Abstract
BACKGROUND
A high throughput, high pressure liquid chromatographic (HPLC) method with triple quadrupole mass spectral detection (LC/MS/MS) was validated for the measurement of 5 endogenous androgens in human plasma and serum and applied to various in vivo and in vitro study samples to pursue a better understanding of the interrelationship of the androgen axis, intracrine metabolism, and castration-recurrent prostate cancer (CaP).
METHODS
A Shimadzu HPLC system interfaced with a Sciex QTRAP 5500 mass spectrometer with electrospray ionization was used with inline column-switching. Samples were liquid/liquid extracted and chromatographed on a Luna C18(2) column at 60°C with a biphasic gradient using a 15-min run time.
RESULTS
The method was validated for five androgens in human plasma and serum, and applied to four sets of samples. Plasma (n = 188) and bone marrow aspirate (n = 129) samples from patients with CaP, who received abiraterone acetate plus prednisone for up to 945 days (135 weeks), had undetectable androgens after 8 weeks of treatment. Plasma dehydroepiandrosterone (DHEA) concentrations were higher in African Americans than Caucasian Americans with newly diagnosed CaP. Analysis of prostate tumor tissue homogenates demonstrated reproducible testosterone (T) and dihydrotestosterone (DHT) concentrations with a minimal sample size of ~1.0–2.0 mg of tissue. Finally, cell pellet and media samples from the LNCaP C4-2 cell line showed conversion of T to DHT.
CONCLUSION
The proposed LC/MS/MS method was validated for quantitation of five endogenous androgens in human plasma and serum, and effectively profiles androgens in clinical specimens and cell culture samples.
Keywords: androgen axis, prostate cancer, testosterone, dihydrotestosterone, steroid 5α-reductase, LC/MS/MS
INTRODUCTION
Prostate cancer (CaP) is heterogeneous and its development and progression is androgen stimulated. Approximately 233,000 men will be diagnosed with CaP and an estimated 29,480 individuals will die from CaP in 2014 [1]. CaP accounts for the largest number of diagnosed cancers (27%) in American men, and 9.6% of all cancer-related deaths in males, second to lung and bronchus cancer at 28.0% [1]. Age appears to be the greatest risk factor with 1 in 16 men diagnosed between ages 60 and 69 years, and 1 in 9 at age 70 years or greater [1,2]. Benign prostatic hyperplasia/hypertrophy (BPH) is a non-malignant enlargement of the prostate that causes lower urinary tract symptoms in 70% of men by age 70 years [2]. In addition, a normal age-related decline in androgenic hormones [3], which may affect overall quality of life [4], can occur concurrently with either benign or malignant prostate disease.
The androgen receptor is a key component of the androgen axis and plays a significant role in the development, maintenance, and survival of normal prostatic tissue, and the pathogenesis of CaP and BPH. The basic mechanisms operating in the androgen axis are: (1) biosynthesis and transport of testosterone (T), (2) 5α-reductase conversion of T to its intracellular metabolite, dihydrotestosterone (DHT), the preferred ligand for the androgen receptor, and (3) mediation of nuclear transcriptional processes by the DHT-bound androgen receptor [5,6]. The pattern of genetic alteration affecting processes related to the androgen axis could provide explanations for the pathogenetic differences between CaP and BPH, and other clinically relevant observations such as the increased incidence of, and mortality from, CaP in African Americans compared to Caucasian Americans. As a result, T, DHT, and the androgen receptor have become primary targets for the development of rational treatment strategies for CaP.
Androgen deprivation therapy (ADT), which suppresses T and its metabolites, can be accomplished either surgically by removal of the testes (orchiectomy), or chemically using anti-androgens or luteinizing hormone releasing hormone (LHRH) agonists or antagonists, and offers palliation for most men with advanced, symptomatic CaP. Alternatively, the 5α-reductase inhibitors finasteride [7] and dutasteride [8], which inhibit conversion of T to DHT, have been shown to reduce tissue androgen concentrations and decrease the frequency of diagnosis of CaP. However, over the long term, ADT is only palliative because the disease progresses and causes death despite castrate levels of circulating T and DHT. This suggests that recurrence of CaP during ADT may result from production of sufficient levels of T and DHT for androgen receptor activation in CR-CaP prostatic tissue by intracrine metabolism [9–11]. Abiraterone acetate (Zytiga®) is a new oral hormonal therapy blocking T production at multiple sites (testes, adrenal glands, and tumor) by inhibiting cytochrome P450 17A1 (CYP17A1), presumably by eliminating intracrine metabolism in target tissue. Studies of abiraterone acetate in combination with prednisone in patients with metastatic CR-CaP have shown reduced androgen levels in plasma and bone marrow [12] and increased median survival [13].
As a result, the accurate, sensitive, and reproducible quantitation of androgens in plasma, CaP tissue and other in vivo and in vitro matrices is essential for the development of appropriate models to understand, characterize and distinguish indolent or aggressive disease-associated alterations of the androgen axis from normal androgen-stimulated gene expression. In addition, other hypo- or hyper-androgenic associative conditions, such as BPH, breast cancer, alopecia, acne, hepatocellular carcinoma, coronary artery disease, and diabetes, could benefit from the availability of androgenic profiling.
