Abstract
Orexins (hypocretins) are two peptides (orexin A and B) produced from the pre-pro-orexin precursor and expressed in a limited region of dorsolateral hypothalamus. Orexins were originally thought to specifically mediate feeding and promote wakefulness, but it is now clear that they participate in a wide range of behavioral and physiological processes under select circumstances. Orexins primarily mediate behavior under situations of high motivational relevance, such as during physiological need states, exposure to threats or reward opportunities. We hypothesize that many behavioral functions of orexins (including regulation of sleep/wake cycling) reflect a fundamentally integrated function for orexins in translating motivational activation into organized suites of psychological and physiological processes supporting adaptive behaviors. We also discuss how numerous forms of neural heterogeneity modulate this function, allowing orexin neurons to organize diverse, adaptive responses in a variety of motivationally relevant situations. Thus, the involvement of orexins in diverse behaviors may reflect a common underlying function for this peptide system.
The essence of the beautiful is unity in variety. —Moses Mendelssohn
Since their simultaneous discovery by two groups1,2, orexin/hypocretin neurons have been implicated in numerous functions, including regulating sleep/wake cycles, arousal, reward seeking, cognition, motor activity and stress responses, and influencing cardiovascular, respiratory, metabolic and homeostatic functions. Such functional diversity is not uncommon in the hypothalamus, which is well known for integrating neural and hormonal signals to promote survival-related behaviors. Orexin neurons also project throughout the CNS, suggesting a potentially widespread influence of this restricted neuronal population. What does this apparent diversity of function signify about the roles for orexin neurons in behavior?
Here, we first briefly summarize the major known functions of orexins (based on animal studies and orexin-deficient human populations) and argue that the orexins’ role in each involves ‘motivational activation’. That is, motivation (the psychological drive underlying goal-directed behavior) is facilitated by orexins during specific environmental challenges or opportunities. Orexin-facilitated motivation is recruited by homeostatic signals of physiological disequilibrium and/or exposure to important environmental stimuli, especially when they are relevant to a current physiological need. We propose that orexins help to transform motivational activation into adaptive behavior directed toward managing/exploiting the challenge/opportunity at hand. They do so by facilitating suites of psychological processes (for example, appetitive or aversive motivation) and physiological processes (for example, cardiovascular activation, suppression of sleep, etc.), all facilitating adaptive behaviors—a variety of responses in service of this unified underlying function. We also suggest that disruption of this integrated orexin function substantially contributes to sleep/wake disturbances seen in narcolepsy/cataplexy (NC). Finally, we discuss how heterogeneity and plasticity among orexin neurons, their afferents, and orexin and co-transmitter signaling in efferent target structures modulate this integrative role for orexins, facilitating appropriate behaviors in many different situations.
The many faces of orexin/hypocretin function
Arousal and sleep/wake transitions
Perhaps the best-known function of orexins is in promoting arousal and waking from sleep. Orexin administration lengthens, and orexin disruption shortens, continuous periods of wakefulness3–5, and loss of orexin neurons or receptors are associated with NC in rodents, dogs and humans4,6–8. Orexin projections excite wake-promoting systems, including locus coeruleus (LC) norepinephrine, dorsal raphe serotonin, tuberomammillary histamine and basal forebrain (BF)/brainstem acetylcholine neurons4,5,9–11. Orexin neurons are more active during wake than sleep, frequently fire just before waking and cause waking within several seconds when optogenetically stimulated3,7,12,13.
However, rather than acting simply as an arousal on/off switch, the orexin system’s role in wakefulness may be more akin to that of its ascending modulatory system targets (for example, dopamine, norepinephrine). Similar to orexin neurons, these systems are active during wakefulness, and stimulating them induces waking from sleep, but this does not imply that they primarily function to cause arousal14,15. Indeed, loss of orexin function does not result in loss of waking, nor does it cause changes in the total daily time spent in sleep versus wake6,16. Instead, humans and animals with NC exhibit unusual intrusions of sleep in the active period and wake in the rest period5,6,16,17.
