Abstract
Background
Inhibition of CC chemokine ligand 20 (CCL20), which is expressed by human keratinocytes after proinflammatory cytokine stimulation, may reduce migration of recipient Langerhans cells into tissue-engineered allogeneic skin grafts and minimize immune rejection by the recipient. Here, we screened CCL20 gene knockout clones in the human immortalized skin keratinocyte line HaCaT and tested multiple transfection methods for optimal efficiency.
Material/Methods
The CCL20 gene was PCR amplified from HaCaT genomic DNA. Both the short arm (1,969 bp) and long arm (2,356 bp) of human CCL20 were cloned into ploxP-targeting vectors at either side of the neomycin resistance cassette, respectively. The resulting ploxP-hCCL20-targeting vector was linearized and electroporated into HaCaT. The positive HaCaT clones were screened under the pressure of both G418 and GANC, and identified by PCR and Southern blot. The ploxP-hCCL20-EGFP fluorescent expression vector was also constructed and transfected into 293FT and HaCaT cells by jetPEI liposome and nucleofection electroporation for evaluating the transfect efficiency under fluorescent microscope.
Results
The replacement targeting vector ploxP-hCCL20 (11.9 kb) for exon 2 of the human CCL20 gene was successfully constructed and transfected into HaCaT cells. The selected HaCaT clones did not show any evidence of CCL20 gene knockout by either PCR or Southern blot analysis. We also successfully constructed a fluorescent expression vector ploxP-hCCL20-EGFP (13.3 kb) to assess possible reasons for gene-targeting failure. Transfection efficiencies of ploxP-hCCL20-EGFP into 293FT and HaCaT cell lines by jetPEI liposome were 75.1±3.4% and 1.3±0.2%, respectively. The transfection efficiency of ploxP-hCCL20-EGFP into HaCaT cells using nucleofection electroporation was 0.3±0.1% (P=0.000), but the positive control vector pmaxGFP (3,490 bp) using the same method was 38.3±2.8%.
Conclusions
Overall low transfection efficiencies of ploxP-hCCL20-EGFP into HaCaT cells, regardless of transfection method, may either be due to the high molecular weight of the vector or to the fact that this particular cell line may be inherently difficult to transfect.
MeSH Keywords: Chemokine CCL20, Gene Targeting, Keratinocytes, Transfection
Background
CC chemokine ligand 20 (CCL20), also known as liver and activation-regulated chemokine (LARC), macrophage inflammatory protein-3α (MIP-3α), and Exodus-1, is the only chemokine known to interact with CC chemokine receptor 6 (CCR6), a property shared with the antimicrobial β-defensins [1]. The human CCL20 gene maps to chromosome 2q35–37 and includes 4 exons and 3 introns with a total length of 4,601 bp. The full-length CCL20 cDNA, corresponding to the exon-encoded human mRNA, is approximately 0.8 kilobases (0.8 kb) and contains both classical (AATAAA) and alternative (AATAAG) polyadenylation signals in the 3′-untranslated region [1]. The ligand-receptor CCL20-CCR6 pair is responsible for chemoattraction among immature dendritic cells (DC), effector/memory T-cells, and B-cells. Additionally, this ligand-receptor pair helps regulate both skin and mucosal surfaces under homeostatic and inflammatory conditions, as well as the pathophysiology of several diseases, including skin rejection, cancer, and rheumatoid arthritis [2–4].
In the skin, CCL20 is primarily produced by active keratinocytes where it serves as a potent chemotactic factor that induces the migration of Langerhans cell precursors, which are derived from CD34+ hematopoietic progenitors, toward the epidermis [5]. Inhibition of CCL20 in keratinocytes can potentially reduce migration of recipient Langerhans cells into allogeneic skin grafts, impair the indirect antigen-presenting pathway, and relieve graft rejection by recipients.