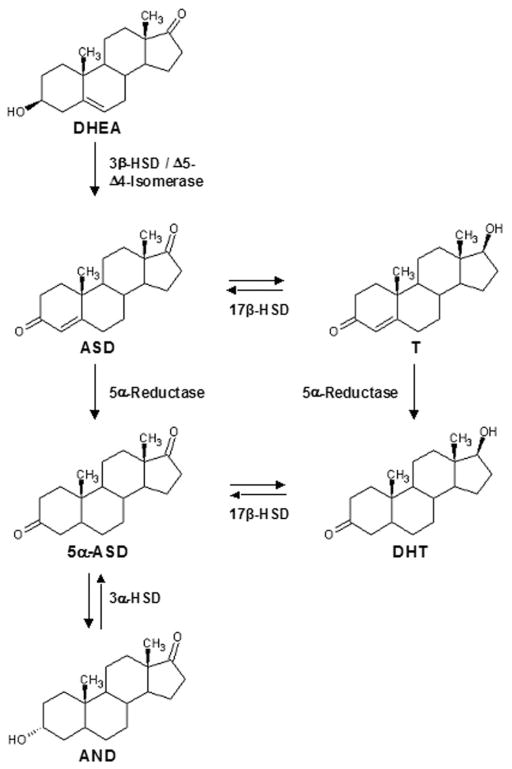
Endogenous androgens have been quantitated using HPLC with ultraviolet detection, with and without derivatization, or by radioimmuoassay (RIA). However, LC/MS/MS has become the preferred method for measurement of physiological levels of androgens. HPLC with ultraviolet detection lacks the sensitivity and selectivity of RIA and LC/MS/MS methods. RIA, although an efficient and sensitive methodology, suffers from lack of selectivity due to cross-reactivity with other androgens. LC/MS/MS using a variety of different ionization sources has become the method of choice because of its efficiency, sensitivity and selectivity. Numerous analytical methods have been reported [14–23], but a quest continues for technologies with an increasing number of analytes, more efficient sample preparation, and improved sensitivity, selectivity, and robustness. Current analytical methods are limited in the scope of detectable compounds, have long run times, or have not presented validated performance data in biological matrices. Herein, we provide validation results of a highly sensitive, high throughput method for the analysis of five androgens in human plasma and serum to further investigations into the role that androgens play in the hydroxysteroid dehydrogenase (HSD) and 5α-reductase pathways (Fig. 1), and pursuit of an overall understanding of the role of intracrine metabolism in the androgen axis and CR-CaP.
Fig. 1.
Hydroxysteroid dehydrogenase (HSD) and 5α-reductase androgenic pathways. Testosterone (T), dihydrotestosterone (DHT), androstenedione (ASD), dehydroepiandrosterone (DHEA), 5α-androstanedione(5α-ASD), and androsterone (AND).
MATERIALS ANDMETHODS
Chemicals and Supplies
T, DHT, DHEA, androstenedione (ASD), 5α-androstanedione (5α-ASD), 4-androsten-17β-ol-3-one-16,16,17-d3 (d3-T), and 5α-androstan-17β-ol-3-one-16,16,17-d3 (d3-DHT) were obtained from Steraloids. Androsterone (AND) and methyl-tert-butyl ether (MTBE, 99.8%) were obtained from Sigma–Aldrich. Ammonium formate and HPLC grade methanol were obtained from J.T. Baker. Formic acid (98%) and Omni-Solv HPLC grade water were obtained from EMD Chemicals. Sodium heparinized plasma and serum from post-menopausal women (double charcoal-stripped) were obtained from Bioreclamation, LLC. The C4-2 cell line [24,25] was obtained from UroCor, Inc. and has been maintained frozen. Aliquots are grown and used for <10 passages. The cell line remains androgen-independent for growth, and growth rates remain similar. SKY karyotyping performed on passage proximate to that used reveals the typical loss of Y chromosome and an extended long arm of chromosome 6 that is unique from the parental LNCaP cell line, whereas the karyotype is similar to that of LNCaP in all other respects.
Preparation of Stock Solutions, Calibration Standards, and QC Samples
Plasma and serum calibration and quality control (QC) samples were prepared by adding the appropriate spiking solution prepared in 70% methanol to blank, charcoal-stripped female human plasma or serum such that the original matrix was not diluted by more than 5%. After preparing the calibrators and QCs in bulk, 600 μl aliquots were transferred to microcentrifuge tubes and stored frozen at −80°C until used. Plasma QC samples (high, medium, and low concentrations) were prepared at concentrations different from the calibrator concentrations. The internal standard (IS) solution was prepared in 70% methanol and contained 75.0 pg/ml d3-T and 225 pg/ml d3-DHT.
Sample Preparation
A 250 μl aliquot of a matrix calibrator, QC, matrix blank, or study sample was added to a 16 × 100 mm glass screw-top tube followed by HPLC grade water (750 μl), 100 μl of IS solution (75.0/225 pg/ml d3-T/d3-DHT), and 6.0 ml of MTBE. The tubes were capped and rotated for 15 min, and centrifuged at 3,000 rpm and 4°C for 15 min. The lower aqueous phase was frozen in a dry ice/acetone bath and the MTBE poured into a glass conical tube. The solvent was evaporated under nitrogen at 37°C, and the residue reconstituted with 75.0 μl of 60% methanol. The resulting mixture was filtered through a 96-well filter plate (0.45 μm; Harvard Apparatus) by centrifuging for 1 min at 3,000 rpm. Samples were maintained at 4°C in the autosampler and 20 μl injected.
LC/MS/MS Analysis
LC/MS/MS analysis was performed using a Prominence UFLC System (Shimadzu Scientific Instruments) interfaced with a QTRAP® 5500 mass spectrometer (AB SCIEX) and equipped with two 10-port switching valves. The first was mounted internally in the column oven for sample cleanup, and the second located between the column oven and mass spectrometer functioning as a waste divert valve. Chromatographic separation was achieved using a reverse phase Phenomenex Luna C18(2) column (3 μm, 2.0 mm × 150 mm, part number 00F-4251-B0) preceded by a Phenomenex C18 SecurityGuard guard cartridge. Sample elution was carried out at 60°C and flow rate of 175 μl/min using a biphasic gradient. Mobile phase A contained 0.4 ml of 1.0 M ammonium formate and 62.0 μl of concentrated formic acid per liter of 65% methanol in water. Mobile phase B contained 0.4 ml of 1.0 M ammonium formate and 62.0 μl of concentrated formic acid per liter of methanol.
