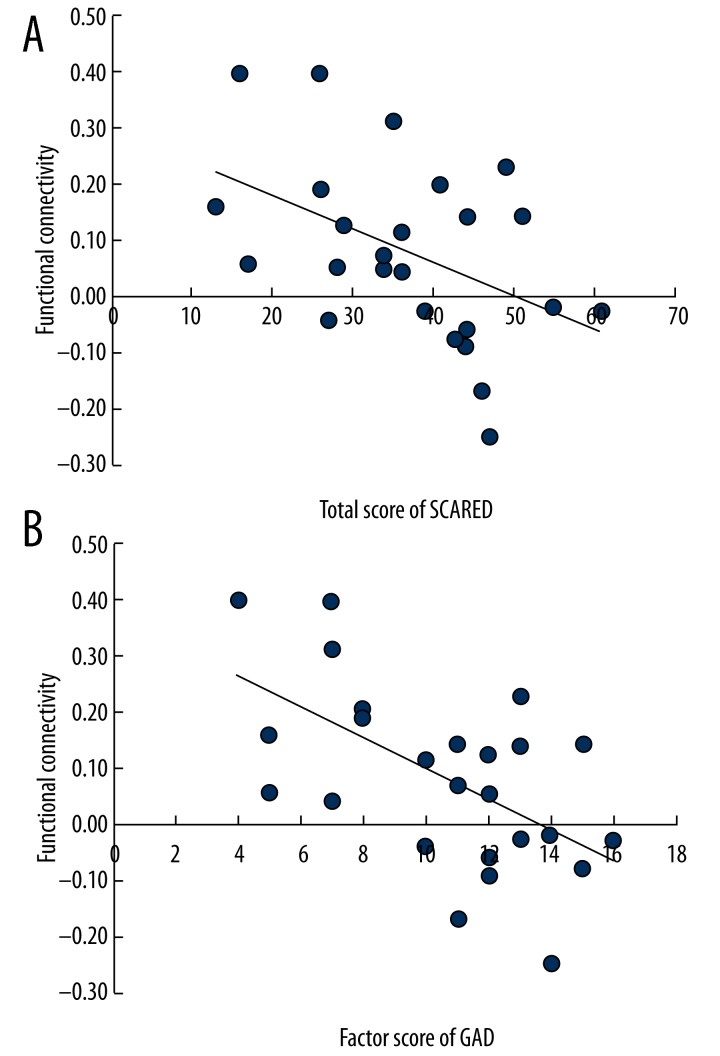
The SCARED total score (Figure 3A) and GAD factor score had a significant negative correlation with functional connectivity of the right amygdala-posterior cerebellum (rho=−0.49, P<0.05; Figure 3B). The CGI-SI score had no significant correlation with amygdala-based functional connectivity (P>0.05). We found that GAD factor score and the total score of SCARED were negatively correlated with the functional connectivity of the right amygdala and posterior cerebellum lobe.
Figure 3.
(A, B) Scores on GAD factor of SCARED and total score of SCARED were negatively correlated with the functional connectivity of the right amygdala and posterior cerebellum lobe. SCARED: Screen for Child Anxiety Related Disorders; GAD: generalized anxiety disorder. FC: functional connectivity.
Discussion
With the bilateral amygdala as ROI, we found disruptions in resting-state functional connectivity of the amygdala with cortex and subcortical regions in GAD adolescents. We found that GAD adolescents exhibited decreased amygdala functional connectivity with the DLPFC, and contralateral amygdala extending to the parahippocampus, while increased functional connectivity with the insula, cerebellum, striatum, superior temporal gyrus, ipsilateral amygdala extending to parahippocampus under the circumstance of resting state. We found that the disrupted functional connectivity of the amygdala-centered emotional processing circuit may be the neuropathological basis of adolescent GAD.
Decreased amygdala functional connectivity with the DLPFC
In our study, the decreased amygdala functional connectivity with the DLPFC were consistent with a study that found decreased amygdala functional connectivity with the DLPFC in patients with social anxiety disorder, both under the condition of fearful facial stimuli and resting state [26]. DLPFC was reported to play an important role in the process of emotional regulation and was involved in working memory, episodic memory, attention, thinking, and task execution [27]. Emotion disorders often occur in patients with prefrontal cortex damage [28]. The ability of DLPFC was weakened in adolescent patients with anxiety disorders during the processing of error messages [31]. Other studies showed insufficient function of the prefrontal cortex (DLPFC and the medial prefrontal lobe) during the process of emotional regulation in adult GAD patients [15]. There was also evidence in studies about metabolism showing the existence of DLPFC abnormalities in GAD patients [15,29–31]. It was reported that the N-acetyl aspartate/creatinine ratio in the DLPFC of adult GAD patients was 16.5% higher than that of normal adults [30]. Based on our findings, the regulatory role of the DLPFC in the amygdala may be reduced in GAD patients under the resting state, which was consistent with the psychological mechanisms of emotional dysregulation in GAD patients [32].