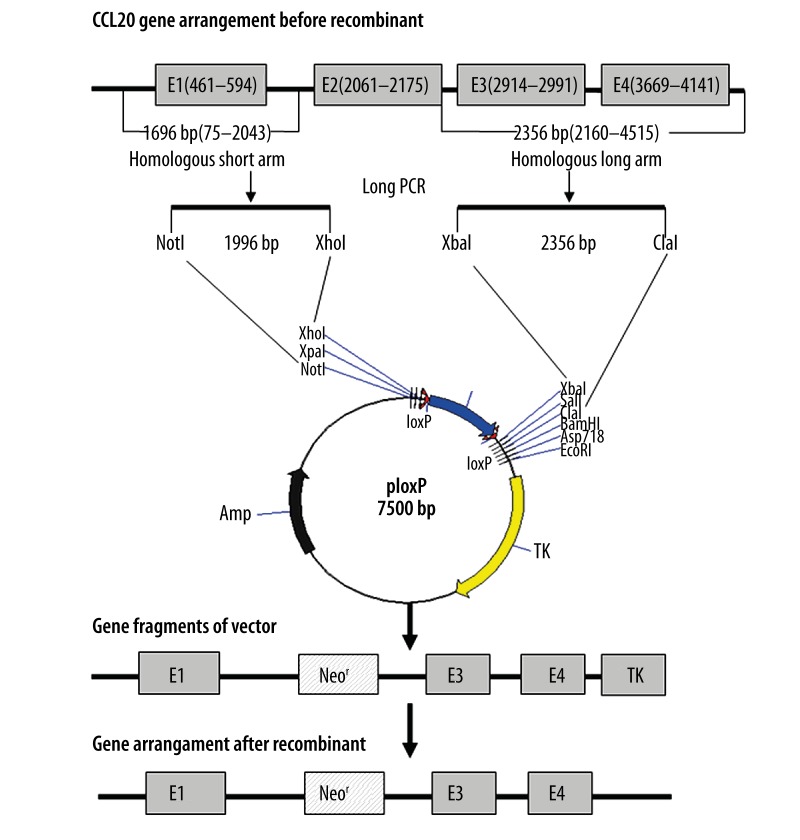
Gene targeting, defined as the introduction of site-specific modifications into the genome by homologous recombination, has revolutionized the field of mouse genetics by allowing analysis of multiple aspects of gene function in vivo. It is now possible to engineer multiple genetic alterations, ranging from point mutations to chromosomal rearrangements. More recently, tissue-specific, inducible gene targeting with temporal-spatial control has even become feasible [6–8]. The purpose of the work we present here was to achieve successful gene targeting of the CCL20 gene into the ploxP vector. First, we inserted exon 1 and its adjacent intron sequences (homologous short arm) of the CCL20 gene into the region between the NotI and XhoI sites of the ploxP vector. Next, we inserted exon 3 and exon 4 and their adjacent introns (homologous long arm) into the region between the XbaI and ClaI sites to construct the targeting vector ploxP-hCCL20, directed against the second exon. The ploxP-hCCL20 construct was transfected into HaCaT cells by electroporation and positive clones were identified via selection with G418 and GANC. Resistant clones were identified by both PCR and Southern blot analyses. We also generated the ploxP-hCCL20-EGFP fluorescent expression vector and transfected this construct into multiple cell lines using various methods to optimize transfection efficiency. The methods and data we describe here provide insight into gene-targeting and transfection strategies when dealing with epidermal cells that may be difficult to transfect. Our construction strategy and gene-targeting approach is illustrated in Figure 1.
Figure 1.
Strategy for construction of the replacement gene-targeting vector. E1, E2, E3, and E4 represent exon 1, exon 2, exon 3, and exon 4 of the CCL20 gene.
Material and Methods
Vectors and cell lines
The ploxP empty vector was kindly provided by Dr. Zhang Wei of the Third Military Medical University. It measures 7,500 bp in length, and was used as the vector for gene targeting in this study. In the vector, there are 9 single restriction sites – NotI, HpaI, XhoI, XbaI, SalI, ClaI, BamHI, Asp718, and EcoRI. The vector includes both ampicillin and neomycin resistance genes, along with a HSV-TK gene and loxP sites. The human immortalized skin keratinocyte line HaCaT and 293FT cells were both preserved by the Institute of Burn Research.
Construction of ploxP-hCCL20-targeting vector
The full-length sequence of the CCL20 gene was obtained through GenBank (NT_005403). CCL20 gene primer sequences were designed using Primer Premier 5.0 software and are provided in Table 1. HaCaT genome DNA was extracted and used as PCR template. Long-distance PCR was used to amplify the human CCL20 gene (4,459bp) using the ExTaq PCR protocol (TaKaRa Biology Company, Dalian, China).
Table 1.
Primer sequences and PCR reaction conditions.
| Gene | Sequence (5′-3′) | Product size (bp) | Annealing (°C) | Cycles |
|---|---|---|---|---|
| CCL20 | Sense: agggtgtaacaataggagttctgg | 4,459 | 55 | 30 |
| Anti-sense: gatggaagaagtgatgcctgaa | ||||
| Short arm | Sense: ataagaatgcggccgctgtaacaataggag Not I |
1,969 | 57 | 30 |
| Anti-sense: agctaccgctcgagtgaaaggtattgga Xho I | ||||
| Long arm | Sense: ctcgctctagaacatcaatgctatc Xba I |
2,356 | 57 | 30 |
| Anti-sense: aaaacccatcgattgcctgaaggtata Cla I |
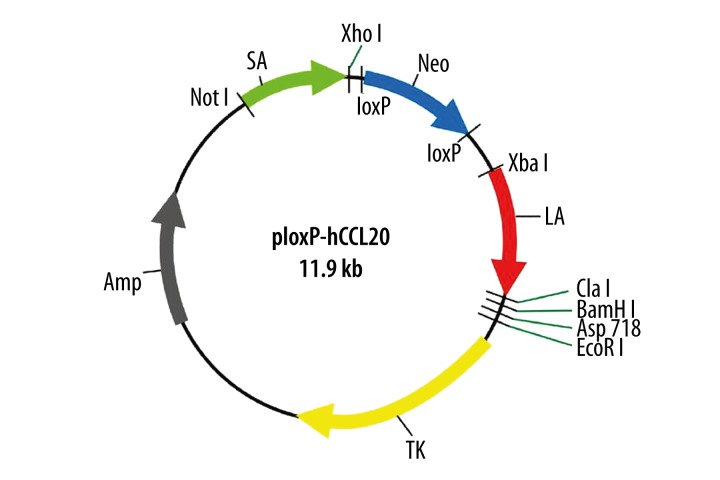
The primer sequences of the homologous short arm and the long arm are also listed in Table 1. Both homologous short arm and long arm of the human CCL20 were amplified by long-distance PCR using HaCaT genomic DNA as template. The short arm was inserted into the upstream of the Neomycin-resistance gene in the ploxP vector, and the long arm was inserted into the downstream of this same gene. These insertions generated the replacement targeting vector ploxP-hCCL20 (11.9 kb) for exon 2 of the human CCL20 gene (Figure 2).