A second C18 SecurityGuard cartridge was attached to the switching valve in the column oven and used for sample cleanup. After the first minute of elution to the analytical column, this cartridge was switched out of line and washed between each injection cycle using a second HPLC pump (Waters, model 600E) and a biphasic gradient consisting of the same mobile phases. Elution of the androgens and ISs was accomplished using the flow rates, gradients, and switching valve time profiles outlined in Table I.
TABLE I.
Timetable for LC/MS/MS Gradient Elution Profiles and Switching Valves
| Time (min) | Shimadzu gradient for analytical column elution
|
Waters gradient for guard column cleanup
|
Switching valves
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Flow rate (μl/min) | Mobile phase A (%) | Mobile phase B (%) | Flow rate (μl/min) | Mobile phase A (%) | Mobile phase B (%) | ID | Position | Flow direction | |
| 0.00 | 175 | 87 | 13 | 200 | 87 | 13 | 1 | A | To cleanup column |
| 0.00 | 2 | B | To waste | ||||||
| 1.00 | 1 | B | To analytical column | ||||||
| 4.50 | 2 | A | To mass spec source | ||||||
| 4.75 | 200 | 87 | 13 | ||||||
| 5.00 | 350 | 0 | 100 | ||||||
| 8.25 | 175 | 20 | 80 | ||||||
| 8.50 | 175 | 5 | 95 | 350 | 0 | 100 | |||
| 8.75 | 350 | 87 | 13 | ||||||
| 9.50 | 175 | 5 | 95 | 2 | B | To waste | |||
| 10.0 | 175 | 87 | 13 | ||||||
| 14.0 | 1 | A | To cleanup column | ||||||
| 15.0 | 175 | 87 | 13 | 350 | 87 | 13 | |||
Mass Spectrometry
The QTRAP® 5500 mass spectrometer with an electrospray ionization source in positive ion mode was used with multiple reaction monitoring (MRM) to detect androgens. System control, peak area measurement, and quantitation was performed using AB SCIEX Analyst® software, version 1.5.1. Mass spectrometer source conditions were ion spray voltage 5,000 V, temperature 650°C for turbo gas (gas 2), and settings of 85, 70, 26, and medium for nebulizer gas (gas 1), turbo gas (gas 2), curtain gas, and collision-activated dissociation (CAD) gas, respectively. Unit mass resolution was used for both Q1 and Q3 mass resolving quadrupoles. Nitrogen gas was employed for nebulizer, turbo, curtain and collision gases. Voltages for maximum parent/fragment ion pair intensities were optimized using solutions of individual androgens with direct infusion and flow injection analysis (FIA). The MRM transitions, dwell times and optimized voltages for each of the androgens are reported in Table II.
TABLE II.
QTRAP 5500 MS Ion Pairs, Dwell Times, and Optimized Voltagesa
| Androgen | Molecular formula | Parent mass + H (m/z) | Fragment mass + H (m/z) | Dwell time (msec) | Declustering potential (V) | Collision energy (V) | Exit potential (V) |
|---|---|---|---|---|---|---|---|
| ASD | C19H26O2 | 286.9 | 97.0 | 125 | 100 | 27.0 | 12.0 |
| T | C19H28O2 | 288.9 | 97.0 | 125 | 112 | 27.0 | 12.0 |
| DHEA | C19H28O2 | 289.0 | 271.1 | 125 | 70.0 | 11.0 | 14.0 |
| DHT | C19H30O2 | 290.9 | 255.1 | 125 | 105 | 21.0 | 12.0 |
| AND | C19H30O2 | 290.9 | 273.2 | 125 | 100 | 13.0 | 12.0 |
| d3-T | C19H25D3O2 | 291.9 | 97.0 | 100 | 112 | 27.0 | 12.0 |
| d3-DHT | C19H27D3O2 | 293.9 | 258.1 | 100 | 105 | 21.0 | 12.0 |
Entrance potential = 10 V; pause time = 5 msec.
Method Validation
The method validation in human plasma was conducted over three analytical runs to assess linearity, accuracy, precision, sensitivity, selectivity, stability, and absolute recovery. Each of the three validation runs included duplicate calibration curves, six replicates of each QC concentration (total n = 18 QCs per run), two plasma blanks, and a reagent blank. Other parameters assessed during validation included within-sample precision (n = 6 injections of a homogeneous sample), absolute recovery (injection of five samples prepared in the reconstitution solvent at each of the post-extracted theoretical QC concentrations), dilution effects (three 25.0 ng/ml plasma QCs that were diluted into the range of the standard curve prior to extraction), pre-processed sample stability (n = 3 high and low concentration QC samples stored at either room temperature or 37°C for 3.5 hr prior to extraction), stability of extracted samples (n = 3 high, medium and low concentration extracted QCs analyzed at the end of a run), re-injection stability (n = 3 high and low concentration QC samples re-injected at the end of a run), and freeze–thaw stability (n = 3 high and low concentration QC samples analyzed after three freeze–thaw cycles). The cross-validation of the method in human serum was performed in a separate validation run to assess accuracy, precision, specificity, stability, and dilution effects. Calibration curves were generated using the analyte/IS area response ratios versus nominal concentrations (ng/ml) and weighted linear regressions with a weighting factor of 1/concentration2. The IS used for T, ASD, DHEA, and 5α-ASD is d3-T whereas d3-DHT was used for DHT and AND. Back-calculated concentrations were generated using the formula x = (y − b)/m where x is the back-calculated concentration, y is the analyte/IS ratio, b is the y-intercept, and m is the slope. Calibrator and QC acceptance criteria required all acceptable concentrations to have accuracy deviations of 15% or less from the nominal concentration with relative standard deviations (% RSD) of 15% or less, except at the lower limit of quantitation (LLOQ), which was allowed a 20% deviation for both parameters. Percent recovery was calculated by dividing the observed concentration by the theoretical concentration ×100.