Etkin et al. reported in 2009 that there was an increased functional connectivity between the bilateral amygdala and DLPFC under the resting state in adult GAD patients [18], and the amygdala functional connectivity with the DLPFC was negatively correlated with anxiety severity, suggesting a compensatory network [18]. Our results were partly different from those of their studies, possibly due to several factors. Firstly, the patients in our study were much younger than that of Etkin’s study. Secondly, the whole amygdala was chosen as the ROI in our study, which was different from the amygdala sub-regions that Etkin chose as the ROI.
Increased amygdala functional connectivity with the insula
In our study, GAD adolescents exhibited an increased amygdala functional connectivity with the insula, which was similar to the results seen in some resting-state and task-state studies. Roy et al. found an increased functional connectivity of the centromedial right amygdala with the insula in GAD adolescents. In addition, the functional connectivity of the centromedial right amygdala with the insula and superior temporal gyrus displayed a significant positive correlation with severity of the disease [19]. There was an increased functional connectivity between the amygdala and the insula under the fearful facial stimuli in GAD adolescents [17]. Volker et al. discovered a positive correlation between the scores in a state of anxiety and amygdala-insula functional connectivity, which was considered to be a biological marker of anxiety [33]. The insula could receive signals of discomfort from the body, and then integrate the signals and send the discomfort information to the amygdala [34]. These discomfort feelings included itching, sense of touch, muscle tension, air hunger, stomach discomfort, and intestinal tension [35]. GAD adolescents often have discomfort symptoms, possibly because the discomfort information was transferred from the insula to the amygdala due to the increasing amygdala functional connectivity with the insula. Decreasing activity of the insula was shown following anxious words stimuli after 7 weeks of citalopram treatment, which further indicates the insula dysfunction in GAD patients [36].
Abnormal functional connectivity between the amygdala and superior temporal gyrus
In this study, our results showed an increased amygdala functional connectivity with the superior temporal gyrus found in GAD adolescents, which is consistent with Miyahara’s study [37]. In the recognition of threatening facial stimuli, a normal adult exhibits a positive correlation of the scores on subjective response to the threatening facial stimuli with functional connectivity between the left amygdala and the superior temporal gyrus, inferior temporal gyrus, and fusiform gyrus [37]. It was reported in the study of De Bellis that the sizes of temporal gyrus, white matter, and gray matter were larger in children and adolescents with GAD compared with normal controls and, the volume of the right temporal gyrus and white matter was significantly larger than that in the left [38]. The increasing volume of the right hemisphere indicated the progressive changes of the superior temporal gyrus in children and adolescents with GAD. The temporal lobe, located beneath the lateral fissure, is responsible for processing auditory information. Recent studies have found that the temporal lobe is associated with memory and emotion processing. In our study, abnormal amygdala functional connectivity with superior temporal gyrus in GAD patients was observed, and we found that the temporal lobe was primarily responsible for processing auditory information. Patients may be more sensitive to noise during the process of data collection under the circumstance of the resting state, requiring the amygdala to co-process the unpleasant emotional response, with the enhanced amygdala functional connectivity with superior temporal gyrus.
Increased amygdala functional connectivity with the striatum (putamen extending to caudata)
It has been found in our study there was an increased amygdala functional connectivity with the putamen extending to the caudate nucleus and parahippocampal gyrus. The striatum which involved the caudate nucleus, putamen and nucleus accumbens is a part of the basal ganglia of brain, a nuclear mass of gray matter buried deep in the cerebral hemispheres bilaterally. It receives afferent fibers from the centromedial region of the amygdala [14], and plays an important role in the process of reward and punishment [39,40]. Striatum can be activated by stimuli associated with reward (the anterior part), and also by failure (the posterior part) [40]. For GAD adolescents, there was an increasing activity of putamen under the stimuli associated with reward, however, those with social anxiety disorder showed no difference comparing with normal controls which indicated that the differences between GAD and social anxiety disorder responses to rewarding stimuli [41]. Normal male subjects (n=15) were found to have elevated activity of the left putamen and bilateral caudate nucleus with increasing levels of expected anxiety [42]. Patients with high-level anxiety could enhance bilateral striatal activation. It has been reported that there was an increased functional connectivity between the striatum and amygdala in patients with social anxiety disorder [43]. We found that adolescent patients with GAD exhibited increased amygdala functional connectivity with the striatum extending to the parahippocampal gyrus, and this may be due to the reason that GAD patients were conditioned to an “all or nothing” mode of thinking and may be more sensitive to reward and punishment stimuli.