Figure 2.
Schematic diagram of ploxP-hCCL20 replacement gene targeting vector. Note: LA and SA represents long arm and short arm, respectively.
Identification of positive clones by PCR and southern blot
The volume of 0.9 ml HaCaT cells at the density of 2.0×107/ml were electroporated with 75 μg linearized ploxP-hCCL20 vector. Selection was performed in the presence of both G418 and GANC to identify positive clones. PCR primers were designed within the 1,852–2,260 bp region of the CCL20 gene sequence across the Neomycin cassette; the size of the PCR product was 409 bp, and the primer sequences were as follows: sense primer 5′-ACT CCT CCT CTA AGT GGT TTA-3′, anti-sense primer: 5′-TAT CTT TGC CAC ATT TCT TTC TCT -3′. HaCaT clones were screened, and genomic DNA from positive clones was used as template for PCR. The probe used for Southern blot was designed against the third exon of the gene. Primers were located in the 2,870–3,078 bp regions and shown as follows: sense primer: 5′-TTG AAA AGC TCA TTA AAC-3′ and antisense primer: 5′-AAG AAT CCC AGA AAA CCT-3′. The PCR product for this reaction was 209 bp in size, and the primer sequences were PCR amplification was used with ploxP-hCCL20 as template, and the purified PCR product was labeled with biotin and used as a homologous probe. Next, DNA hybridization was performed according to the protocol described in the North2South kit (Pierce) [9].
Construction of ploxP-hCCL20-EGFP fluorescent expression vector
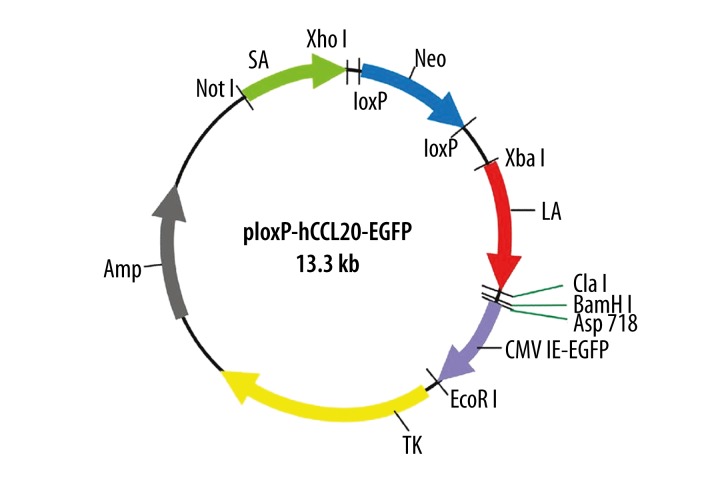
Both the CMV promoter and EGFP coding sequence of the pEGFP-N2 plasmid were cloned into ploxP-hCCL20 between the Asp718 and EcoRI restriction sites to construct the ploxP-hCCL20-EGFP green fluorescent expression vector (size: 13.3 kb). The resulting construct is provided in Figure 3.
Figure 3.
Schematic diagram of ploxP-hCCL20-EGFP fluorescent expression plasmid. Note: LA and SA represents long arm and short arm, respectively.
293FT and HaCaT cell transfection using jetPEI liposome
293FT and HaCaT cells were seeded in 24-well culture plates at a density of 2×104 cells/well. Cells were allowed to adhere for 24 h prior to transfection. Cells were approximately 80% confluent at time of transfection. A total of 2 μg of ploxP-hCCL20-EGFP vector DNA (either circular or linearized with EcoRI) was diluted to 50 μl with 150 mM NaCl. Fifty microliters of jetPEI (Polyplus) transfection reagent was diluted in NaCl and added to the plasmid mixture. The DNA: lipid combination was incubated at room temperature for 30 min after gently mixing. A total of 100 μl of the mixture was added to each well, and the final culture volume per well was 1 ml. Cells were maintained at 37°C and 5% CO2 for 12 h, at which point, the medium was changed.
Nucleofection of HaCaT cells using Amaxa electroporation
Linearized ploxP-hCCL20-EGFP DNA (2 μg) was prepared in water; the pmaxGFP plasmid provided with the kit (also 2 μg) served as a positive control. Approximately 1.0×106 HaCaT cells were suspended in preheated Nucleofector Solution V (100 μl). The plasmid DNA solution was combined with 100 μl cell suspension. The combination was then transferred into an electroporation cup. Electroporation was carried out according to the instructions detailed by the Amaxa electroporation instrument U-20 program. Next, the sample solution was transferred to a 6-well culture plate. Following 12 h of incubation, medium was changed once for continuous cultivation at 37°C and 5%CO2. Transfected cells were observed under an inverted fluorescent microscope. Digital images were captured 12 h and 72 h after transfection.