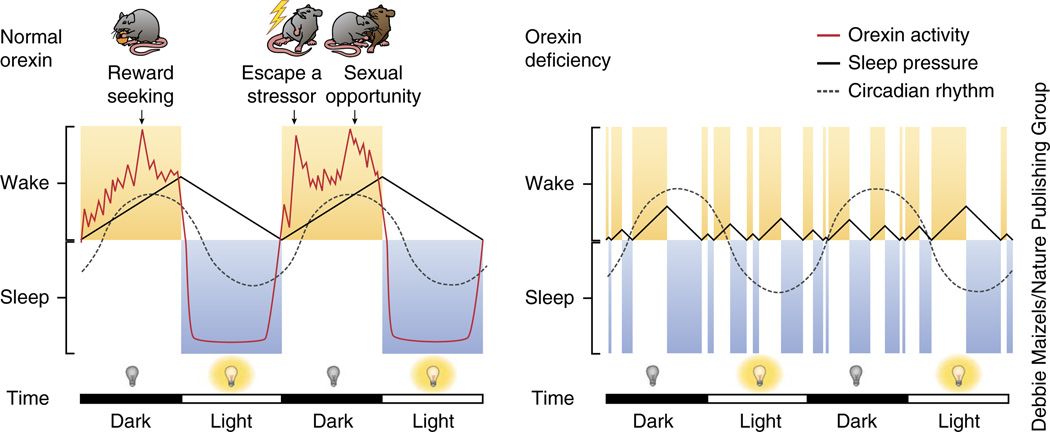
We propose that this results from a role for orexins in motivational activation, superimposed on a circadian oscillation in orexin neuron excitability. The orexin system exhibits two arousal-related patterns of activity: long-term circadian oscillations in orexin release/cerebrospinal fluid levels and phasic, behavior-related bursts of orexin neuron firing. Orexin circadian rhythmicity is controlled closely (though likely indirectly) by the suprachiasmatic nucleus (SCN), which causes basal excitation of orexin neurons during the active period6,11. Orexin neuron firing also varies substantially throughout wakefulness, with both tonic activity13,18 and rapid firing specifically during periods of adaptive behavior, such as exploration, play, predation and grooming, but not during other behaviors matched for physiological arousal and locomotor activity12,19,20. This is consistent with the role we propose for orexins in the motivational processes underlying these adaptive behaviors (associated with phasic firing), which is overlaid on circadian oscillations in tonic activity, excitability or peptide release (Fig. 1).
Figure 1.
Model of orexins’ functions in sleep/wake regulation. Left, sleep/wake activity is homeostatically controlled, with sleep pressure (black solid line) increasing as a function of time awake and subsiding gradually over time asleep until sufficiently reduced to allow waking. Conversely, wakefulness is driven by a circadian signal (dashed gray line) that activates orexins and other arousal systems3,4,6,10,25. Orexin neuron activity (red line) has a circadian pattern, but also phasic bursts during waking as a function of motivational state and adaptive behavior. Orexins excite wake-active (for example, LC and dorsal raphe) and inhibit sleep-active brain regions (for example, medullary REM muscle atonia circuit), so discharge of orexin neurons during wakefulness helps to counteract sleep pressure and decrease the probability of sleep initiation, especially during emotionally arousing situations4,11,19. For purposes of clarity, sleep and wake periods have been depicted as uninterrupted, although rodents typically display multiple sleep/wake transitions in both light and dark phases. Right, in orexin-deficient subjects (for example, NC humans, or animal models of NC), even relatively low levels of unopposed sleep pressure can result in inappropriate intrusions of sleep, regardless of circadian phase or motivational state. Conversely, lower peaks of homeostatic sleep pressure (resulting from decreased wake epoch durations) result in shorter periods of recovery sleep to regain homeostasis, allowing inappropriate intrusion of waking in the rest phase. This mechanism might account for rapid sleep/wake transitions seen in narcoleptics and orexin-deficient animals.
These roles for orexins in motivated behaviors and in arousal are mutually supportive, as adaptive, motivated behavior necessarily requires wakefulness. We propose that SCN-dependent tonic excitation during the active period not only increases orexin baseline firing and release6,11, but also increases the ability of other excitatory inputs to activate orexin neurons21, potentiating phasic firing during adaptive behaviors in vivo12. Thus, we hypothesize that both SCN-dependent tonic excitation and phasic orexin neuron burst firing help to produce and maintain wakefulness—the latter as a consequence of increased motivation to engage adaptive challenges or opportunities. This is consistent with the view that orexins prolong arousal in the service of managing environmental threats or opportunities4,10,19,22–24.
The orexin system’s ability to promote wakefulness is opposed by a homeostatic sleep pressure signal that accumulates in a linear fashion with wakefulness duration. Such sleep pressure increases until the onset of sleep, and subsides during sleep until reaching a nadir, when waking can occur25. Notably, orexins compete with sleep pressure to control activity in sleep- and wake-generating brain circuits6,11,26. For example, optogenetic orexin neuron stimulation that normally produces waking fails to do so after induction of sleep pressure via sleep deprivation7 (although rebound sleep after deprivation is fragmented in orexin knockout mice, despite accumulated sleep pressure16).
