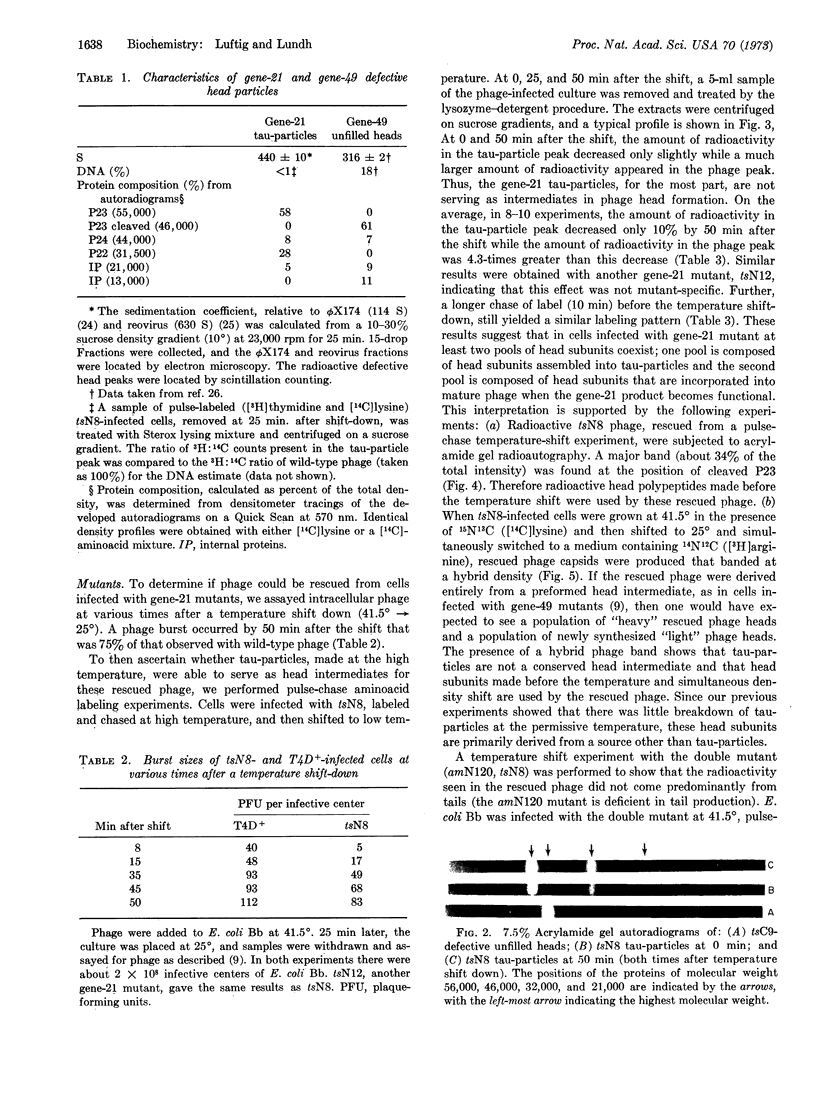
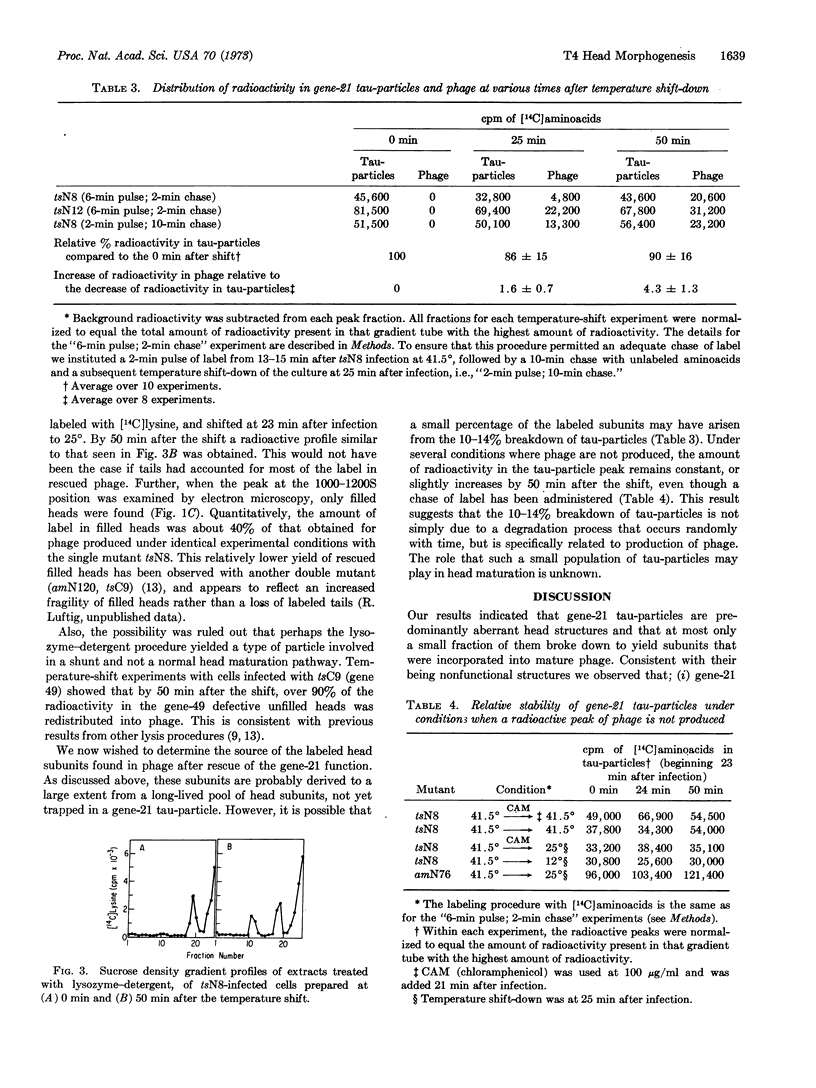
Abstract
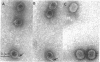
A lysozyme-detergent procedure was developed for isolation of tau-particles from cells infected by gene-21 mutants of T4 bacteriophage. These particles have a sedimentation coefficient of 440 ± 10 S. They contain less than 1% detectable nuclease-resistant DNA, are smaller (650 × 850 Å) than normal bacteriophage heads (800 × 1100 Å), and exhibit two major bands on 7.5% Na dodecyl sulfate-acrylamide gels. The more prominent band (55,000 daltons) corresponds to the uncleaved, major capsid polypeptide (P23); the other band (32,000 daltons) corresponds to the gene-22 product (P22). Temperature-shift experiments with cells infected with tsN8 (gene 21) mutants were used to study the fate of tau-particles accumulated under nonpermissive conditions. 50 Min after ts N8-infected cells were shifted from the nonpermissive (41.5°) to the permissive (25°) temperature, a phage burst occurred that was 75% of that observed with wild-type phage. However, in “pulse-chase” temperature-shift experiments, the radioactive tau-particle peak only slightly decreased (by 10-14%) by 50 min after the shift, whereas an increased amount of radioactivity (about four times as much as the tau-particle decrease) appeared in phage particles. The results suggest that at least two pools of head polypeptides coexist in cells infected with gene-21 mutants. One pool is composed of head subunits assembled into tau-particles, which are mostly aberrant structures; the second pool is composed of head subunits that are incorporated into mature phage when the gene-21 product becomes functional.
Keywords: capsids, plasma membranes, polypeptides
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cummings D. J., Couse N. L., Forrest G. L. Structural defects of T-even bacteriophages. Adv Virus Res. 1970;16:1–41. doi: 10.1016/s0065-3527(08)60020-2. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Lielausis I. Some steps in the assembly of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):263–276. doi: 10.1016/0022-2836(68)90008-9. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Gomatos P. J., Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton D. L., Luftig R. B. Bacteriophage T4 head morphogenesis. 