Abstract
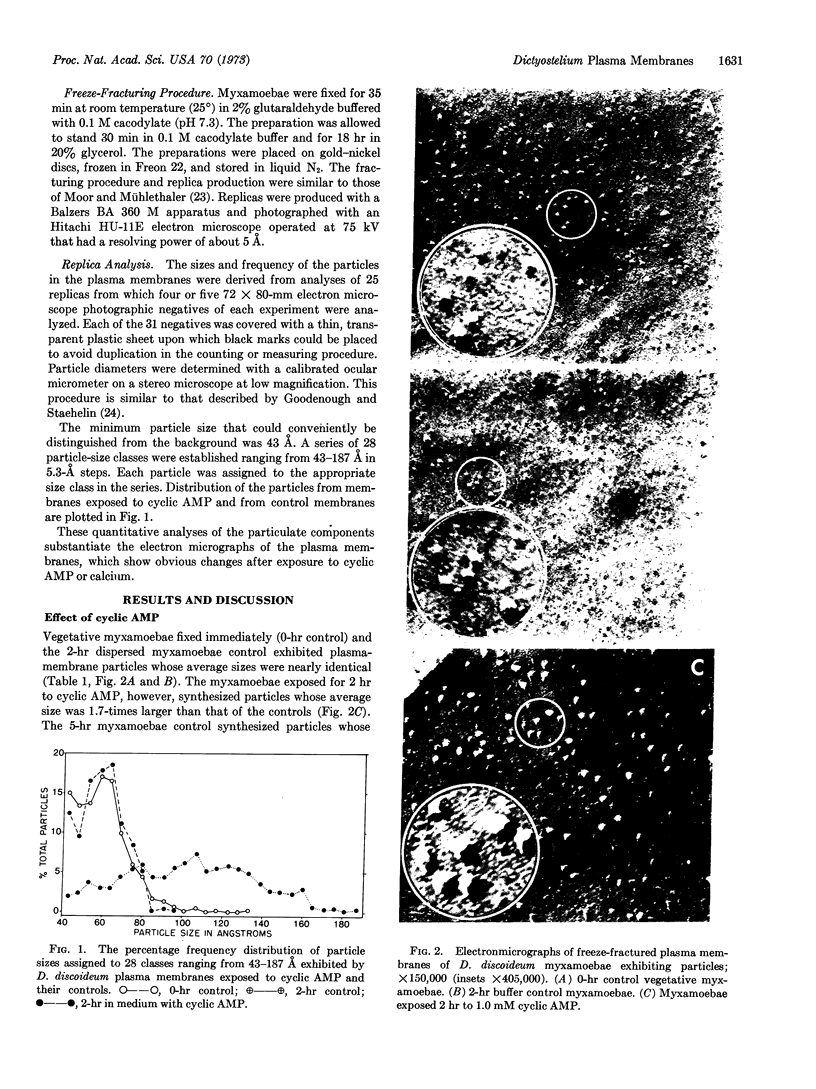
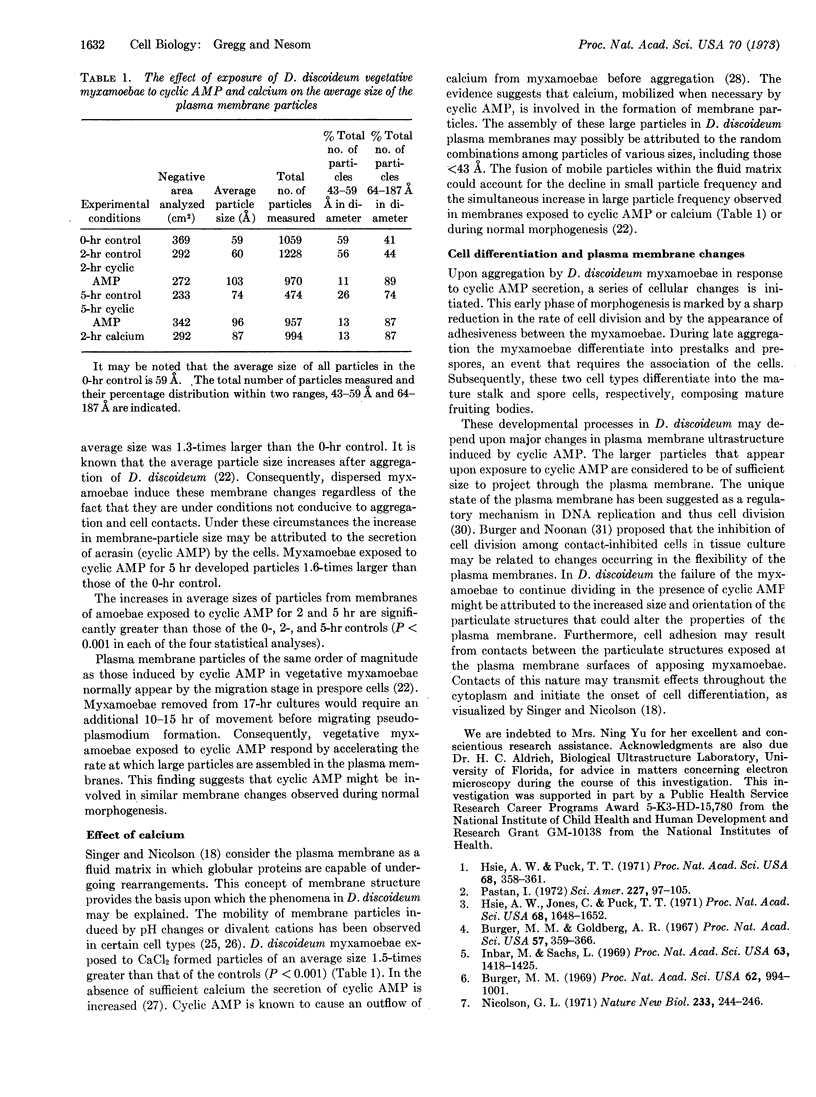
Myxamoebae of the cellular slime mold Dicytostelium discoideum aggregate in response to a chemotactic substance identified as adenosine 3′:5′-cyclic monophosphate. Upon aggregation cell division is suppressed, the cells become adhesive, and differentiation is initiated. Freeze-fracture studies of myxamoebae were conducted to determine the effect of cyclic AMP and calcium on plasma membrane ultrastructure. The inner surfaces of the plasma membranes exhibited particulate structures whose sizes (43-187 Å) and frequency distribution were determined. Cyclic AMP and calcium induced within 2 hr the formation of particles having average diameters 1.7-times and 1.5-times greater, respectively, than those of the vegetative myxamoebae controls. These data suggest that cyclic AMP mobilizes the intracellular calcium which may be effective in changing plasma membrane structure.