Increased amygdala functional connectivity with the cerebellum
The cerebellum is linked with the cerebrum, brainstem, and spinal cord through efferent and afferent fibers. In animals, the cerebellar verm is connected with the amygdala anatomically [44]. In recent years, a growing number of studies have suggested that the cerebellum is functionally related to expressing fears and processing fear memory [45,46]. The cerebellum performs functions in language processing, cognitive processing, and emotional control [47,48]. Cerebellar cognitive affective syndrome was observed in patients with cerebellar damage [49], expressed as: executive dysfunction in planning, scene transitions, abstract reasoning and working memory, visuospatial disorders, language disorders, and behavioral and affective disorders [49]. Subjects receiving the transcranial magnetic stimulation to the cerebellum (15 minutes per day, for 3 consecutive days) can show stronger emotional response to happy facial expressions, indicating that the transcranial magnetic stimulation may damage the cerebellum effect on emotion regulation [50]. It has been reported that the left basolateral amygdala was positively correlated with the brainstem and cerebellum; however, the right superficial amygdala was negatively correlated with the cerebellum in GAD adolescents. There was no difference in the functional connectivity between the cerebellum and amygdala subregions in the normal controls and GAD patients [19]. This phenomenon is different from our findings that GAD adolescents exhibited increased amygdala functional connectivity with the cerebellum, which may be because our selection was stricter. Severe GAD patients can mobilize emotional memory from the cerebellum to alleviate the symptoms of anxiety under the resting state. Our study found that the amygdala-cerebellum functional connectivity is significantly negatively correlated with the severity of anxiety, indicating that there is a compensatory adaptation between the amygdala and cerebellum in anxiety patients.
There were some limitations in this study. There was a wide range of ages (13–18 years) in our study. Obvious differences existed in the activated levels of brain functional regions in anxiety patients in early youth and middle and late adulthood because adolescence has a rapid brain development, with a large amount of synaptic pruning [51]. In the future, we will explore the abnormal amygdala functional connectivity in GAD patients at adolescence, divided into 3 phases: 13–14 years old, 15–16 years old, and 17–18 years old. However, only patients with severe or very severe GAD based on CGI-SI scores were enrolled in our study. The abnormal amygdala functional connectivity with the DLPFC, cerebellum, temporal lobe, insula, and striatum observed in the present study may simply represent a change in brain function in patients with severe GAD. However, further study is needed to determine if patients with mild severity disease show similar results.
Conclusions
We found that there was an abnormal amygdala-based functional connectivity, particularly decreased functional connections, between the amygdala and DLPFC in accordance with the psychological model of emotional dysregulation in adolescent patients with GAD. In the resting state, increased amygdala functional connectivity with the insula, cerebellum, striatum, and temporal lobe, and the decreased amygdala functional connectivity with the DLPFC may be the pathological basis of adolescent GAD. Furthermore, the amygdala functional connectivity showed a significantly negative correlation with severity of disease in GAD adolescents, indicating that amygdala-cerebellum functional connectivity may play a compensatory role in the pathogenesis of GAD. Our study results may provide a theoretical basis for the clinical treatment of GAD.
Acknowledgments
We would like to thank Yun Qian, Yi Liu, Huiqin Tang, and Jing Chen for their help in case collection, and also give thanks to the voluntary participants and their guardians in this study.
Footnotes
Ethics statement
The study was approved by the Ethics Committee of the Shanghai Mental Health Center (number: 2013-02). All participants and guardians were informed of the study’s purpose and significance, and signed a written informed consent before enrollment. This study obtained informed consent from the relatives, caretakers, and guardians on behalf of all minors and children enrolled.
Conflict of Interest
None.
