Abstract
Patient: Male, 77
Final Diagnosis: Deere strongyloidiasis
Symptoms: Abdominal pain • apetite loss • diarrhea
Medication: Prednisolon
Clinical Procedure: Upper endoscopy
Specialty: Gastroenterology and Hepatology
Objective:
Unusual clinical course
Background:
Strongyloidiasis usually presents as a chronic and limited disease, but in some immunocompromised patients it may become a life-threatening disease.
Case Report:
A 77-year-old Haitian male, with history of temporal arteritis on 40 mg of oral prednisone presented complaining of decreased oral intake, epigastric pain, and non-bloody diarrhea. He had bi-temporal wasting and a distended abdomen but without guarding or tenderness.
Laboratory examination included mild leukocytosis, anemia, negative HIV antibody, negative parasite stool exam, and negative serology for Giardia and Strongyloides. CT of the abdomen showed multiple distended loops, without obstruction.
During the admission he had a 4 g hemoglobin drop and a positive occult blood test, requiring blood transfusions, IV pantoprazole, and upper endoscopy. Findings included severe duodenitis, blunted villi, and intramucosal and luminal helminthic worms and eggs. Pathology showed Strongyloides stercoralis infection, confirmed by subsequent PCR.
He was given 1 day of 15 mg oral ivermectin, diarrhea resolved, and was discharged with a percutaneous endoscopic gastrostomy tube because of the persistent lack of appetite.
Conclusions:
Given the persistent nature of strongyloidiasis and its high susceptibility to ivermectin, it potentially would be worth consider treating high-risk patients in the appropriate clinical and epidemiological setting, irrespective of screening test results, in order to avoid false-negative result consequences.
MeSH Keywords: Duodenitis, Strongyloides stercoralis, Strongyloidiasis
Background
The current estimates of 30 to 100 million persons infected by Strongyloides stercoralis (S. stercoralis) dates back to review articles published between 1989 and 1996. However, these figures lack the more sensitive diagnostic techniques currently in use [1].
It is mostly a tropical and subtropical infection, being also endemic in some temperate areas such as the southeastern United States, and other areas of Europe and Japan [2].
It usually presents as a chronic and limited disease, but in some immunocompromised patients it may become a life-threatening disease [3]. The following case emphasizes the importance of identifying risk factors for some parasitic infections prior to starting immunosuppressive agents.
Case Report
A 77-year-old male originally from Haiti but in the United States for the last 10 years and with history of temporal arteritis on 40 mg of oral prednisone for the last 4 months presented to the emergency room complaining of 3 months of decreased oral intake, intermittent epigastric pain, and non-bloody diarrhea.
On examination, vital signs were within normal limits but he appeared fatigued. Head exam revealed bi-temporal wasting and his abdomen was distended, with sluggish bowel sounds but without guarding or tenderness.
Laboratory examination was significant for elevated white count at 12 300 mm3, decreased hemoglobin at 10.3 g/dL with MCV 92 fl, and eosinophils within normal limits. Iron and TIBC were within normal limits and albumin was decreased at 2.9 g/dL. Baermann test for stool examination was repeated 3 times in order to increase the yield of the test and all of them came back negative for larva or ova. Blood and urine cultures were negative. HIV test came back negative. CT of the abdomen showed multiple distended loops and enzyme-linked immunosorbent assay for immunoglobulin M and G antibodies against Giardia and Strongyloides came back negative as well.
The patient was admitted and started on intravenous fluids, underwent nasogastric tube placement, and oral intake was withheld. He improved clinically but by the third day of admission, had a hemoglobin drop of 4 g and positive occult blood test, requiring 2 packs of red blood cells and IV pantoprazole. When hemodynamically stable, he underwent an upper endoscopy which demonstrated severely inflamed duodenal mucosa, with focal superficial mucosal erosions, blunted and atrophic villi, and possible intramucosal and luminal helminthic worms and eggs present (Figure 1). Further gastrointestinal pathology evaluation demonstrated S. stercoralis infection (Figure 2). Subsequent PCR study of the tissue confirmed the diagnosis.
Figure 1.
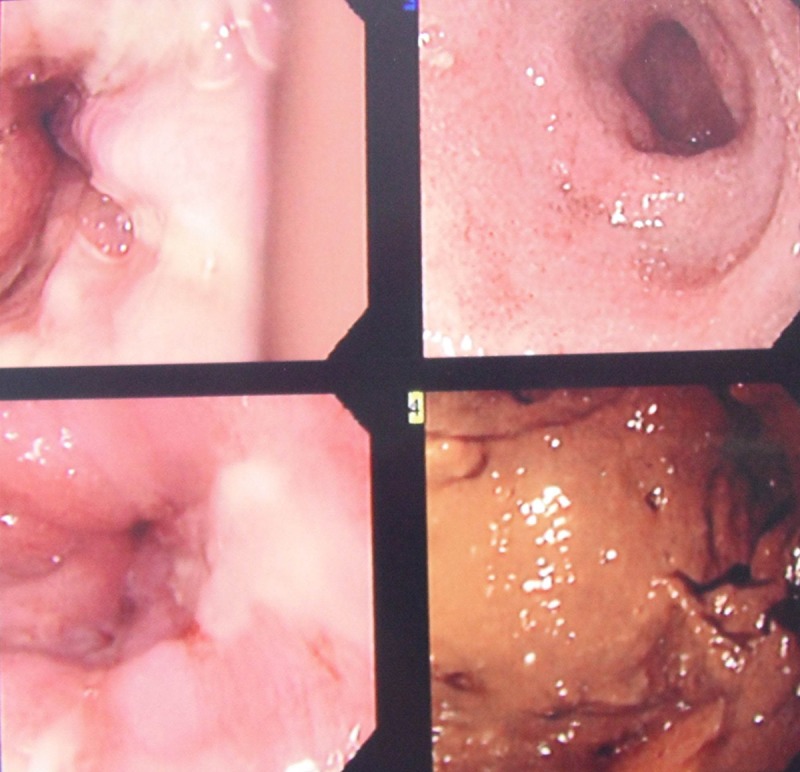
Upper endoscopy showing severe duodenitis.