Method Applications
The validated method was applied to four studies. In the first, plasma (n = 188) and BM aspirate (n = 129) samples were obtained from patients with metastatic CaP pre- and post-treatment with abiraterone acetate (1,000 mg QD) and prednisone (5 mg BID) for up to 945 days (median treatment 233 days) [12]. The second study analyzed 90 plasma samples from African and Caucasian Americans with newly diagnosed CaP. Blood samples (EDTA anticoagulant) were collected during home visits and transported to a PCaP Blood and Tissue Core Laboratory at the University of North Carolina or the Louisiana State University Health Science Center for processing and plasma collection. A third application entailed the analysis of prostatic tumor tissue to determine assay reproducibility and the minimum amount of tissue required for analysis with the ultimate goal of being able to analyze laser capture microdissection samples (~50 μg tissue). Lastly, cell pellet and media samples were analyzed from the lymph node carcinoma of the prostate (LNCaP) C4-2 cell line to develop methodologies for in vitro evaluation of novel treatment strategies directed at interdiction of androgen metabolism. Clinical samples from the abiraterone acetate study and the African American/Caucasian American PCaP study were analyzed using calibrators and QCs that were the same biological matrix as the study samples (i.e., human plasma). The exploratory prostatic tissue and cell pellet studies were performed using water-based extracted calibrators to provide added sensitivity, which is limited in plasma by the endogenous androgen concentrations. The accuracy of recovery of the water-based calibration curves was based on the incorporation of plasma QC samples. Androgen concentrations in the study samples that were below the LLOQ were treated as zero. Data analysis in the abiraterone acetate study compared post-treatment androgen concentrations to pretreatment levels to determine the effectiveness of the abiraterone acetate/prednisone combination on hormonal blockade. For the African American, Caucasian American comparison study, the results of the individual androgens from the two groups were compared using a Student’s t-test (two-tailed, homoscedastic).
The abiraterone acetate study and the racial comparison serum androgen study (a non-clinical trial) were performed after institutional review board approval with informed consent obtained from each subject prior to their enrolment. The abiraterone acetate study (trial registration ID: NCT00544440) was conducted in accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services.
RESULTS
Method Validations
The analytical method was validated for T, DHT, ASD, DHEA, and AND; however, 5α-ASD, a sixth androgen that was attempted, failed to achieve acceptable performance statistics for accuracy and precision at lower concentrations. Chromatographic resolution was established for all androgens to prevent overlapping peak responses of androgens with similar molecular weights and fragmentation patterns. Retention times for ASD, T, DHEA, 5α-ASD, DHT, and AND were 5.4, 5.8, 6.3, 6.8, 7.1, and 8.2 min, respectively. A single sample analysis cycle required 16 min allowing 80 sample injections over 21.4 hr. Duplicate plasma calibration curves were analyzed in each of the three validation runs and correlation coefficients for all five androgens were >0.9953. The calibrator and QC accuracy and precision results for T, DHT, ASD, DHEA, and AND passed the intended acceptance criteria (Table III) with mean imprecision (% RSD) <7.75% and overall analytical recovery ranging 93.3–106% for all androgens. Other parameters assessed during validation included within-sample precision, absolute recovery, dilution effects, and stability. All results passed validation criteria (Table IV) except those at the lower concentrations of DHT and AND for three-cycle freeze–thaw stability, and for AND stability in plasma maintained at an elevated temperature of 37°C for 3.5 hr.
TABLE III.
Plasma Calibrator and QC Validation Results
| Sample type | Androgen | Concentrations (ng/ml) | Precision (% RSD)
|
Accuracy (% AR)
|
||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Min | Max | Mean | |||
| Calibrators | ASD | 0.0125–3.75 | 0.83 | 6.76 | 3.89 | 93.2 | 106 | 100 |
| T | 0.00625–3.75 | 0.77 | 5.32 | 2.53 | 92.6 | 105 | 100 | |
| DHEA | 0.250–7.50 | 1.33 | 7.36 | 3.99 | 98.2 | 104 | 100 | |
| DHT | 0.0250–7.50 | 1.47 | 7.66 | 3.76 | 95.6 | 105 | 99.8 | |
| AND | 0.500–7.50 | 3.32 | 6.26 | 3.93 | 94.2 | 107 | 99.8 | |
| QCs | ASD | 0.0600, 0.420, 2.94 | 1.77 | 3.07 | 2.42 | 95.6 | 101 | 99.4 |
| T | 0.0270, 0.270, 2.70 | 2.04 | 2.30 | 2.19 | 94.0 | 101 | 96.8 | |
| DHEA | 0.600, 1.80, 5.40 | 1.96 | 5.79 | 4.28 | 92.9 | 96.5 | 94.6 | |
| DHT | 0.0540, 0.540, 5.40 | 3.56 | 6.76 | 5.08 | 92.2 | 95.4 | 93.3 | |
| AND | 0.600, 1.80, 5.40 | 3.66 | 13.4 | 7.75 | 92.6 | 114 | 106 | |
RSD, relative standard deviation; AR, analytical recovery.
TABLE IV.
