Abstract
A critical property for tissue adhesives is a controllable degradation rate so that these adhesives do not act as barriers to wound healing. Typical degradation tests require large amount of samples, which can be tedious and expensive to perform. Additionally, current degradation tests are carried out in vitro under simulated physiological conditions and may not accurately reflect the complex environment that an adhesive would experience in vivo. As a means to develop a simple technique for testing tissue adhesive, a rapidly degrading adhesive hydrogel that mimics mussel adhesive proteins was coated onto magnetoelastic (ME) sensor strips to track the degradation of the adhesive remotely and in real time. Adhesive-coated ME sensors were submerged in phosphate buffer saline solution (pH 7.4) at body temperature (37 °C). Based on the change in the resonant amplitude, the degradation time was determined to be 22 min, which was in agreement with qualitative monitoring of the bulk adhesive hydrogel. Additionally, when the adhesive-coated ME sensor was incubated in a slightly acidic medium (pH 5.7), the degradation rate was drastically lengthened (3 hrs) as the hydrolysis of ester bonds is faster under basic conditions. Oscillatory rheological testing confirmed the formation and degradation of the adhesive. However, rheological test results did not accurately reflect the degradation rate of the adhesive hydrogel, potentially due to a slow exchange of acidic degradation products with the surrounding medium. ME sensor was demonstrated as a potential useful tool for evaluating the degradation rate of bioadhesives.
Keywords: Magnetoelastic sensor, Biodegradation, Bioadhesive, Nitrodopamine
1. Introduction
Rapid and effective wound closure remains an important goal of virtually all modern endoscopic and conventional surgical procedures. Additionally, surgical reconnection of injured tissues is essential for restoration of their structure and function. While the discontinuity in soft tissues is traditionally secured with mechanical perforating devices (e.g., sutures, tacks, and staples), these devices are also a source of complications. The application of mechanical devices is inherently traumatic to the surrounding tissues, which can result in neural irritation and persistent pain [1–4] or leakage of bodily fluid [5]. They are also not suitable for repairing tissues with low cohesive properties such as lung, liver, spleen, and kidney.
Tissue adhesives can potentially simplify complex procedures, reduce surgery time, and minimize trauma [6, 7]. However, unlike sutures and other commonly used surgical devices, tissue adhesives can act as a barrier for tissue healing and the union of wound edges. Thus, tissue adhesives must be able to degrade at a rate that approximates the rate of tissue healing for satisfactory wound repair. Traditionally, the evaluation of adhesive degradation involves tracking the mass loss of the adhesive over time [8, 9], which utilizes a large amount of samples. Additionally, experiments carried out in vitro under simulated physiological conditions may not accurately reflect the complex environment and foreign body response that an adhesive would experience in vivo. Currently, there are no accurate way to quantitatively evaluate the degradation of tissue adhesives in vivo.
Metglas 2826MB (Fe40Ni38Mo4B18) has a large magnetoelastic (ME) coupling factor (0.98) and a magnetostriction on the order of 10−5 [10–12]. As a result, when the ME sensor is excited by a magnetic AC field, its vibration frequency and amplitude can be remotely detected by capturing the secondary magnetic field generated by the sensor [13]. An applied mass loading onto the ME sensor causes a shift in the sensor’s resonant frequency and amplitude, which allows them to detect chemical and biological agents [14, 15] and changes in materials viscosity [16, 17]. Most importantly, with proper surface functionalization, the ME sensors remained functional either in cell culture or when they were implanted in vivo to monitor biointerfacial binding events (e.g., cellular attachment and proliferation) [18, 19]. The long-term durability and functionality of the ME sensors in an aqueous and biological environment makes them suitable for monitoring adhesive degradation in real time.