Calculation of transfection efficiency of different cell lines using multiple transfection methods [10]
Using a high-power fluorescent microscope, 3 non-overlapping vision fields were randomly selected. The number of positive cells out of every 100 cells in total was determined, and the corresponding transfection efficiency was then calculated according to the following formula:
Statistical analysis
Data are expressed as mean ±SD. The Mann-Whitney test for non-related samples was used for statistical analyses. A P value less than 0.05 was considered statistically significant.
Results
PCR amplification of both homologous long and short arms of the human CCL20 gene
PCR amplified products were analyzed on a 1% agarose gel. A single band (4,459 bp) corresponding to the CCL20 amplicon was detected. Following Asp718 digestion, two bands (3,529 bp and 930 bp) were detected. Moreover, a 1,969 bp band corresponding to the homologous short arm and a 2,356 bp band corresponding to the long arm were also observed; all bands obtained were at predicted sizes.
Restriction analysis identification of the ploxP-hCCL20-targeting vector and sequencing analysis of the inserted fragments
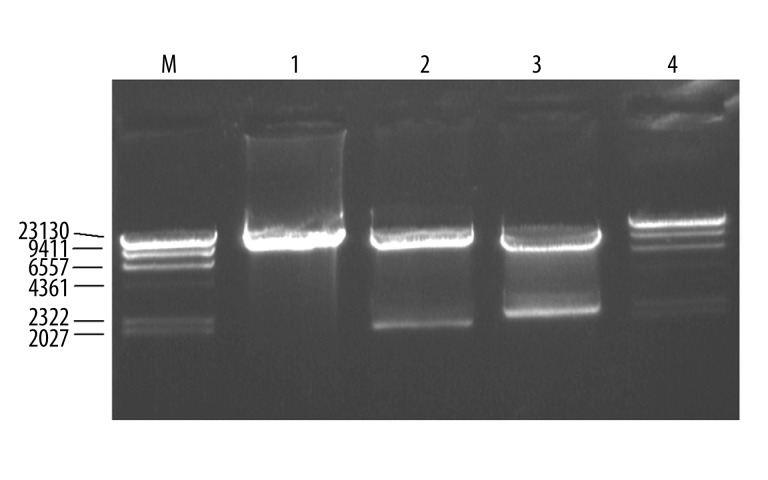
The ploxP-hCCL20 vector was double digested with NotI and XhoI. Following this digestion, 2 bands measuring 9.9 kb and 2.0 kb could be observed in an agarose gel. Furthermore, double digestion with XbaI and ClaI gave rise to 9.5 kb and 2.4 kb fragments, as expected (Figure 4).
Figure 4.
Identification of ploxP-hCCL20 targeting vector by restriction endonuclease digestion. M, λDNA/Hind III Marker; 1, ploxP-CCL20 targeting vector; 2, XbaI/ClaI digestion; 3, NotI/XhoI digestion; M, λDNA/Hind III marker.
Compared to the annotated sequence in GenBank (NT_005403), sequencing of the CCL20 gene from HaCaT cells indicated 2 mutations within the homologous short arm: site 115 G→A and site 1274 A→G. Despite this, restriction sites at both ends were unaltered. The NotI recognition sequence (GC↓GGCCGC) was found at the 5′ end, and the XhoI recognition sequence (C↓TCGAG) was located at the 3′ end. We identified 3 mutations within the homologous long arm: site 2228 T→C, site 3310 T→A, and site 4208 A→G. As in the short arm, the restriction sites on both ends were preserved (XbaI at the 5′ end, recognition sequence T↓CTAGA; and ClaI at the 3′ end, recognition sequence AT↓CGAT). None of the mutations occurred in exons. The homologous short arm contained exon 1 and some intronic sequence, and the homologous long arm contained exons 3 and 4 as well as some intronic sequence.
PCR and Southern blot identification of formed clones after pressure screening
Bu electroporation followed by both positive and negative selection, 81 potentially positive clones were selected and then subjected to PCR and Southern blot analyses. Agarose gel electrophoresis of the PCR products from genomic DNA of these clones identified only a 409 bp band but not a 2,150 bp band, indicating that CCL20 gene knockout was not successful. Similarly, Southern blot analysis failed to identify expected band sizes of 2,943 bp and 4,684 bp, again supporting the fact that gene knockout was unsuccessful. Importantly, our positive control worked, confirming that the technique itself was successfully executed (data not shown).
Sequencing analysis of fragments inserted into ploxP-hCCL20-EGFP
Vector sequence data was obtained from both Dalian TaKaRa Biology Company and from Clontech (pEGFP-N2). Based on the sequence, there is an Asp718 restriction site between nucleotides 122 to 127 (recognition sequence GGTAC↓C). There is an EcoRI site from 1,572 to 1,577 (recognition sequence G↓AATTC). The CMV IE promoter sequence is located between nucleotides 194 and 782, and the EGFP coding region is found from 852 to 1,571. All sequence nucleotides and positions were completely consistent, indicating that construction was successful.