Additional Androgen Validation Studies in Heparinized Human Plasma
| Test parameter | ASD | T | DHEA | DHT | AND |
|---|---|---|---|---|---|
| Within-sample | |||||
| % RSD | 1.30 | 1.29 | 1.28 | 2.12 | 2.96 |
| % AR | 94.3 | 98.5 | 93.7 | 97.7 | 105 |
| Dilution QC (25 ng/ml) | |||||
| % RSD | 3.58 | 2.26 | 5.24 | 3.72 | 6.69 |
| % AR | 104 | 94.4 | 99.3 | 97.4 | 104 |
| Three-cycle freeze–thaw | |||||
| QC1: % RSD | 1.71 | 0.22 | 2.71 | 1.55 | 2.12 |
| QC1: % Change | −0.70 | −3.80 | −1.50 | −7.30 | −6.60 |
| QC3: % RSD | 2.92 | 6.14 | 5.47 | 3.34 | 4.06 |
| QC3: % Change | 3.00 | −5.30 | −9.60 | −18.8 | −29.6 |
| Preprocessed stability (3.5 hr) | |||||
| RT: % RSD | 3.68 | 1.82 | 14.5 | 6.52 | 13.1 |
| RT: % Change | −1.70 | −3.60 | 12.0 | −8.20 | −12.9 |
| 37°C: % RSD | 3.53 | 1.52 | 7.34 | 3.7 | 14.3 |
| 37°C: % Change | −0.70 | −3.00 | 8.00 | −10.7 | −19.6 |
| Reinjection stability (23.4 hr) | |||||
| % RSD | 4.88 | 3.82 | 4.56 | 5.73 | 11.1 |
| % AR | 107 | 97.6 | 96.3 | 94.2 | 108 |
| Extracted sample stability (25.7 hr) | |||||
| % RSD | 5.72 | 2.60 | 3.90 | 3.46 | 8.90 |
| % AR | 107 | 95.8 | 94.3 | 90.8 | 80.1 |
| Absolute recovery | |||||
| % RSD | 2.00 | 6.31 | 5.39 | 14.3 | 13.9 |
| % Recovery | 106 | 95.5 | 114 | 87.8 | 89.2 |
AR, analytical recovery; RSD, relative standard deviation; RT, room temperature.
Assay specificity was assessed using both biological matrices and reagent blanks prepared during each validation run. Blank plasma and serum contain low levels of endogenous androgenic compounds despite charcoal-stripping, which limits the achievable LLOQ for the individual androgens. Therefore, the LLOQ was defined as the lowest calibrator concentration having a signal-to-baseline response ratio >3, which was equivalent to on-column masses of 1.08 (T), 4.31 (DHT), 2.18 (ASD), 43.4 (DHEA), and 86.1 fmol (AND). In contrast, non-matrix, water-based extracted samples had on-column masses of 0.349 (T), 0.689 (DHT), 0.349 (ASD), 3.47 (DHEA), and 1.72 fmol (AND) at S/N = 3.
A cross-validation study in charcoal-stripped human serum was conducted in a single validation run and the results were similar to those obtained for plasma. However, large interferences were observed in clinical samples collected in serum separator tubes (SST). SSTs contain a silicone-based gel whose components can leach into whole blood and the resulting plasma during processing. As a result, SSTs cannot be used to collect clinical specimens for quantitation of androgens using this method.
Method Applications
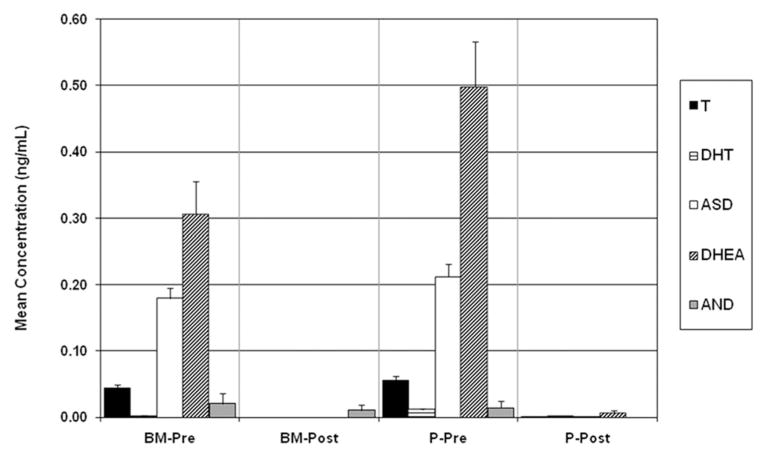
Prior to abiraterone acetate treatment, the mean (±standard error of mean; SEM) androgen concentrations for T, DHT, ASD, DHEA, and AND in plasma were 55.9 (±6.17), 11.7 (±1.93), 212 (±18.2), 498 (±67.3), and 14.6 (±10.5) pg/ml, and in BM aspirates 43.8 (±5.94), 1.62 (±1.15), 179 (±15.3), 306 (±50.1), and 21.1 (±15.1) pg/ml (Fig. 2). Androgen concentrations were undetectable after 8 weeks of treatment. Clinical specimens were analyzed over nine analytical runs using duplicate calibration curves. The precision and accuracy of the QCs were 4.21% and 98.1% for T, 5.88% and 97.2% for DHT, 5.72% and 98.4% for ASD, 4.37% and 94.3% for DHEA, and 11.8% and 95.9% for AND, respectively.
Fig. 2.
Mean androgen concentrations (±SEM) in human plasma (P) and bone marrow (BM) aspirate pre- and post-treatment with abirateroneacetate and prednisone.
Androgen concentrations were also evaluated in 90 plasma samples from newly diagnosed African and Caucasian Americans with CaP from North Carolina and Louisiana. Of the five androgens that were quantitated, only DHEA was statistically higher (Table V) in African Americans compared to Caucasian Americans [t-test, DHEA (87) = 0.00293; P <0.05]. However, samples from this study were stored at −80°C and not immediately quantitated for DHEA or DHEA-sulfate. Therefore, the possibility exists that DHEA-sulfate may have degraded to DHEA during storage to influence the non-sulfated concentrations and statistical outcome of this analyte. Samples for this study were analyzed using duplicate calibration curves and duplicate QCs in each analytical run. The precision and accuracy of the QCs were 2.43% and 102% for T, 4.83% and 101% for DHT, 10.8% and 107% for ASD, 6.82% and 97.2% for DHEA, and 9.19% and 94.7% for AND, respectively.