Figure 2.
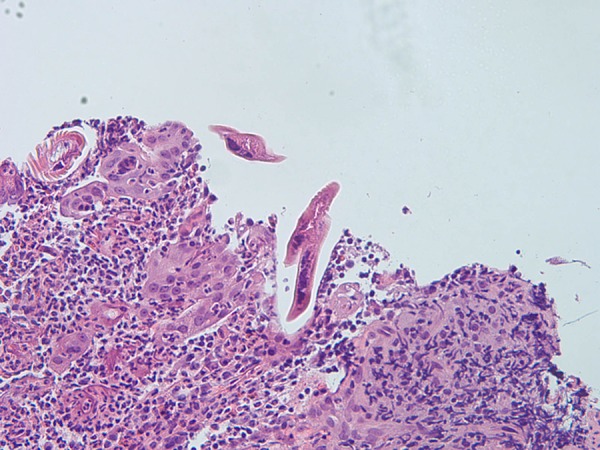
Histology showing the presence of S. stercoralis.
He was given 1 day of ivermectin 15 mg and was started on sucralfate. Diarrhea resolved but the poor oral intake continued. Appetite stimulants were tried without success and ultimately the patient elected percutaneous endoscopic gastrostomy tube placement, which he used until his discharge. The patient was told to follow up at the clinic but he did not return.
Discussion
S. stercoralis possesses the ability to complete its life cycle entirely within the human host and/or the soil. This feature may lead to increased number of organisms in an infected host even without reinfection and potentially may persist indefinitely. Patients are at increased risk of extreme intestinal parasite replication, leading to opportunistic behavior of the organism, resulting in severe and complicated gastrointestinal manifestations and potential dissemination to various anatomical locations [4,5].
When the parasite burden remains balanced, chronic infections may remain asymptomatic and eosinophilia can be present in about 50% to 80% of patients with mild infection. In immuno-compromised states such as with the use of corticosteroids, cytotoxic agents, tumor factor inhibitors, or the presence of hematological malignancies, as well as the coinfection with HTLV1, severe symptomatic strongyloidiasis can occur. The exact mechanism that leads to this severity is not known, but events that inhibit Th2-directed immune responses can release eosinophil-mediated control of the parasites [6–8].
Bush et al. [6] described a paradoxical effect of being infected with the human immunodeficiency virus (HIV) or having the acquired immunodeficiency syndrome (AIDS), as it does not seem to be an independent risk factor for aggressive Strongyloides infection. At the same time, although strongyloidiasis was once considered an AIDS-defining illness, there is no evidence that a low CD4 will increase the risk of dissemination or decrease the chance of clearing an infection [9,10]. However, recent series and case reports show the opposite – that HIV is an actual risk factor for strongyloidiasis in immunocompromised patients [11–13].
Routine single-stool ova and parasite testing has been reported to have a rather low diagnostic yield and thus repeat sample studies might be needed to improve the sensitivity of the test.
Enzyme-linked immunosorbent assay for the detection of circulating anti-Strongyloides serum antibodies has a reported sensitivity of up to 95%, but can be lower in immunocompromised patients [14,15]. A systematic review of case reports found 2 cases of negative serology: a HIV-infected patient with larvae in stool and sputum and a patient with dermatomyositis on chronic prednisone and methotrexate [16]. In addition, single serologic tests do not reliably distinguish past from currently active infections. Thus, examination of fecal samples should follow a positive serologic test [17].
Endoscopic evaluation of gastrointestinal symptoms in an immunocompromised patient is another alternative in the diagnostic workup, especially if the results of prior studies have been negative or inconclusive. Upper endoscopic findings are not pathognomonic and include hemorrhagic stigmata, ulcers, erythema, swollen folds, stenosis, ulcer, or even normal mucosa [18]. For our patient, we performed endoscopy because of the sudden drop in hemoglobin and the positive result of the occult blood test.
Regarding the treatment, our patient was given 1 dose of ivermectin and clinically improved. However, according to recent reviews such as the one by Mejia et al. [9], patients with uncomplicated Strongyloides infection should receive ivermectin for 2 days and in cases of disseminated infections immunosuppressive therapy should be reduced and they should extend the treatment, usually until negative stool exam results persist for 2 weeks [17].