Fluorescent expression and transfection efficiency of linearized ploxP-hCCL20-EGFP plasmid in 293FT and HaCaT cells by liposome-mediated transfection
Liposome-mediated transfection was used to introduce linearized ploxP-hCCL20-EGFP into both 293FT and HaCaT cells. Green fluorescence was detected under an inverted fluorescent microscope 12 h after transfection. Fluorescent intensity increased after 72 h. Additionally, fluorescent expression in 293FT cells was significantly stronger than in HaCaT cells. In 293FT cells, the transfection efficiency by this method was 75.1±3.4%, and 1.3±0.2% in HaCaT cells was lower compared to 293FT cells (P=0.000) (Figures 5 and 6).
Figure 5.
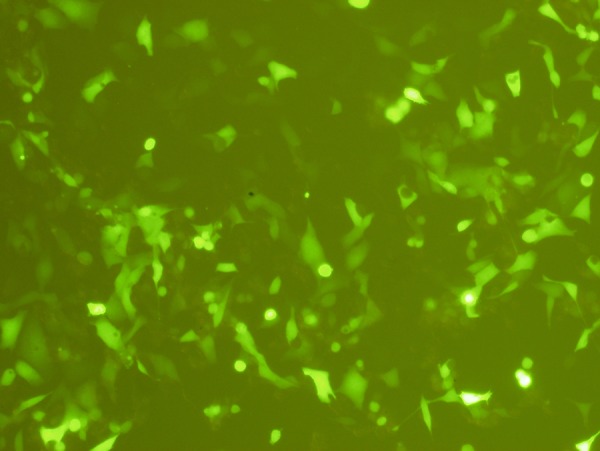
Linearized ploxP-hCCL20-EGFP plasmid was transfected into 293FT cells using liposome-mediated delivery. Images were taken 72 h after transfection. Fluorescence (100×).
Figure 6.
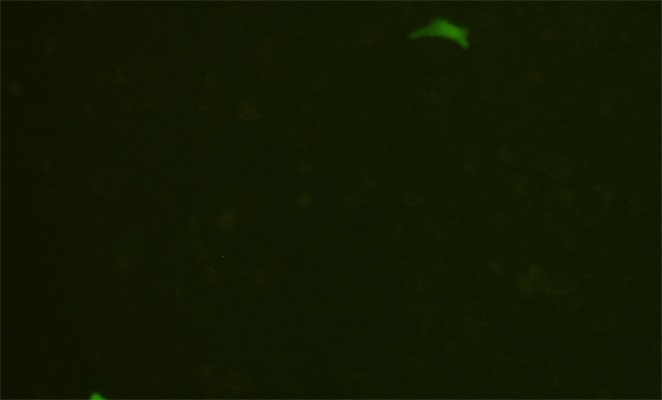
Linearized ploxP-hCCL20-EGFP plasmid was transfected into HaCaT cells using liposome-mediated delivery. Images were taken 72 h after transfection. Fluorescence (100×).
Liposome-mediated delivery could also be successfully used to transfect circular ploxP-hCCL20-EGFP plasmid into 293FT cells. Although the transfection rate was somewhat lower compared to linearized vector, the transfection efficiency was still 53.2±7.8% (Figures 7 and 8).
Figure 7.
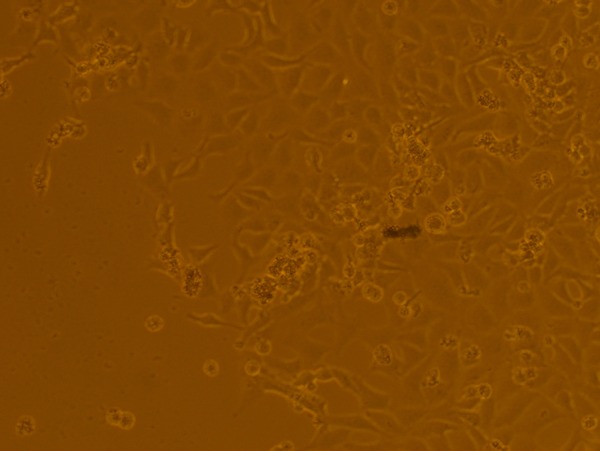
Circular ploxP-hCCL20-EGFP plasmid liposome transfected into 293FT cells for 72 h. Bright field (100×).
Figure 8.
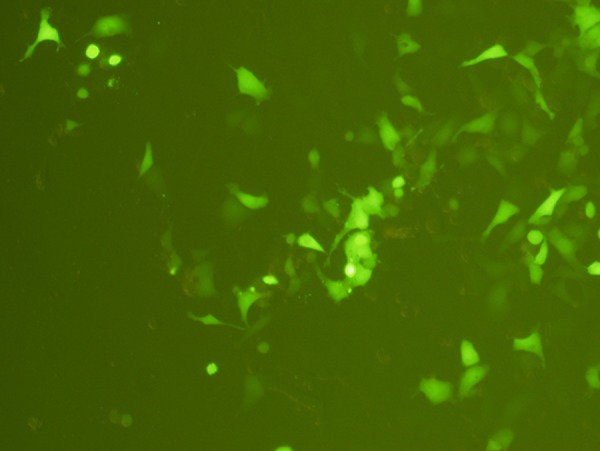
Circular ploxP-hCCL20-EGFP plasmid liposome transfected into 293FT cells for 72 h. Fluorescence (100×).