TABLE V.
Mean Androgen Plasma Concentrations (±SEM) in African Americans (AA) and Caucasian Americans (CA)
| Race | Mean plasma concentration (ng/ml) ± standard error of mean (SEM)
|
||||
|---|---|---|---|---|---|
| T | DHT | ASD | DHEAa | AND | |
| AA | 2.94 ± 0.190 | 0.297 ± 0.0320 | 0.528 ± 0.0411 | 1.62 ± 0.167 | 0.0221 ± 0.00869 |
| CA | 2.77 ± 0.239 | 0.245 ± 0.0251 | 0.527 ± 0.0370 | 0.973 ± 0.116 | 0.0333 ± 0.0124 |
t-Test, DHEA (87) = 0.00293; P < 0.05.
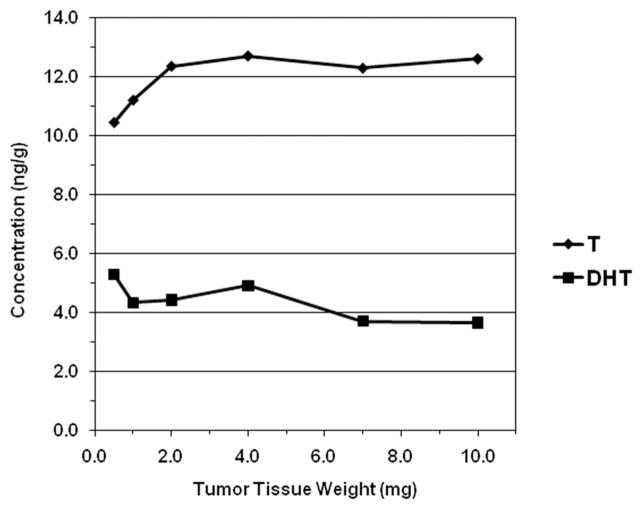
Varying aliquots of human CaP tissue homogenate, functionally representing different weights of tissue matrix, were extracted, and quantitated to determine the minimal tissue sample size required for reproducible results. Observed T, DHT, and AND concentrations ranged 10.4–12.7, 3.65–5.28, and 8.10–27.3 ng/g, respectively (Fig. 3). The procedure was reproducible for T and DHT using a minimum of 1.0–2.0 mg of tumor tissue; however, AND results were more variable.
Fig. 3.
Extraction reproducibility of testosterone (T) and dihydrotestosterone (DHT) from a single prostate tissue sample of varying representative sample weights (1 determination per weight).
Media and cell pellet samples from LNCaP C4-2 treated with 1.0 nM T were analyzed to assess the time-course of conversion of T to DHT by 5α-reductase. The initial media concentration of T was 1.05 nM (302 pg/ml; 3,020 pg/10 ml culture; 10.5 pmol). The measured T and DHT concentrations in the 36-hr cell pellet sample were 148 pg/ml (0.513 pmol; 4.9%) and 20.4 pg/ml (0.0702 pmol; 0.7%), respectively.
DISCUSSION
Normal human plasma contains endogenous levels of androgens that prevents its use as a bioanalytical matrix for the preparation of calibration and QC samples without preliminary treatment. Some investigators [18,21,22] have used bovine serum albumin (BSA) to prepare calibration and QC samples, but BSA alone does not challenge an analytical method due to absence of endogenous compounds that complicate the chromatography, produce ionization suppression, or are long-retained causing overlapping responses in subsequent injections. In this study, double charcoal-stripped female plasma and serum were used, which provided sufficiently reduced levels of endogenous androgens to prepare matrix standards and QCs at concentrations required to measure both physiologic and castrate levels of androgens. These matrices also provided the necessary complexity to adequately evaluate assay performance of in vivo and in vitro samples. However, despite double charcoal-stripping, low levels of androgens remain in these matrices, which varies from batch to batch and affects the attainable LLOQ. This requires the background of each matrix lot to be assessed prior to preparing calibrators and QCs. Serum generated by the standard clotting procedure followed by charcoal stripping was similar to plasma; however, serum collected in SST contained significant interferences from the silicone gel making these devices unusable to collect research specimens for analysis.
The developed method uses a switching valve and an additional guard column prior to the analytical column for sample cleanup. After sample injection, eluate from the first guard column was directed to the analytical column for 1.0 min, at which time it was switched out-of-line. This process minimized accumulation of long-retained and/or ion-suppressing compounds on the analytical column, and eliminated the need for an extended gradient wash between injections. Chromatographic separation continued on the analytical column at the same time the first guard column was washed and re-equilibrated using a secondary pump. Eluate from the analytical column was directed to the mass spectrometer from 4.5 to 9.5 min while eluate before and after this time period was directed to waste. Analytical throughput was 80 samples per 21.4 hr.
The quantitation was linear over different concentration ranges for the various androgens based on differences in their ionization and fragmentation profiles. Five of six androgens had acceptable validation accuracy and precision results, but data for 5α-ASD were too variable to pass acceptance criteria. The validation precision and accuracy results were acceptable; however, low concentrations of DHT or AND were found to be unstable during three-cycle freeze–thaw studies, and low concentrations of AND were sensitive to elevated temperature (37°C at 3.5 hr). Therefore, sample processing should limit freeze–thaw cycles to less than 3, and reduce exposure to elevated temperatures during plasma processing. With regard to 5α-ASD, inadequate chromatographic resolution may be a contributing factor since studies with cell pellet and media samples subsequent to the validation demonstrated that epi-T, the 17α-epimer of testosterone, elutes at almost the same retention time as 5α-ASD. Since the two compounds have the same molecular weight and similar fragmentation patterns, co-elution could introduce sample-to-sample variability. Additional method development may improve resolution of these compounds and allow incorporation of both epi-T and 5α-ASD into a future modification of the assay.