In NC humans or animals, an inability of circadian or motivation-related orexin activity to oppose homeostatic sleep pressure and maintain wakefulness can explain the deconsolidation of both waking and sleep seen in the disorder, in accordance with the two-process model of sleep-wake regulation25 (Fig. 1). This hypothesis explains dysregulated sleep/wake cycling associated with orexin neuron loss in NC, but does not explain cataplexy symptoms. How cataplexy relates to motivational or arousal functions of the orexin system is controversial, but a link to emotional arousal is clear7,11,19,23,27–29 (Box 1).
Box 1. What can NC tell us about orexin function?
Key observations
Cataplexy involves brief episodes of muscle atonia (paralysis) with maintained consciousness, often triggered by strong positive emotional stimuli in humans and animals3,6,8,11,28,29.
Orexin cerebrospinal fluid levels in NC are very low or absent, but this is not necessarily the case in narcolepsy without cataplexy6,17.
NC-like symptoms occur with orexin disruption in humans, dogs and rodents. Both OX1 and OX2 receptors, as well as co-transmitters lost with orexin cells, may be involved in NC4,8,68.
Mounting evidence indicates that an autoimmune response causes orexin neuron loss in human NC69.
NC patients show abnormal emotional processing and decision-making, likely involving amygdala17,70–72, and orexin is released in human amygdala during positive emotions73. mPFC and amygdala are required for emotional stimuli to induce cataplexy in orexin knockout mice28,29.
NC patients are often prescribed stimulant or sedative drugs for sleep disturbances and may be less likely to abuse these drugs4, although additional testing of this hypothesis is needed17.
Muscle atonia in cataplexy shares similarities to atonia in REM sleep, including a common effector circuit involving the medulla and sublaterodorsal nucleus projections to spinal motor neurons11. This indicates that cataplexy may involve inappropriate intrusion of REM sleep features (atonia, hypnogogic hallucinations) into the waking period11. However, it is unclear why emotional activation in particular would trigger intrusions of REM-like features27.
Hypothesis: cataplexy as a feature of orexins’ role in motivational activation
Cataplexy may result from poor coordination of adaptive behaviors during motivational/emotional activation.
To facilitate appropriate responses during threats or opportunities, orexins promote adaptive behaviors and inhibit competing behaviors. Corresponding to this, orexins facilitate top-down control of muscle activity and suppress competing networks, including the medullary-spinal atonia circuit27,74.
In NC and animal models of orexin dysfunction, emotional/motivational activation fails to engage orexin signaling, and atonia circuits are not adaptively inhibited, yielding episodes of cataplexy.
In sum, we propose that, although orexins are closely associated with arousal and wakefulness, they are neither necessary nor sufficient for either. Instead, orexins promote arousal/wakefulness as a component of their role in motivational activation.
Reward seeking
Food intake functions were proposed by researchers that initially described orexins/hypocretins because orexin administration produces feeding, and food deprivation increases orexin mRNA1,2. This hypothesis is also consistent with the fact that this region of hypothalamus is well known to mediate feeding and other appetitive behaviors1,30,31. Subsequent studies demonstrated that orexin neurons are excited by peripheral signals of nutrient need (for example, ghrelin), inhibited by satiety signals (for example, glucose), and interact with feeding peptides to promote food consumption and seeking4,32–35.
However, recent evidence has clarified that the orexin system is not indiscriminately linked to feeding, but instead mediates seeking and consumption of palatable foods, food seeking that is highly motivated by hunger, or seeking that is elicited by Pavlovian cues30,32,34,36. Notably, orexin neurons are active during hunger and help to translate peripheral hunger signals into increased appetitive responding for food and food cues32,34,37,38. Thus, orexins facilitate food seeking and feeding especially in motivationally charged circumstances.
Orexins have a parallel role in motivation for addictive drugs. Our laboratory first demonstrated that orexin neurons respond to drug cues and mediate drug seeking via projections to the ventral tegmental area (VTA)30,31,39,40. Numerous reports have since shown that seeking for all major addictive drugs involves orexin neurons36. Reminiscent of their role in food seeking, orexins are preferentially involved in highly motivated drug seeking (for example, under progressive ratio reinforcement schedules or seeking that is triggered by salient external stimuli such as drug-paired cues or stressors)30,36,39,41–43. Acute drug withdrawal also involves orexins, leading some to speculate that orexin neuron activity during withdrawal facilitates drug seeking to alleviate this specific ‘drug need state’42. In sum, orexins have a crucial, but conditional, role in facilitating reward seeking, primarily when the external world and/or the internal milieu signals a need for highly motivated action.