L2 Fluorescent expression and transfection efficiency of linearized ploxP-hCCL20-EGFP plasmid in HaCaT cells using nucleofection electroporation
Electroporation using nucleofection reagent resulted in fairly good transfection efficiency of the pmaxGFP control plasmid (3,490 bp) into HaCaT cells by 12 h. Fluorescent expression increased up to 72 h with transfection efficiency of 38.3±2.8%. In contrast, transfection efficiency of ploxP-hCCL20-EGFP (13.3 kb) by the same method into HaCaT cells was extremely poor at the level of 0.3 ± 0.1% compared to the pmaxGFP control plasmid (P=0.000) (Figures 9 and 10).
Figure 9.
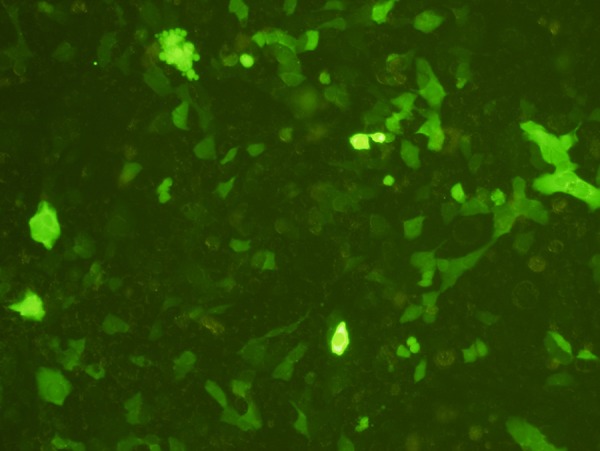
pmaxGFP plasmid electroporated into HaCaT cells for 72 h. Fluorescence (100×).
Figure 10.
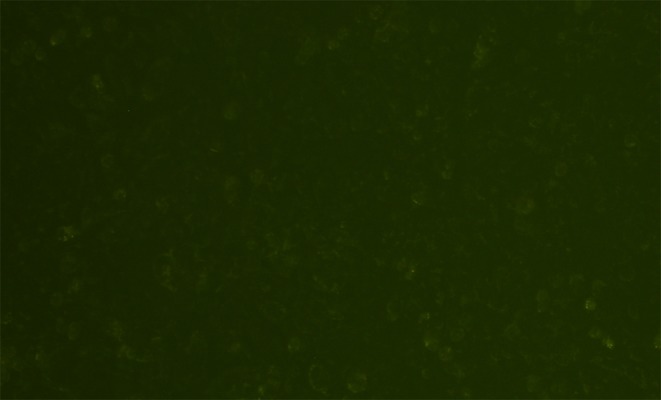
ploxP-hCCL20-EGFP plasmid electroporated into HaCaT cells for 72 h. Fluorescence (100×).
Discussion
Somatic gene targeting is a highly effective, fast, and specific method that can be used to disrupt normal gene function under physiological conditions [11]. Somatic targeting is widely accepted by the research community and has several advantages over similar methodologies in embryonic stem cells, including relative ease of operation and shorter procedure time. As early as the 1990s, there have been several publications from around the world describing the use of somatic cell gene knockout technology [12–14]. The HaCaT cell line is the first permanent epithelial cell line from adult human skin that exhibits normal differentiation and no tumorigenicity, thus providing a useful tool for studying the regulation of keratinization in human cells [15,16]. Flow cytometric analysis of HaCaT cells has revealed that this cell line displays a high keratinocyte stem cell phenotype, a transient amplifying cell ratio, strong adhesive basement membrane features, and a high proliferative capacity, all of which indicate that these cells can be used for human skin tissue engineering and for gene modification [17]. Human skin tissue engineering can be performed on an acellular dermal scaffold from Sprague-Dawley rats using HaCaT cells as the seed cell [18]. Here, we aimed to establish a CCL20 knockout HaCaT cell line to generate a seed cell with decreased rejection capacity.
In this study, the CCL20 gene replacement targeting vector was constructed from a ploxP vector, initially derived from the pPNT vector. We cloned the homologous short arm (1–2 kb) between the PGKneo cassette and the PGKtk gene of the pPNT vector. In contrast, the homologous long arm was cloned between the NotI restriction site and the PGKneo cassette. The overall length of the CCL20 coding region is 4,601 bp; thus, gene targeting using conventional techniques is sufficient to result in integration with the HSV-TK gene down-stream of the ploxP vector. As a result, it is increasingly difficult to screen for positive clones [19–21]. In order to explore the influence of fragment length on homologous recombination frequency and to decrease potential problems, we increased the length of the 3′ homologous long arm to 2,356 bp and inserted this fragment between the neomycin resistance cassette and the HSV-TK gene. We cloned the homologous short arm (1,969 bp) between the NotI restriction site and the neo cassette. The purpose of this was to reduce the integration frequency by increasing the length of the homologous arm upstream of the HSV-TK gene. We aimed to improve the screening frequency of homologous recombination when carrying out gene targeting in the next step. We performed PCR, restriction digest, and sequencing to confirm that the 2 homologous arms contained exon 1, exon 2, exon 4, and some intronic sequence of the human CCL20 gene. Thus, the construction of the ploxP-hCCL20-targeting vector was successful.