In the first application of the method, plasma and BM samples were analyzed from patients who had bone metastases and serum T levels <50 ng/dl. Thirty-six of the 56 enrolled patients had prior ADT therapy with estrogens and/or ketoconazole as described [12]. Plasma samples had one detectable T and two detectable DHT concentrations during the 8th week of treatment [12]. In addition to these reported concentrations, only one sample had detectable DHEA at 8 weeks treatment, and none of the plasma samples had quantifiable ASD or AND concentrations after initiation of treatment. For BM aspirates, concentrations of T and DHT were undetectable for all patients after initiation of treatment [12]. ASD and DHEA also were undetectable, whereas AND was detectable in two samples at 8 weeks. After the 8-week time point, all plasma and BM sample concentrations were below the LLOQ for the remainder of the study (945 days). These data are consistent with abiraterone acetate’s mechanism of action of inhibiting CYP17A1, which reduces T biosynthesis in the testes, adrenal glands and CaP cells.
In the racial comparison study of 90 individuals with CaP, DHEA was the only androgen that demonstrated a statistical difference between African and Caucasian Americans. The results for T agree with those of Kubricht et al. [26] who reported no difference in serum T concentrations in men of either race who underwent prostate biopsies. Mohler et al. [27] demonstrated no racial differences in T or DHT levels in localized CaP tissue, but African Americans had higher prostate tissue ASD levels than Caucasian Americans.
The third application was a feasibility study to assess assay reproducibility and determine minimum sample size required for analysis of prostate biopsy core samples, which generally range in size from 5 to 10 mg, with an ultimate goal of analyzing laser capture microdissection samples (50–100 μg). The results demonstrated reproducibility for specimens down to 1.0–2.0 mg for both T and DHT, which is well within the weight of prostate biopsy samples. Concentrations (n = 1) observed in this study for the 1.0 and 2.0 mg tissue quantities were 11.2 and 24.7 ng/g for T, and 4.33 and 8.84 ng/g for DHT, respectively. The results for AND were variable, possibly due to its more lipophilic nature. Additional studies are required to enhance accuracy and precision. Although the T and DHT results are acceptable for prostate biopsy samples, further improvement in sensitivity will be required to measure microdissected samples.
The last study analyzed media and cell pellet samples from the LNCaP C4-2 cell line. Only two of five androgens were detected intracellularly in cells grown for 36 hr: 0.513 pmol T and 0.0702 pmol DHT. The corresponding 36-hr media sample was not analyzed so no mass balance calculations were possible.
CONCLUSIONS
The continued investigation of androgenic pathways, and the development of appropriate models to characterize and monitor androgen-stimulated gene expression in various disease states, require improved analytical methods for more accurate, reproducible, and sensitive quantitation of androgens. The reported assay was designed with appropriate surrogate matrices for quantitation of five androgens (T, DHT, ASD, DHEA, and AND) and successfully validated for accuracy, precision, linearity, sensitivity, selectivity, stability, and recovery. The simple liquid/liquid extraction and use of a second switching valve were effective in sustaining high daily throughput. Application to various sample types showed that plasma and BM aspirates from patients treated with abiraterone acetate had no detectable androgen levels after 8 or more weeks of treatment; African and Caucasian Americans only demonstrated a discernible difference in plasma DHEA concentrations; a minimal sample size of ~1.0–2.0 mg is required for analysis of prostate biopsy samples; and LNCaP C4-2 cell pellet/media samples showed metabolism of T to DHT.
Acknowledgments
Grant sponsor: Department of Defense; Grant number: DAMD 17-03-2-0052; Grant sponsor: Department of Defense Prostate Cancer Research Program; Grant numbers: W81XWH-10-1-0273; W81XWH-10-1-1000; Grant sponsor: PCF Challenge Award; Grant sponsor: National Cancer Institute; Grant number: NCI-CCSG-CA016056.
The North Carolina—Louisiana Prostate Cancer Project (PCaP) is carried out as a collaborative study supported by the Department of Defense contract DAMD 17-03-2-0052. The research was also funded in part by Department of Defense Prostate Cancer Research Program Grants W81XWH-10-1-0273 (M.A.T.) and W81XWH-10-1-1000 (J.L.M.), PCF Challenge Award (M.A.T. and E.E.), and Grant NCI-CCSG-CA016056 (Roswell Park Cancer Institute). Gary J. Smith, Ph.D., Roswell Park Cancer Institute, reviewed the manuscript and provided many helpful comments. The authors thank the staff, advisory committees, and research subjects participating in the PCaP study for their important contributions. We would like to acknowledge the UNC BioSpecimen Facility and the LSUHSC Pathology Lab for our DNA extractions, blood processing, storage, and sample disbursement (https://genome.unc.edu/bsp).
Footnotes
Conflicts of interest: No potential conflicts of interest were disclosed.