Source of support: This study was supported by the Large Instruments Open Foundation of East China Normal University and the National Technology Support Program during the Eleventh Five-year Plan (2009BAI77B05)
References
- 1.Costello EJ, Egger HL, Angold A. The developmental epidemiology of anxiety disorders: phenomenology, prevalence, and comorbidity. Child Adolesc Psychiatr Clin N Am. 2005;14(4):631–48. doi: 10.1016/j.chc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Beesdo K, Pine DS, Lieb R, Wittchen HU. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry. 2010;67(1):47–57. doi: 10.1001/archgenpsychiatry.2009.177. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson CM, Muehlenkamp JJ, Miller AL, Turner JB. Psychiatric impairment among adolescents engaging in different types of deliberate self-harm. J Clin Child Adolesc Psychol. 2008;37(2):363–75. doi: 10.1080/15374410801955771. [DOI] [PubMed] [Google Scholar]
- 4.Foley DL, Goldston DB, Costello EJ, Angold A. Proxiamal psychiatric risk factors for suicidality in youth: the Great Smoky Moutains Study. Arch Gen Psychiatry. 2006;63(9):1017–24. doi: 10.1001/archpsyc.63.9.1017. [DOI] [PubMed] [Google Scholar]
- 5.Asbahr FR. Anxiety disorders in childhood and adolescence: clinical and neurobiological aspects. J Pediatr. 2004;80(2 Suppl):28–34. doi: 10.2223/1166. [DOI] [PubMed] [Google Scholar]
- 6.Birmaher B, Yelovich AK, Renaud J. Pharmacologic treatment for children and adolescents with anxiety disorders. Pediatr Clin North Ann. 1998;45:1187–204. doi: 10.1016/s0031-3955(05)70069-9. [DOI] [PubMed] [Google Scholar]
- 7.Chavira DA, Stein MB, Bailey K, Stein MT. Child anxiety in primary care: prevalent but untreated. Depress Anxiety. 2004;20(4):155–64. doi: 10.1002/da.20039. [DOI] [PubMed] [Google Scholar]
- 8.Etkin A, Wager T. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Procesing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003;37(4):8–25. [PubMed] [Google Scholar]
- 10.Strawn JR, Adler CM, Chu W, et al. Adolescent generalized anxiety disorder: neurophysiology and neurochemistry. Annual Meeting of the American Academy of Child and Adolescent Psychiatry(AACAP); New York, NY. 2010 October; pp. 26–31. [Google Scholar]
- 11.Behar E, DiMarco ID, Hekler EB, et al. Current theoretical models of generalized anxiety disorder (GAD): Conceptual review and treatment implications. J Anxiety Disord. 2009;23:1011–23. doi: 10.1016/j.janxdis.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Strawn JR, Wehry AM, DelBello MP, et al. Establishing the neurobiologic basis of treatment in children and adolescents with generalized anxiety disorder. Depress Anxiety. 2012;29:328–39. doi: 10.1002/da.21913. [DOI] [PubMed] [Google Scholar]
- 13.Strawn JR, Bitter SM, Weber WA, et al. Neurocircuitry of generalized anxiety disorder in adolescents: a pilot functional neuroimaging and functional connectivity study. Depress Anxiety. 2012;29:939–47. doi: 10.1002/da.21961. [DOI] [PubMed] [Google Scholar]
- 14.McClure EB, Monk CS, Nelson EE, et al. Abormal attention Modulation of fear circuit functiona in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 15.Etkin A, Katherine E, Alan FS, et al. Disrupted Amygdala Subregein Functional Connectivity in Generalized Anxiety Disorder. Arch Gen Psychiatry. 2009;66(12):1361–72. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy AK, Fudge JL, Kelly C, et al. Intrinsic Functional Connectivity of Amygdala-Based Networks in Adolescent Generalized Anxiety Disorder. J Am Acad Child Adolesc Psychiatry. 2013;2(3):290–99.e2. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoehn-Saric R, Schlund MW, Wong SH. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Res. 2004;131(1):11–21. doi: 10.1016/j.pscychresns.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of MentalDisorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 19.Wang K, Su LY, Zhu Z, et al. Norms of the Screen for Child Anxiety Related Emotional Disorders in Chinese Urban Children. Chinese Journal of Clinical Psychology. 2002;10(4):270–72. [Google Scholar]
- 20.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YX, Liu J, Wang YF. Reliability and validity of Chinese version of the Mini International Neuropsychiatric Interview for Children and Adolescents (Child Version) Chinese Mental Health Journal. 2001;25(1):8–13. [Google Scholar]
- 22.Chao Gan Y, Yu Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2001;4(13):1–7. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song XW, Zhang YD, Xiang YL, et al. Rest: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS One. 2011;6(9):e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampson M, Peterson BS, Skudlarski P, et al. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15(4):247–62. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prater KE, Hosanagar A, Klumpp H, et al. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety. 2013;30(3):234–41. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balconi M. Dorsolateral prefrontal cortex, working memory and episodic memory processes: insight through transcranial magnetic stimulation techniques. Neurosci Bull. 2013;29(3):381–89. doi: 10.1007/s12264-013-1309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yiniu W, Yuejia L. Emotional Disorders in Patients with Prefrontal Cortex Lesions. 2004;12(2):161–67. [Google Scholar]