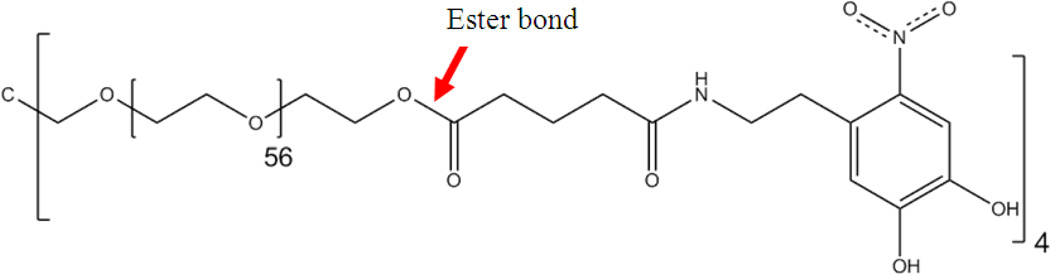
Here, we evaluated the feasibility of using the ME sensor to monitor the degradation of a synthetic adhesive that mimics the proteins secreted by marine mussels. Mussel adhesive proteins (MAPs) enable mussels to adhere tightly to various wet surfaces such as rocks, ships, piers, and other natural or manmade structures [20, 21]. A catechol amino acid, 3,4-dihydroxyphenylalanine (DOPA), is found in large abundance (as much as 25 mol%) in MAPs and is believed to function as a crosslinking precursor and interfacial binding [22]. Various synthetic mimics of MAPs have demonstrated promise in sealing fetal membranes [23, 24], Achilles tendon repair [25], suture-less wound closure [26], immobilization and delivery of therapeutic cells [27, 28], and targeted local delivery of drugs [29]. This study uses a 4-armed poly(ethylene glycol) (PEG) end-modified with glutaric acid (Glu) and nitrodopamine (ND) (Figure 1). PEG and Glu are linked by a hydrolysable ester linkage, and breaking of this bond results in the degradation of the adhesive. ND consists of catechol group that is further modified with an electron withdrawing nitro group, which enhances interfacial binding [30, 31]. Nitro-functionalization also renders the adhesive to degrade at a significantly faster rate as compared to dopamine functionalized adhesive.
Figure 1.
Chemical structure of PEG-(Glu-ND)4.
PEG-(Glu-ND)4 was spin-coated onto ME sensors and its degradation was monitored wirelessly in real time by tracking the changes in the resonant frequency and amplitude. Additionally, oscillatory rheometry was performed to confirm the formation and degradation of the adhesive hydrogel.
2. Experiment Details
2.1. Materials
3,4-dihydroxyphenethylamine hydrochloride (dopamine HCl) and sodium periodate (NaIO4) were obtained from Acros Organics (Geel, Belgium). Diethyl ether (Et2O), magnesium sulfate (MgSO4), phosphate buffered saline (PBS), Tris HCl, chloroform, and N,N'-Dimethylformamide (DMF) were obtained from Fisher Scientific (Fair Lawn, New Jersey). N,N'-dicyclohexyl carboiimide (DCC), N-hydroxysuccinimide (NHS), 4-(dimethylamino) pyridine (DMAP), and pyridine were purchased from Sigma aldrich (St. Louis, MO). Parylene-C was obtained from Specialty Coating Systems Inc, Indianapolis, IN. Metglas 2826MB (Fe40Ni38Mo4B18) was purchased from Metglas, Inc. (Conway, SC) as a continuous ribbon of 12.7 mm in width and 30 µm in thickness. Nitrodopamine hemisulfate [32] and PEG end-modified with glutaric acid (PEG-(Glu)4) [33] were synthesized following previously published protocols.