Keywords: cellular slime molds, freeze-fracturing, membrane particle alteration
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., Gerisch G., Kempff S., Riedel V., Cremer G. Specific inhibition of cell contact formation in Dictyostelium by univalent antibodies. Exp Cell Res. 1970 Nov;63(1):147–158. doi: 10.1016/0014-4827(70)90343-5. [DOI] [PubMed] [Google Scholar]
- Bonner J. T., Barkley D. S., Hall E. M., Konijn T. M., Mason J. W., O'Keefe G., 3rd, Wolfe P. B. Acrasin, Acrasinase, and the sensitivity to acrasin in Dictyostelium discoideum. Dev Biol. 1969 Jul;20(1):72–87. doi: 10.1016/0012-1606(69)90005-0. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Noonan K. D. Restoration of normal growth by covering of agglutinin sites on tumour cell surface. Nature. 1970 Nov 7;228(5271):512–515. doi: 10.1038/228512a0. [DOI] [PubMed] [Google Scholar]
- Chi Y. Y., Francis D. Cyclic AMP and calcium exchange in a cellular slime mold. J Cell Physiol. 1971 Apr;77(2):169–174. doi: 10.1002/jcp.1040770206. [DOI] [PubMed] [Google Scholar]
- Fiil A., Branton D. Changes in the plasma membrane of Escherichia coli during magnesium starvation. J Bacteriol. 1969 Jun;98(3):1320–1327. doi: 10.1128/jb.98.3.1320-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGG J. H. Serological investigations of cell adhesion in the slime molds, Dictyostelium discoideum, Dictyostelium purpureum, and Polysphondylium violaceum. J Gen Physiol. 1956 May 20;39(5):813–820. doi: 10.1085/jgp.39.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg J. H. Developmental potential of isolated Dictyostelium myxamoebae. Dev Biol. 1971 Nov;26(3):478–485. doi: 10.1016/0012-1606(71)90077-7. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Jones C., Puck T. T. Further changes in differentiation state accompanying the conversion of Chinese hamster cells of fibroblastic form by dibutyryl adenosine cyclic 3':5'-monophosphate and hormones. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1648–1652. doi: 10.1073/pnas.68.7.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konijn T. M., Van De Meene J. G., Bonner J. T., Barkley D. S. The acrasin activity of adenosine-3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1152–1154. doi: 10.1073/pnas.58.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B. CELL DIVISION AND A HYPOTHESIS OF CANCER. Natl Cancer Inst Monogr. 1964 May;14:7–20. [PubMed] [Google Scholar]
- Pastan I. Cyclic AMP. Sci Am. 1972 Aug;227(2):97–105. doi: 10.1038/scientificamerican0872-97. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane intercalated particles: the plasma membrane as a planar fluid domain. Chem Phys Lipids. 1972 May;8(4):265–278. doi: 10.1016/0009-3084(72)90054-0. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Douglas S. D., Branton D. Localization of A antigen sites on human erythrocyte ghosts. Nature. 1971 Jul 16;232(5307):194–196. doi: 10.1038/232194a0. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P. Translational mobility of the membrane intercalated particles of human erythrocyte ghosts. pH-dependent, reversible aggregation. J Cell Biol. 1972 Jun;53(3):777–787. doi: 10.1083/jcb.53.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- SONNEBORN D. R., SUSSMAN M., LEVINE L. SEROLOGICAL ANALYSES OF CELLULAR SLIME-MOLD DEVELOPMENT. I. CHANGES IN ANTIGENIC ACTIVITY DURING CELL AGGREATION. J Bacteriol. 1964 Jun;87:1321–1329. doi: 10.1128/jb.87.6.1321-1329.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]