Following selection with both G418 and GANC, 81 potential clones were isolated. However, PCR and Southern blot analysis revealed no true-positive clones with CCL20 gene knockout. To try to understand why this occurred, we next constructed the ploxP-hCCL20-EGFP fluorescent expression plasmid (13.3 kb). We linearized the vector and used jetPEI liposome to transfect 293FT cells. We observed strong green fluorescence (transfection efficiency=75.1±3.4%). We performed similar transfection in HaCaT cells but the efficiency was only 1.3±0.2% (P=0.000). Transfection efficiency of 293FT cells with a circular plasmid was 53.2±7.8%, which was lower than the efficiency obtained with the linearized plasmid. Thus, a linearized plasmid likely enters 293FT cells more easily than a circular plasmid. We therefore used linearized plasmid for subsequent transfection experiments.
The 293 cell line is a permanent line established from primary embryonic human kidney and transformed with sheared human adenovirus type 5 DNA. The 293FT cell line is derived from the 293F cell line and stably expresses the SV40 large T antigen from the pCMVSPORT6TAg.neo plasmid. It displays relatively strong re-absorption and endocytosis functions, and is thus easily transfected [22,23]. More than 95% of epidermal cells are composed of keratinocytes. Keratinocytes constitute one of the most important cell types of the epidermis. They are the main functional cells of the skin, serving as a barrier against outside pathogens and other stimuli. They also play an important role in maintaining the normal physiological function of the skin [23]. HaCaT cells were formed from the immortalization of keratinocytes. HaCaT cells share many similar biological characteristics with primary human keratinocytes; thus, they have become a primary tool for studying human epidermis [16,25]. Since they serve as a barrier against pathogens, HaCaT cells intrinsically resist incorporation of exogenous substances, making them difficult to transfect [24].
Nucleofection technology introduces foreign DNA to nucleus of primary cells and passaged cells by combining traditional electroporation technology and patented cell-specific transfection reagents. Compared to other transfection technologies, nucleofection is more suitable for transfecting primary cells and for other cell lines that are difficult to transfect. This technology is not limited by slowly dividing cells, such as primary lines, and it is widely used in cancer research, immunology, tissue engineering, and cardiovascular disease research [26,27]. Previous work has shown that it can be used to transfect pEGFP-C1 plasmid with molecular weight of 4,700 bp into normal human keratinocytes. Flow cytometry and observation under a fluorescent microscope showed that the transfection efficiency was approximately 56 ± 9%; this value is significantly higher compared to transfection with other liposome-based reagents from another 3 companies (11±2%). Moreover, cell viability after transfection is high with only 14–16% dead cells and no cell showing terminal differentiation [28]. Amaxa nucleofection reagent could successfully introduce the positive control plasmid pmaxGFP with molecular weight of 3490 bp into HaCaT cells with a transfection efficiency of 38.3±2.8% after 72 h. This is in accordance with specifications described with the kit. There were a few cells expressing weak fluorescence following transfection with the ploxP-hCCL20-EGFP plasmid. The targeting vector has a low homologous recombination frequency in this particular cell line (approximately 10−3–10−7) [21]. Thus, it is necessary for a large amount of linearized targeting vector to be transferred into target cells to increase the occurrence of homologous recombination.
Conlusions
Based on the above results we conclude that HaCaT cells are intrinsically difficult to transfect. The large sizes of ploxP-hCCL20 and ploxP-hCCL20-EGFP targeting vectors (11.9 kb and 13.3 kb, respectively) pose a difficulty to HaCaT cell entry. Therefore, we were unable to achieve levels of DNA required for recombination and subsequent gene knockout.
Despite its successes in other cell lines, nucleofection was unable to achieve high transfection efficiency in HaCaT cells. Therefore, we suggest that other transfection strategies should be tested in order to achieve positive HaCaT clones with successful CCL20 gene knockout.