References
- 1.Siegel R, Ma J, Zou Z. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Prostate Cancer Foundation; Santa Monica, CA: Website www.pcf.org. [Google Scholar]
- 3.Mohr B, Guay A, O’Donnell A, McKinlay J. Normal, bound and nonbound testosterone levels in normally ageing men: Results from the Massachusetts Male Ageing Study. Clin Endocrinol. 2005;62:64–73. doi: 10.1111/j.1365-2265.2004.02174.x. [DOI] [PubMed] [Google Scholar]
- 4.Practice Committee of the American Society for Reproductive Medicine. Androgen deficiency in the aging male. Fertil Steril. 2008;90(3):S83–S87. doi: 10.1016/j.fertnstert.2008.08.094. [DOI] [PubMed] [Google Scholar]
- 5.Dehm S, Tindall D. Androgen receptor structural and functional elements: Role and regulation in prostate cancer. Mol Endocrinol. 2007;21:2855–2863. doi: 10.1210/me.2007-0223. [DOI] [PubMed] [Google Scholar]
- 6.Deslypere J, Young M, Wilson J, McPhaul M. Testosterone and 5-alpha-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol Cell Endocrinol. 1992;88:15–22. doi: 10.1016/0303-7207(92)90004-p. [DOI] [PubMed] [Google Scholar]
- 7.Thompson I, Goodman P, Tangen C, Lucia M, Miller G, Ford L, Lieber M, Cespedes D, Atkins J, Lippman S, Carlin S, Ryan A, Szczepanek C, Crowley J, Coltman C. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 8.Andriole G, Bostwick D, Brawley O, Gomella L, Marberger M, Montorsi F, Pettaway C, Tammela T, Teloken C, Tindall D, Somerville M, Wilson T, Fowler I, Rittmaster R. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 9.Heracek J, Hampl R, Hill M, Starka L, Sachova J, Kuncova J, Eis V, Urban M, Mandys V. Tissue and serum levels of principal androgens in benign prostatic hyperplasia and prostate cancer. Steroids. 2007;72(4):375–380. doi: 10.1016/j.steroids.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Mohler J, Gregory C, Ford O, Kim D, Weaver C, Petrusz P, Wilson E, French F. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 11.Titus M, Schell M, Lih F, Tomer K, Mohler J. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11(13):4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 12.Efstathiou E, Titus M, Tsavachidou D, Tzelepi V, Wen S, Hoang A, Molina A, Chieffo N, Smith L, Karlou M, Troncoso P, Logothetis C. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30:637–643. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bono J, Logothetis C, Molina A, Fizazi K, North S, Chu L, Chi K, Jones R, Goodman O, Saad F, Staffurth J, Mainwaring P, Harland S, Flaig T, Hutson T, Cheng T, Patterson H, Hainsworth J, Ryan C, Sternberg C, Ellard S, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq C, Scher H. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lih F, Titus M, Mohler J, Tomer K. Atmospheric pressure photoionization tandem mass spectrometry of androgens in prostate cancer. Anal Chem. 2010;82:6000–6007. doi: 10.1021/ac100460x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Titus M, Tomer KB. Androgen quantitation in prostate cancer tissue using liquid chromatography tandem mass spectrometry. Methods Mol Biol. 2011;776:47–57. doi: 10.1007/978-1-61779-243-4_3. [DOI] [PubMed] [Google Scholar]
- 16.Kushnir M, Rockwood A, Roberts W, Pattison E, Owen W, Bunker A, Meikle A. Development and performance evaluation of a tandem mass spectrometry assay for 4 adrenal steroids. Clin Chem. 2006;52(8):1559–1567. doi: 10.1373/clinchem.2006.068445. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien Z, Post N, Brown M, Madan A, Coon T, Luo R, Kokout T. Validation and application of a liquid chromatography-tandem mass spectrometric method for the simultaneous determination of testosterone and dihydrotestosterone in rat prostatic tissue using a 96-well format. J Chromatogr B. 2009;877(29):3515–3521. doi: 10.1016/j.jchromb.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 18.McNamara K, Harwood D, Simanainen U, Walters K, Jimenez M, Handelsman D. Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol. 2010;121(3–5):611–618. doi: 10.1016/j.jsbmb.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Cruz M, Lopez de Alda M, Lopez R, Barcelo D. Determination of estrogens and progestogens by mass spectrometric techniques (GC/MS, LC/MS and LC/MS/MS) J Mass Spectrom. 2003;38:917–923. doi: 10.1002/jms.529. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Catlin D, Demers L, Starcevic B, Swerdloff R. Measurement of total serum testosterone in adult men: Comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89(2):534–543. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]
- 21.Guo T, Taylor R, Singh R, Soldin S. Simultaneous determination of 12 steroids by isotopic dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clin Chim Acta. 2006;372:76–82. doi: 10.1016/j.cca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 22.Guo T, Chan M, Soldin S. Steroid profiles using liquid chromatography-tandem mass spectrometry with atmospheric pressure photoionization source. Arch Pathol Lab Med. 2004;128:469–475. doi: 10.5858/2004-128-469-SPULCM. [DOI] [PubMed] [Google Scholar]
- 23.Schulman C, Irani J, Morote J, Schalken J, Montorsi F, Chlosta P, Heidenreich A. Testosterone measurements in patients with prostate cancer. Eur Urol. 2010;58:65–74. doi: 10.1016/j.eururo.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Thalmann G, Anezinis P, Chang S, Zhau H, Kim E, Hopwood V, Pathak S, von Eschenbach A, Chung L. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54(10):2577–2581. [PubMed] [Google Scholar]
- 25.Wu H, Hsieh J, Gleave M, Brown N, Pathak S, Chung L. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: Role of bone stromal cells. Int J Cancer. 1994;57(3):406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 26.Kubricht W, Williams B, Whatley T, Pinckard P, Eastham J. Serum testosterone levels in African-American and white men undergoing prostate biopsy. Urology. 1999;54:1035–1038. doi: 10.1016/s0090-4295(99)00290-3. [DOI] [PubMed] [Google Scholar]
- 27.Mohler J, Gaston K, Moore D, Schell M, Cohen B, Weaver C, Petrusz P. Racial differences in prostate androgen levels in men with clinically localized prostate cancer. J Urol. 2004;171:2277–2280. doi: 10.1097/01.ju.0000127739.88383.79. [DOI] [PubMed] [Google Scholar]