2.2. Synthesis of PEG-(Glu-ND)4
PEG-(Glu-ND)4 was prepared in two steps: 1) preparation of NHS-activated PEG (PEG-(Glu-NHS)4) and 2) end-modification with nitrodopamine. To synthesize PEG-(Glu-NHS)4, PEG-(Glu)4 (10 g, 0.96 mmol) was dried with azeotropic evaporation of toluene and redissolved in 60 mL of chloroform along with DCC (1.19 g, 5.75 mmol), NHS (1.32 g, 11.5 mmol), and DMAP (46.7 mg, 0.383 mmol). The reaction was stirred for overnight with N2 purging. The insoluble DCC urea was removed through filtration and the filtrate was further added to 800 mL of Et2O. The precipitate was collected through vacuum filtration and dried in a vacuum desiccator to yield 8.3 g of PEG-(Glu-NHS)4. 1H NMR: (400 MHz, D2O) δ 4.16 (t, 2H, PEG-CH2-O-C(=O)-), 3.70–3.37 (m, PEG), 2.82 (s, 4H, NHS –CH2–), 2.69 (t, 2H, PEG-O-C(=O)-CH2-CH2-CH2-C(=O)-NHS), 2.44 (t, 2H, PEG-O-C(=O)-CH2-CH2-CH2-C(=O)-NHS), 1.92 (m, 2H, PEG-O-C(=O)-CH2-CH2-CH2-C(=O)-NHS).
Nitrodopamine hemisulfate (756 mg, 3.06 mmol) in 10 mL of DMF was added to PEG-(Glu-NHS)4 (4.0 g, 0.38 mmol) dissolved in 15 mL each of chloroform and DMF. 1 mL of pyridine (12.4 mmol) was added and the mixture was stirred for overnight with N2 purging. The reaction mixture was washed with saturated NaCl and the organic portion of the mixture was dried over MgSO4. The polymer was further purified by precipitation in Et2O to yield 2.9 g of PEG-(Glu-ND)4. 1H NMR: (400 MHz, D2O) δ 7.47 (d, 1H, ND -C6HH-), 6.62 (s, 1H, ND -C6HH-), 4.08 (t, 2H, PEG-CH2-O-C(=O)-), 3.70–3.30 (m, PEG), 3.25 (t, 2H, C6H2-CH2-CH2-(NH)-C(=O)-), 2.85 (t, 2H, C6H2-CH2-CH2-(NH)-C(=O)-), 2.62 (t, 2H, PEG-O-C(=O)-CH2-CH2-CH2-C(=O)-ND), 2.37 (t, 2H, PEG-O-C(=O)-CH2-CH2-CH2-C(=O)-ND), 1.85 (m, 2H, PEG-O-C(=O)-CH2-CH2-CH2-C(=O)-ND). 93% ND coupling efficiency based on integral values of ND phenyl and Glu methylene peaks.
2.3. Preparing adhesive-coated sensor
ME sensors were prepared by mechanically shearing of the Metglas ribbon into 12.7 mm × 5 mm × 30 µm strips. After cleaning by sonication in ethanol for two minutes, the ME sensor strips were coated with Parylene-C using a parylene deposition system (PDS 2010 Labcoter® 2, Special Coating Systems, Inc.) and then oxygen plasma (200 mTorr) etched (Jupiter II Reactive Ion Etcher, March Instruments) following published protocols [18]. Sensors were further sonicated in ethanol for two minutes, rinsed with deionized (DI) water, and dried. The mass of the sensors and their resonant responses were measured using a customized detector, similar to the one used in [18], to determine the initial resonant frequency.
PEG-(Glu-ND)4 was coated onto the ME sensors using a published protocol with minor modifications [34]. Sensors were first submerged in a 10 mg/mL solution of dopamine HCl in 10 mM Tris-HCl (pH 8.5) for 30 minutes to form a thin polydopamine layer that provide a robust adhesive interface for subsequent covalent attachment of PEG-(Glu-ND)4 [34]. After rinsing with deionized (DI) water and dried with a nitrogen stream, 60 µL of PEG-(Glu-ND)4 (1 wt% in deionized water) was added to surface and allowed to react for one hour. Sensors were then rinsed with deionized water and dried with a nitrogen stream. Once the sensor was pretreated, the bulk of the adhesive was coated using 6 µL of 200 mg/mL of PEG-(Glu-ND)4 in DI water, 6 µL of 10 mM NaIO4, and 12 µL of ethanol were combined onto the sensor surface and spun at 1000–1500 RPM for 3.75 minutes using a Chemat technology KW-4A spin coater. The adhesive-coated sensors were dried and stored under vacuum until use. The presence of the coating was verified by determining the change in mass and the resonant frequency before and after the coating process. Additionally, the surface of the sensors was characterized using FTIR spectroscopy (Perkin Elmer Spectrum One).