Stress
Orexins orchestrate various aspects of stress responses, including arousal, attention, anxiety- or panic-like states, and grooming, as well as hypothalamic-pituitary-adrenal axis, cardiovascular and respiratory activation10,44–46. Orexins interact with classical brain stress systems (for example, corticotropin releasing factor (CRF), norepinephrine, extended amygdala)5,7,10 and reside in a part of the hypothalamus associated with fight or flight responses46. They are also required for stress-related aspects of addiction42.
Although the orexin system can promote stress responses, its role is neither exclusive to stress (for example, orexin signaling facilitates non–stress-related reward seeking) nor common to all types of stress. Only behavioral/physiological responses to certain acute stressors are mediated by orexin in rodents, including foot shocks, shock-associated or novel contexts, short-term forced swimming or restraint, food restriction, panic-like states, and social stress31,41,45–47. These stressors all elicit adaptive coping responses, such as escape attempts, conspecific aggression/submission, novelty exploration or freezing in a shock-associated context.
In contrast, chronic, inescapable stressors (such as the types used in animal models of depression) generally yield both cessation of coping responses and downregulation of orexin transmission. For example, acute (but not chronic and predictable) stress is associated with orexin neuron activation41 and chronic stress can disrupt orexin signaling in target regions48. In humans (including NC patients), depression symptoms have been associated with orexin system hypoactivity in some (but not all) studies17,41,46,49. Conversely, increased orexin function is associated with antidepressant effects in chronically stressed animals50.
Thus, increased signaling by orexins may help to organize stress responses, but only when motivated, adaptive coping behaviors are undertaken during potentially escapable stressors. In contrast, when a stressor is chronic, predictable and impossible to escape, orexin system hypoactivity could yield depression-like, amotivational symptoms41,46,51.
Homeostatic regulation
Orexin neurons respond not just to homeostatic feeding signals, but also to signals of several physiological need states. For example, orexin neurons depolarize when blood CO2 levels rise, causing increased respiration via brainstem projections33,44. As with other orexin-dependent behaviors, homeostatic modulation of breathing is conditional, occurring predominantly when animals are awake and active44. Intriguingly, application of CO2 to orexin cells reduces their responsiveness to glucose, meaning that at least some orexin cells respond to multiple homeostatic signals, potentially to help prioritize different behavioral strategies (breathing/feeding) on the basis of need hierarchies33.
Orexins have a similar conditional role in other peripheral physiological functions. For example, the orexin system activates cardiopulmonary responses specifically during motivational activation, and orexins increase peripheral thermogenesis specifically when animals are cold44,52. This sensitivity of orexin neurons to multiple specific needs implies a common role in promoting homeostasis by sensing physiological needs and promoting corresponding adaptive responses to address them.
Cognition: attention, learning and memory
Orexin neurons facilitate cognition, especially attention and certain types of learning53. This is achieved via direct projections to medial prefrontal cortex (mPFC), where orexins increase sustained attention54, and modulation of BF neurons, facilitating attention by causing mPFC acetylcholine release9. However, systemic orexin 1 receptor blockade does not affect attention, instead reducing impulsivity, in a five-choice reaction time task55, consistent with a role for orexins in facilitating appetitive motivation.
Orexins may also have a role in aversive and appetitive learning, although evidence for this is limited and somewhat mixed. Acquisition of Pavlovian fear conditioning involves orexinergic projections to LC56, and orexin neurons are activated via CRF during an unconditioned shock stimulus47. Hippocampal orexins are also required for learning and performance in a Morris water maze task, which involves using spatial memory to escape from forced swimming57. Orexin neurons are required for attentional aspects of motivated learning53, and acquisition of morphine conditioned place preference activates and requires LH orexinergic projections to VTA31 (although orexin 1 receptors are not required for Pavlovian cue/cocaine learning in a self-administration/reinstatement procedure58).
Orexins facilitate attention and some types of learning, but little more is known about their roles in cognition, learning and memory. On the basis of orexin functions in several processes described above, we predict that orexins primarily promote learning and cognition that involves emotionally or motivationally relevant stimuli (for example, fear or place preference conditioning), but not non-emotional/motivational forms of learning (for example, stimulus-response habits or habituation learning).
A unified underlying function for orexin neurons?