Footnotes
Source of support: Supported by grants from National Key Basic Research and Development Project of China (973 Project) (No.2005CB522605), National High Technology Research and Development Project of China (863 Project) (No.2006AA02A121), and National Natural Science Foundation of China (No. 39290700-1)
References
- 1.Hieshima K, Imai T, Opdenakker G, et al. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem. 1997;272:5846–53. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
- 2.Li B1, Xu W, Xu L, Jiang Z, et al. I-TAC is a dominant chemokine in controlling skin intragraft inflammation via recruiting CXCR3+ cells into the graft. Cell Immunol. 2010;260:83–91. doi: 10.1016/j.cellimm.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Rubie C, Kruse B, Frick VO, et al. Chemokine receptor CCR6 expression is regulated by miR-518a-5p in colorectal cancer cells. J Transl Med. 2014;12:48. doi: 10.1186/1479-5876-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AY, Körner H. CCR6 and CCL20: emerging players in the pathogenesis of rheumatoid arthritis. Immunol Cell Biol. 2014;92(4):354–58. doi: 10.1038/icb.2013.97. [DOI] [PubMed] [Google Scholar]
- 5.Schutvser E, Struvf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–26. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 6.Evans MJ, Kaufmarm MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–56. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 7.Muller U. Ten years of gene targeting: targeted mouse mutants, from vector design to phenotype analysis. Mech Dev. 1999;82:3–21. doi: 10.1016/s0925-4773(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 8.Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cells. 1987;51:503–12. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 9.Adilakshmi T, Laine RO. Ribosomal protein S25 mRNA partners with MTF-1 and La to provide a p53-mediated mechanism for survival or death. J Biol Chem. 2002;277:4147–51. doi: 10.1074/jbc.M109785200. [DOI] [PubMed] [Google Scholar]
- 10.Deyrieux AF, Wilson VG. In vitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotechnology. 2007;54:77–83. doi: 10.1007/s10616-007-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedivy JM, Dutriaux A. Gene targeting and somatic genetics: a rebirth or a coming of age? Trends Genet. 1999;15:88–90. doi: 10.1016/s0168-9525(98)01689-8. [DOI] [PubMed] [Google Scholar]
- 12.Park BH, Vogelstein B, Kinzler KW. Genetic disruption of PPARdelta decreases the tumorigenicity of human colon cancer cells. Proc Natl Acad Sci USA. 2001;98:2598–603. doi: 10.1073/pnas.051630998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li G, Nelsen C, Hendrickson EA. Ku86 is essential in human somatic cells. Proc Natl Acad Sci USA. 2002;99:832–37. doi: 10.1073/pnas.022649699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIPI/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–34. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 15.Boukamp P, Petrussevska RT, Breitkreutz D, et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boukamp P, Stanbridge EJ, Foo DY, et al. c-Ha-ras oncogene expression in immortalized human keratinocytes (HaCaT) alter growth potential in vivo but lacks correlation with malignancy. Cancer Res. 1990;50:2840–47. [PubMed] [Google Scholar]
- 17.Zhu CT, Peng DZ, Pan F, et al. [Stem cell phenotypic analysis of human immortal keratinocyte line HaCaT by flow cytometry]. Xiandai Shengwu Yixue Jinzhan (Progress in Modern Biomedicine) 2007;7:499–503. [in Chinese] [Google Scholar]
- 18.Zhu CT, Peng DZ, Zhou X, et al. [Constrution of human tissue engineering skin by seed cell HaCaT]. Xiandai Shengwu Yixue Jinzhan (Progress in Modern Biomedicine) 2007;7:817–19. [in Chinese] [Google Scholar]
- 19.Shulman MJ, Nissen L, Collins C. Homologous recombination in hybridoma cells: dependence on time and fragment length. Mol Cell Biol. 1990;10:4466–72. doi: 10.1128/mcb.10.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas KR, Deng C, Capecchi MR. High-fidelity gene targeting in embryonic stem cells by using sequence replacement vectors. Mol Cell Biol. 1992;12:2919–23. doi: 10.1128/mcb.12.7.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Te Riele H, Maandag ER, Berns A. Highly efficient gene targeting in embryonic stem cells through homologous recombination with isogenic DNA constructs. Proc Natl Acad Sci USA. 1992;89:5128–32. doi: 10.1073/pnas.89.11.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham FL, Smiley J, Russell WC, et al. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira AC, Ferraz MP, Monteiro FJ, et al. Cationic liposome-DNA complexes as gene delivery vectors: Development and behaviour towards bone-like cells. Acta Biomater. 2009;5:2142–51. doi: 10.1016/j.actbio.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 24.Proksch E, Brandner JM, Jensen JM. The skin: An indispensable barrier. Exp Dermatol. 2008;17:1063–72. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 25.Boukamp P, Popp S, Bleuel K, et al. Tumorigenic conversion of immortal human skin keratinocytes (HaCaT) by elevated temperature. Oncogene. 1999;18:5638–45. doi: 10.1038/sj.onc.1202934. [DOI] [PubMed] [Google Scholar]
- 26.Zeitelhofer M, Vessey JP, Thomas S, et al. Transfection of cultured primary neurons via nucleofection. Curr Protoc Neurosci. 2009;4:32. doi: 10.1002/0471142301.ns0432s47. [DOI] [PubMed] [Google Scholar]
- 27.Wu K, Zhao XJ, Wong KW, et al. Comparison of plasmid DNA versus PCR amplified gene of insert DNA for nucleofection in Kasumi-1 cells. Cytotechnology. 2014 doi: 10.1007/s10616-013-9683-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Distler JH, Jungel A, Kurowska-Stolarska M, et al. Nucleofection: a new, highly efficient transfection method for primary human keratinocytes. Exp Dermatol. 2005;14:315–20. doi: 10.1111/j.0906-6705.2005.00276.x. [DOI] [PubMed] [Google Scholar]