- 28.Fitzgerald KD, Liu Y, Stern ER, et al. Reduced error-related activation of dorsolateral prefrontal cortex across pediatric anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2013;52(11):1183–91. doi: 10.1016/j.jaac.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ball TM, Ramsawh HJ, Campbell-Sills L, et al. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol Med. 2013;43(7):1475–86. doi: 10.1017/S0033291712002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathew SJ, Mao X, Coplan JD, et al. Dorsolateral prefrontal cortical pathology in generalized anxiety disorder: a proton magnetic resonance spectroscopic imaging study. Am J Psychiatry. 2004;161(6):1119–21. doi: 10.1176/appi.ajp.161.6.1119. [DOI] [PubMed] [Google Scholar]
- 31.Behar E, DiMarco ID, Hekler EB, et al. Current theoretical models of generalized anxiety disorder (GAD): conceptual review and treatment implications. J Anxiety Disord. 2009;23(8):1011–23. doi: 10.1016/j.janxdis.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Pfeifer JH, Blakemore SJ. Adolescent social cognitive and affective neuroscience: past, prescent, and future. Soc Cogn Affect Neurosci. 2012;7(1):1–10. doi: 10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClure EB, Monk CS, Nelson EE, et al. Abormal attention Modulation of fear circuit functiona in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 34.Baur V, Hänggi J, Langer N, et al. Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry. 2013;73(1):85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Nieuwenhuys R. The insular cortex: a review. Prog Brain Res. 2012;195:123–63. doi: 10.1016/B978-0-444-53860-4.00007-6. [DOI] [PubMed] [Google Scholar]
- 36.Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451–63. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(45):727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seymour B, Daw N, Dayan P, et al. Differential encoding of losses and gains in the human striatum. J Neurosci. 2007;27(18):4826–31. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pohlack ST, Nees F, Ruttorf M, Jäncke L. Activation of the ventral striatum during aversive contextual conditioning in humans. Biological Psychology. 2012;91:74–80. doi: 10.1016/j.biopsycho.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Guyer AE, Choate VR, Detloff A, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry. 2012;169(2):205–12. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H, Spence JS, Devous MD, Sr, et al. Striatal-limbic activation is associated with intensity of anticipatory anxiety. Psychiatry Res. 2012;204(2–3):123–31. doi: 10.1016/j.pscychresns.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao W, Qiu CJ, Gentill C, et al. Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state fMRI study. Plos One. 2010;5(12):e15238. doi: 10.1371/journal.pone.0015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyahara M, Harada T, Ruffman T, et al. Functional connectivity between amygdala and facial regions involved in recognitionof facial threat. Soc Cogn Affect Neurosci. 2013;8(2):181–89. doi: 10.1093/scan/nsr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Bellis MD, Keshavan MS, Shifflett H, et al. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2012;51(7):553–62. doi: 10.1016/s0006-3223(01)01375-0. [DOI] [PubMed] [Google Scholar]
- 45.Supple WF, Leaton RN, Fanselow MS. Effects of cerebellar vermal lesions on species-specific fear responses, neophobia, and taste-aversion learning in rats. Psysiol Behav. 1987;39:579–86. doi: 10.1016/0031-9384(87)90156-9. [DOI] [PubMed] [Google Scholar]
- 46.Sacchetti B, Scelfo B, Strata P. The cerebellum: synaptic changes and fear conditioning. Neuroscientist. 2005;11(3):217–27. doi: 10.1177/1073858405276428. [DOI] [PubMed] [Google Scholar]
- 47.Sacchetti B, Sacco T, Strata P. Reversible inactivation of amygdala and cerebellum but not perirhinal cortex impairs reactivated fear memories. Eur J Neurosci. 2007;25(9):2875–84. doi: 10.1111/j.1460-9568.2007.05508.x. [DOI] [PubMed] [Google Scholar]
- 48.Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–92. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- 49.Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11(2):352–65. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- 50.De Smet HJ, Paquier P, Verhoeven J, Mariën P. The cerebellum: Its role in language and related cognitive and affective functions. Brain Lang. 2013;127(3):334–42. doi: 10.1016/j.bandl.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Schutter DJ, Enter D, Hoppenbrouwers SS. High-frequency repetitive transcranial magnetic stimulation to the cerebellum and implicit processing of happy facial expressions. J Psychiatry Neurosci. 2009;34(1):60–65. [PMC free article] [PubMed] [Google Scholar]