We have summarized some of the primary behavioral and physiological processes in which orexins participate and note that orexins’ roles are conditional for each process. Orexin neurons are involved in arousal, sleep/wake, homeostatic and metabolic regulation, but these functions vary according to motivational state, sleep pressure, circadian rhythms and other variables. Orexins facilitate reward seeking, but only when this seeking is highly motivated by a physiological need, such as hunger, and/or by a psychological need triggered by substantial external stimuli, such as cues or stressors. Orexins help coordinate stress responses, but only for certain acute stressors in which escape or other coping strategies occur, and not when stress is chronic, predictable and inescapable. Orexins can also facilitate attention, but are only involved in certain types of emotional learning.
We propose that a common theme underlying these diverse processes is recruitment of the orexin system during motivational activation triggered by internal (homeostatic) or external (motivationally relevant) signals of threat or opportunity. We also propose that orexins fundamentally function to facilitate adaptive, often highly motivated behavior by coordinating psychological and physiological responses supporting such behaviors to address the threat or opportunity at hand. However, if orexins function in this integrated manner, heterogeneity at some level must modulate the orexin system to allow coordination of diverse, contextually appropriate behaviors, adding flexibility and variety to orexins’ unified function.
Sources of variety in the orexin system
Heterogeneity in the orexin system depends on at least three factors: diversity of the cells themselves, diversity in their inputs and/or diversity in efferent target responses to orexin neuron activity.
Heterogeneity amongst orexin neurons
Several groups have documented heterogeneity in orexin neuron function based on anatomical position in the mediolateral axis of the orexin field. Orexin neurons medial and dorsal to the fornix (dorsomedial and perifornical hypothalamus) express Fos in relation to wakefulness and aversive stimuli, whereas those lateral to fornix (lateral hypothalamus) are instead activated during conditioned food or drug reward seeking3,31,59, although some studies have not found a clear aversion/reward dichotomy10. Topographical differences could reflect differential connectivity of medial versus lateral orexin cells with wider avoidance- versus approach-related circuits. However, although some connectional specificity has been reported9,60, these differences are frequently subtle10.
Orexin neuron heterogeneity may also result from differential or plastic orexin peptide expression and/or trafficking of orexin A and B, which have different affinities for the two orexin receptors2. In addition, orexin peptide expression is up- or downregulated by many factors, including motivational or physiological state and prior stress or reward exposure, perhaps reflecting epigenetic or transcription factor–based modifications of orexin production50,61. This dynamic modulation of orexin expression implies that the mere identity of a neuron as orexinergic or non-orexinergic could be mutable.
The term orexin neuron also obscures the fact that these neurons coexpress other transmitters that can modulate the synaptic and behavioral effects of orexins. For example, virtually all orexin neurons coexpress the opioid peptide dynorphin55,62. Orexins and dynorphin can be packaged in the same synaptic vesicles and can have directly opposing electrophysiological and behavioral actions (at least in VTA55). However, orexin and kappa opioid receptors are heterogeneously distributed among synaptic compartments and cell types across target regions, yielding substantial heterogeneity in orexin/dynorphin effects33,63. Because most prior experiments have disrupted orexin signaling (for example, with antagonists or receptor/peptide knockout) while sparing dynorphin and other signaling, there is still much to learn about orexin/co-transmitter interactions.
The majority of orexin neurons also contain glutamate, which can signal independently from orexin64. As with other neuropeptides, orexin neurons may signal predominantly with classical transmitters when firing slowly, and peptides such as orexins and dynorphin when firing in a rapid bursting pattern64. This could imply functionally distinct slow- and fast-firing modes for orexin neurons, as previously proposed7.
In addition, at least two subpopulations of orexin neurons (termed H and D neurons) exist33. These subtypes vary in their anatomical and electrophysiological characteristics, but not in their likelihood of projecting to LC or VTA or their expression across the mediolateral extent of the orexin field. This newly discovered diversity is intriguing, but its functional consequences are not yet clear.
Heterogeneity in orexin inputs
Several factors can influence how and when orexin neurons are recruited during behavior. First, orexin neuron afferents may selectively target subsets of orexin neurons, potentially allowing for differential recruitment of functional subpopulations of orexin neurons59,60. Signals of diverse physiological and psychological needs also regulate orexin neuron activity, and some orexin neurons respond to multiple such signals (for example, CO2 and glucose33). However, heterogeneity in receptor expression also exists. For example, ~60% of orexin neurons express CRF receptors, about 25% respond to CRF in vitro47 and glucose responses are similarly variable33,35. In addition, motivationally important experiences such as cocaine exposure, food restriction or sleep deprivation elicit plasticity in excitatory inputs to orexin neurons, indicating that stimuli capable of recruiting orexin neurons change on the basis of learning and motivational state41,65. However, there remains much to be learned about the contribution of input heterogeneity and plasticity in orexin functions (Box 2).
Box 2. Outstanding questions about integration and heterogeneity in the orexin system.