3. Some novel properties of gene 13-defective heads. J Virol. 1972 Jun;9(6):1047–1056. doi: 10.1128/jvi.9.6.1047-1056.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger E., Eiserling F. A., Boy de la Tour E. Studies on the morphopoiesis of the head of phage T-even. 3. The cores of head-related structures. J Ultrastruct Res. 1967 Dec 12;21(3):335–360. doi: 10.1016/s0022-5320(67)80099-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Mölbert E., Showe M., Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970 Apr 14;49(1):99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- Luftig R. B. Further studies on the dimensions of viral and protein structures using the catalase crystal internal marker technique. J Ultrastruct Res. 1968 Apr;23(1):178–181. doi: 10.1016/s0022-5320(68)80041-3. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. II. Studies on the maturation of gene 49-defective head intermediates. J Virol. 1972 Feb;9(2):377–389. doi: 10.1128/jvi.9.2.377-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. IV. Comparison of gene 16-, 17-, and 49-defective head structures. J Virol. 1972 Sep;10(3):545–554. doi: 10.1128/jvi.10.3.545-554.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Black L. W. Assembly core of bacteriophage T4: an intermediate in head formation. Nat New Biol. 1973 Mar 21;242(116):70–75. doi: 10.1038/newbio242070a0. [DOI] [PubMed] [Google Scholar]
- Simon L. D. Infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope: T4 head morphogenesis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):907–911. doi: 10.1073/pnas.69.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Champe S. P. Genetic determinant of an internal peptide of bacteriophage T4. J Mol Biol. 1969 Dec 28;46(3):377–392. doi: 10.1016/0022-2836(69)90183-1. [DOI] [PubMed] [Google Scholar]
- Stromberg K. Surface-active agents for isolation of the core component of avian myeloblastosis virus. J Virol. 1972 Apr;9(4):684–697. doi: 10.1128/jvi.9.4.684-697.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weigle J. Studies on head-tail union in bacteriophage lambda. J Mol Biol. 1968 Apr 28;33(2):483–489. doi: 10.1016/0022-2836(68)90204-0. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Edgar R. S., King J., Lielausis I., Henninger M. Bacteriophage assembly. Fed Proc. 1968 Sep-Oct;27(5):1160–1166. [PubMed] [Google Scholar]