It has been argued that immunocompromised populations should be screened before starting immunosuppressive drugs, as they can develop potentially fatal presentations such as hyperinfection and disseminated strongyloidiasis [5]. Patients from Strongyloides endemic areas taking these medications should also be considered as at high risk for these severe presentations of strongyloidiasis and require a broad and thorough screening, particularly if prevention of life-threatening dissemination is the goal [9].
Conclusions
Given the persistent nature of S. stercoralis and its high susceptibility to ivermectin, it potentially would be worth consider treating high-risk patients in the appropriate clinical and epidemiological setting, irrespective of screening test results, in order to avoid false-negative result consequences [15,19,20].
Footnotes
Statement
No financial support was needed in order to prepare this case report.
References:
- 1.Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl trop Dis. 2013;7(5):e2214. doi: 10.1371/journal.pntd.0002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis – the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967–72. doi: 10.1016/j.trstmh.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Altintop L, Cakar B, Hokelek M, et al. Strongyloides stercoralis hyperinfection in a patient with rheumatoid arthritisand bronchial asthma: a case report. Ann Clin Microbiol Antimicrob. 2010;9:27. doi: 10.1186/1476-0711-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grove DI. Human strongyloidiasis. Adv Parasitol. 1996;38:251–309. doi: 10.1016/s0065-308x(08)60036-6. [DOI] [PubMed] [Google Scholar]
- 5.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–17. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush LM, De Almeida KNF, Perez MT. Severe strongyloidiasis associated with subclinical Human T-cell leukemia/lymphoma virus-1 infection. Infect Dis Clin Pract. 2009;17:84–89. [Google Scholar]
- 7.Ross AG, Olds GR, Cripps AW, et al. Enteropathogens and chronic illness in returning travelers. N Engl J Med. 2013;368(19):1817–25. doi: 10.1056/NEJMra1207777. [DOI] [PubMed] [Google Scholar]
- 8.Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5:345–55. doi: 10.1128/cmr.5.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mejia R, Nutman TB. Screening, prevention, and treatment for hyperinfection síndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis. 2012;25(4):458–63. doi: 10.1097/QCO.0b013e3283551dbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walson JL, Stewart BT, Sangare L, et al. Prevalence and correlates of helminth co-infection in Kenyan HIV-1 infected adults. PLoS Negl Trop Dis. 2010;4:e644. doi: 10.1371/journal.pntd.0000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonwutiwes U, Waywa D, Sjilpasakorn S, et al. Prevalence and risk factors of acquiring Strongloides stercolaris infection among patients attending a tertiary hospital in Thailand. Pathog Glob Health. 2014;108(3):137–40. doi: 10.1179/2047773214Y.0000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollela VR, Feliciano C, Teixeira AC, et al. Fulminant gastrointestinal hemorrhage due to Strongyloides stercoralis hyperinfection in an AIDS patient. Rev Soc Bras Med Trop. 2013;46(1):111–13. doi: 10.1590/0037-868215522013. [DOI] [PubMed] [Google Scholar]
- 13.Ursini T, Polilli E, Fazii P, et al. Late diagnosis of central nervous system involvement associated with lethal dissemination of Strongyloides stercoralis in an advanced HIV patient from Nigeria. Int J Infect Dis. 2013;17(4):e280–82. doi: 10.1016/j.ijid.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Van Doorn HR, Koelewijn R, Hofwegen H, et al. Use of enzyme-linked immunosorbent assay and dipstick assay for detection of Strongyloides stercoralis infection in humans. J Clin Microbiol. 2007;45:438–42. doi: 10.1128/JCM.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loutfy MR, Wilson M, Keystone JS, et al. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg. 2002;66(6):749–52. doi: 10.4269/ajtmh.2002.66.749. [DOI] [PubMed] [Google Scholar]
- 16.Buonfrate D, Ruqeuna-Mendez A, Angheben A, et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis. 2013;13:78. doi: 10.1186/1471-2334-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcos LA, Terashima A, Dupont HL, et al. Strongyloides hyperinfection síndrome: en emerging global infectious disease. Trans R Soc Trop Med Hyg. 2008;102(4):314–18. doi: 10.1016/j.trstmh.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto K, Hokama A, Hirata T, et al. J.Endoscopic and histopathological study on the duodenum of Strongyloides stercoralis hyperinfection. World Gastroenterol. 2008;14(11):1768–73. doi: 10.3748/wjg.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam CS, Tong MK, Chan KM, et al. Disseminated strongyloidiasis: a retrospective study of clinical course and outcome. Eur J Clin Microbiol Infect Dis. 2006;25(1):14–18. doi: 10.1007/s10096-005-0070-2. [DOI] [PubMed] [Google Scholar]
- 20.Klein RA, Cleri DJ, Doshi V, et al. Disseminated Strongyloides stercoralis: A fatal case eluding diagnosis. South Med J. 1983;76(11):1438–40. doi: 10.1097/00007611-198311000-00030. [DOI] [PubMed] [Google Scholar]