Do subpopulations of orexin cells mediate different aspects of its integrative function in motivational activation?
If so, do these subpopulations differentially connect with wider circuits? Does this functional connectivity relate to medial versus lateral, or other orexin neuron subpopulations?33,59
Orexin neurons show variability and plasticity in their afferent inputs, expression of orexin peptides (A versus B), co-transmitters, receptors and other factors. How is this heterogeneity regulated and how does it relate to other known sources of orexin heterogeneity, such as anatomical connectivity patterns?
How do orexins and their co-transmitters interact and regulate target structure synaptic signaling? Do such interactions vary by target structure and/or is it based on dynamic changes in transmitter production, trafficking and release?
Dynorphin is coexpressed in nearly all orexin neurons and can have opposite effects on synaptic signaling and behavior55,62. Is this ‘push-pull’ feature a fundamental property of orexin signaling? Are orexin/dynorphin peptides or transmitters dynamically regulated to favor predominant signaling by orexins/dynorphin? If so, what are the factors that influence this regulation?
How does orexin neuron firing rate affect orexin function? Do these neurons show clear tonic and phasic firing modes, perhaps related to circadian influences and motivated behaviors, respectively? Do subpopulations of orexin neurons differ in their firing properties?
What is the connection between dysregulated sleep/wake transitioning and cataplexy in narcolepsy with cataplexy? Do these effects of disrupting orexin signaling reflect separate roles for orexin in basal sleep/wake regulation and atonia suppression during emotional activation, or do both result from loss of a common underlying orexin function?
Heterogeneous processing in efferent targets
Orexin function is modulated at the level of efferent targets in several different ways. First, not all orexin neurons project to all regions known to receive orexinergic input. Individual orexin axons are collateralized and frequently target several adjacent structures, but few neurons project both to BF and LC (for example, see ref. 10). This implies that orexins participate in multiple parallel, but anatomically segregated neural circuits. The principles governing this heterogeneity are still largely unknown10,33.
Orexin receptor distribution also determines orexins’ effects in efferent target regions. Orexin peptides act at OX1 and OX2 receptors, which have broad and partially non-overlapping distributions, allowing for target-specific effects of orexin release4. Both receptors can also be expressed pre- or postsynaptically, and although they usually couple to Gq proteins, they can also engage Gs, or Gi pathways33,63. In addition, synaptic and behavioral effects of orexins can be dynamic; for example, orexins can inhibit target neurons via preand postsynaptic mechanisms during day and night, respectively66. Numerous studies have suggested that stimulation of OX1 and OX2 receptors, independently or together, produces different functional results3,4,7,10,36,63. In general, sleep/wake regulation is most closely tied to OX2 receptors, whereas reward-related behaviors are more closely associated with OX1 receptor activation36.
Finally, interaction of orexins with other synaptic activity in target regions also crucially modulates its synaptic and behavioral effects. For example, orexins facilitate glutamatergic transmission via longterm potentiation–like processes in VTA dopamine neurons, and this interaction is required for reinstatement of cocaine seeking22,30,36. We hypothesize that orexins increase the gain of motivation-related glutamatergic inputs to dopamine cells (from mPFC or other sources), facilitating conditioned reward seeking36,40. This neuromodulatory role may be a common principle of orexin signaling36,64,67.
In sum, orexin signaling is complex and heterogeneous and is subject to input and output plasticity modulated by circadian and experiential factors. We propose that such heterogeneity refines the orexin system’s underlying function in motivation, facilitating specific behaviors and physiological processes appropriate for the threat or opportunity at hand.
Integration among multiple orexin functions: unity in variety
Here, we propose that orexins have a fundamentally unified role that spans a variety of processes, including arousal, reward seeking, homeostatic regulation and stress, which we term motivational activation. This underlying function facilitates appetitive or aversive motivation and related physiological responses in service of producing behaviors to address pressing threats or opportunities.
However, if orexins have an integrative role as we have proposed, this must be embedded in heterogeneous functional connectivity with wider circuits for orexins to generate diverse behaviors. Heterogeneity in orexin cell subpopulations and their inputs, as well as synaptic consequences of orexin transmission in efferent targets, may therefore result in parallel information streams, each involving orexins in a qualitatively similar manner, but differentially interacting with separate wider circuits (for example, of approach versus avoidance). A tremendous amount has been learned about the functions and properties of orexin neurons since their discovery 16 years ago, and rapid development of neuroscience tools7,12,23,48,50,66,68 gives hope for answering many remaining questions about this deceptively complex peptide (Box 2).
ACKNOWLEDGMENTS
We thank E. Vought for assistance with figure art and design, and A. Koller for helpful comments. Funding was provided by US National Institutes of Health grants F32 DA026692, K99 DA035251, R01-DA006214, P50 DA015369, R21 DA037744, R21 DA032005 and C06 RR015455, and National Health and Medical Research Council CJ Martin Fellowship 1072706.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.de Lecea L, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein–coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Alexandre C, Andermann ML, Scammell TE. Control of arousal by the orexin neurons. Curr. Opin. Neurobiol. 2013;23:752–759. doi: 10.1016/j.conb.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat. Rev. Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 5.Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- 6.Mignot E. Sleep, sleep disorders and hypocretin (orexin) Sleep Med. 2004;5(suppl. 1):S2–S8. doi: 10.1016/s1389-9457(04)90001-9. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Hu Z, de Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. Br. J. Pharmacol. 2014;171:332–350. doi: 10.1111/bph.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel JM. Narcolepsy: a key role for hypocretins (orexins) Cell. 1999;98:409–412. doi: 10.1016/s0092-8674(00)81969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fadel J, Burk JA. Orexin/hypocretin modulation of the basal forebrain cholinergic system: role in attention. Brain Res. 2010;1314:112–123. doi: 10.1016/j.brainres.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berridge CW, Espana RA, Vittoz NM. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J. Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 16.Mochizuki T, et al. Behavioral state instability in orexin knock-out mice. J. Neurosci. 2004;24:6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayard S, Dauvilliers YA. Reward-based behaviors and emotional processing in human with narcolepsy-cataplexy. Front. Behav. Neurosci. 2013;7:50. doi: 10.3389/fnbeh.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 19.Chase MH. A unified survival theory of the functioning of the hypocretinergic system. J. Appl. Physiol. 2013;115:954–971. doi: 10.1152/japplphysiol.00700.2012. [DOI] [PubMed] [Google Scholar]
- 20.Wu MF, Nienhuis R, Maidment N, Lam HA, Siegel JM. Cerebrospinal fluid hypocretin (orexin) levels are elevated by play but are not raised by exercise and its associated heart rate, blood pressure, respiration or body temperature changes. Arch. Ital. Biol. 2011;149:492–498. doi: 10.4449/aib.v149i4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appelbaum L, et al. Circadian and homeostatic regulation of structural synaptic plasticity in hypocretin neurons. Neuron. 2010;68:87–98. doi: 10.1016/j.neuron.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson JL, Borgland SL. A role for hypocretin/orexin in motivation. Behav. Brain Res. 2011;217:446–453. doi: 10.1016/j.bbr.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding and motivation. Front. Behav. Neurosci. 2013;7:28. doi: 10.3389/fnbeh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao XB, Horvath T. Function and dysfunction of hypocretin/orexin: an energetics point of view. Annu. Rev. Neurosci. 2014;37:101–116. doi: 10.1146/annurev-neuro-071013-013855. [DOI] [PubMed] [Google Scholar]
- 25.Borbély AA. A two process model of sleep regulation. Hum. Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 26.Zeitzer JM, et al. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J. Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overeem S, Lammers GJ, van Dijk JG. Cataplexy: ‘tonic immobility’ rather than ‘REM-sleep atonia’? Sleep Med. 2002;3:471–477. doi: 10.1016/s1389-9457(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 28.Oishi Y, et al. Role of the medial prefrontal cortex in cataplexy. J. Neurosci. 2013;33:9743–9751. doi: 10.1523/JNEUROSCI.0499-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess CR, Oishi Y, Mochizuki T, Peever JH, Scammell TE. Amygdala lesions reduce cataplexy in orexin knock-out mice. J. Neurosci. 2013;33:9734–9742. doi: 10.1523/JNEUROSCI.5632-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borgland SL, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J. Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 32.Berthoud HR, Munzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol. Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burdakov D, Karnani MM, Gonzalez A. Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control. Physiol. Behav. 2013;121:117–124. doi: 10.1016/j.physbeh.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cason AM, et al. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol. Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng Z, Santiago AM, Thomas MP, Routh VH. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Mol. Cell. Neurosci. 2014 Aug 6; doi: 10.1016/j.mcn.2014.08.001. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog. Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamanaka A, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 38.Perello M, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol. Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calipari ES, Espana RA. Hypocretin/orexin regulation of dopamine signaling: implications for reward and reinforcement mechanisms. Front. Behav. Neurosci. 2012;6:54. doi: 10.3389/fnbeh.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl.) 2013;226:687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV. Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Front. Neurosci. 2014;8:36. doi: 10.3389/fnins.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boutrel B, Steiner N, Halfon O. The hypocretins and the reward function: what have we learned so far? Front. Behav. Neurosci. 2013;7:59. doi: 10.3389/fnbeh.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchant NJ, Millan EZ, McNally GP. The hypothalamus and the neurobiology of drug seeking. Cell. Mol. Life Sci. 2012;69:581–597. doi: 10.1007/s00018-011-0817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuwaki T, Zhang W. Orexin neurons as arousal-associated modulators of central cardiorespiratory regulation. Respir. Physiol. Neurobiol. 2010;174:43–54. doi: 10.1016/j.resp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Carrive P. Orexin, orexin receptor antagonists and central cardiovascular control. Front. Neurosci. 2013;7:257. doi: 10.3389/fnins.2013.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A. Orexin, stress and anxiety/panic states. Prog. Brain Res. 2012;198:133–161. doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winsky-Sommerer R, et al. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J. Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc. Natl. Acad. Sci. USA. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salomon RM, et al. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol. Psychiatry. 2003;54:96–104. doi: 10.1016/s0006-3223(02)01740-7. [DOI] [PubMed] [Google Scholar]
- 50.Lutter M, et al. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J. Neurosci. 2008;28:3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.James MH, et al. Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats. Front. Behav. Neurosci. 2014;8:244. doi: 10.3389/fnbeh.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J. Neurosci. 2011;31:15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wheeler DS, et al. Role of lateral hypothalamus in two aspects of attention in associative learning. Eur. J. Neurosci. 2014;40:2359–2377. doi: 10.1111/ejn.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J. Neurosci. 2005;25:5225–5229. doi: 10.1523/JNEUROSCI.0719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muschamp JW, et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc. Natl. Acad. Sci. USA. 2014;111:E1648–E1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sears RM, et al. Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proc. Natl. Acad. Sci. USA. 2013;110:20260–20265. doi: 10.1073/pnas.1320325110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akbari E, Naghdi N, Motamedi F. Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav. Brain Res. 2006;173:47–52. doi: 10.1016/j.bbr.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 58.Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur. J. Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J. Comp. Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deutch AY, Bubser M. The orexins/hypocretins and schizophrenia. Schizophr. Bull. 2007;33:1277–1283. doi: 10.1093/schbul/sbm096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chou TC, et al. Orexin (hypocretin) neurons contain dynorphin. J. Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kukkonen JP, Leonard CS. Orexin/hypocretin receptor signaling cascades. Br. J. Pharmacol. 2014;171:314–331. doi: 10.1111/bph.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schöne C, Burdakov D. Glutamate and GABA as rapid effectors of hypothalamic “peptidergic” neurons. Front. Behav. Neurosci. 2012;6:81. doi: 10.3389/fnbeh.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao XB, Horvath T. Function and dysfunction of hypocretin/orexin: an energetics point of view. Annu. Rev. Neurosci. 2014;37:101–116. doi: 10.1146/annurev-neuro-071013-013855. [DOI] [PubMed] [Google Scholar]
- 66.Belle MD, et al. Acute suppressive and long-term phase modulation actions of orexin on the mammalian circadian clock. J. Neurosci. 2014;34:3607–3621. doi: 10.1523/JNEUROSCI.3388-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borgland SL, Ungless MA, Bonci A. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: emerging players in addiction. Brain Res. 2010;1314:139–144. doi: 10.1016/j.brainres.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 68.Tabuchi S, et al. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J. Neurosci. 2014;34:6495–6509. doi: 10.1523/JNEUROSCI.0073-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahlios J, De la Herran-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr. Opin. Neurobiol. 2013;23:767–773. doi: 10.1016/j.conb.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khatami R, Birkmann S, Bassetti CL. Amygdala dysfunction in narcolepsy-cataplexy. J. Sleep Res. 2007;16:226–229. doi: 10.1111/j.1365-2869.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 71.Ponz A, et al. Reduced amygdala activity during aversive conditioning in human narcolepsy. Ann. Neurol. 2010;67:394–398. doi: 10.1002/ana.21881. [DOI] [PubMed] [Google Scholar]
- 72.Morein-Zamir S, Turner DC, Sahakian BJ. A review of the effects of modafinil on cognition in schizophrenia. Schizophr. Bull. 2007;33:1298–1306. doi: 10.1093/schbul/sbm090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blouin AM, et al. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat. Commun. 2013;4:1547. doi: 10.1038/ncomms2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siegel JM, et al. Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla. Science. 1991;252:1315–1318. doi: 10.1126/science.1925546. [DOI] [PMC free article] [PubMed] [Google Scholar]