2.4. Monitoring the degradation of the adhesive
A PEG-(Glu-ND)4-coated sensor was placed in a vial containing phosphate buffered saline solution (PBS, pH 5.7 or 7.4, 37 °C) and placed in the customized ME sensor detector, which scanned the sensor every ten seconds for 24 hours to record the resonant frequency and amplitude. The sensor detector performed a frequency sweep operation, which involved the sending of AC magnetic fields with increasing frequency, and then monitoring the returned magnetic flux generated by the sensor vibration. The frequency sweep operation yielded a resonance plot, from which the detector determined the resonant frequency (the frequency where the returned field was maximum) and the corresponding signal amplitude (resonant amplitude). Prior to the start of the experiment, the vial containing PBS and the adhesive-coated sensor were placed in the incubator for at least 15 and 5 minutes, respectively, to allow each of them to reach thermal equilibrium at 37 °C. The ME sensor detector used in this project was a portable system that can be placed on a desktop. For in vivo monitoring, a wearable system can be fabricated. The test subject can be put on the wearable system for continuous monitoring of the sensor in vivo.
2.5. Oscillatory rheometry
The viscoelastic properties of the adhesive hydrogels were characterized with a TA Instrument HR-2 rheometer, using a 2 degree cone and plate setup with a diameter of 20 mm. Equal volume (200–300 µL) of 200 mg/mL PEG-(Glu-ND)4 and 30 mM of NaIO4 solutions were added directly onto the rheometer and the cone fixture was lowered to position to conduct experiment immediately after mixing the precursor solutions. Frequency sweep (0.01–100 Hz at 10% strain) experiment was performed to determine both the storage (G’) and loss (G’’) moduli (n = 4) of the samples immediately after curing. A time sweep (1 Hz, 10% strain) experiment was performed to monitor the change in G’ as PEG-(Glu-ND)4 degraded over time (n = 3) and G’ was normalized to the maximum G’ value. To simulate degradation under physiological conditions, the time sweep experiment was performed on a Peltier plate fixture containing 30 mL of PBS (pH 7.4) and heated to 37°C for the duration of the degradation test.
3. Results and Discussion
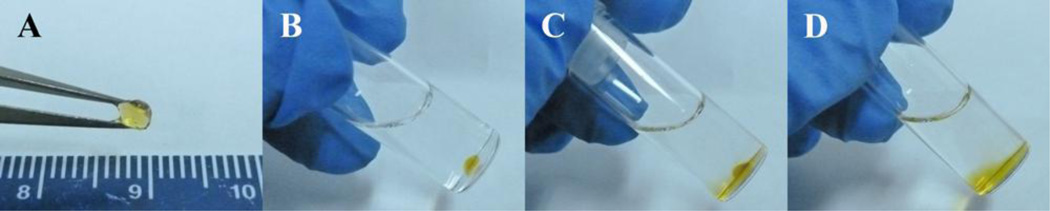
PEG-(Glu-ND)4 was prepared with a nitro-substituted dopamine end group. The presence of an electron withdrawing nitro-functional group lowered the dissociation constant (pKa) of the catechol hydroxyl groups (9.1 and 14 for dopamine and 6.5 and 10.3 for nitrodopamine) and drastically affects its reactivity [31, 35]. Nitrodopamine forms stronger complexes with metal oxides [30, 31] and metal ions [35] when compared to dopamine. When PEG-(Glu-ND)4 was mixed with NaIO4, the polymeric solution rapidly transitioned into a hydrogel network (Figure 2A). When incubated in PBS (pH 7.4) at 37 °C, PEG-(Glu-ND)4 degraded within 23 min (Figure 2B to D). This fast degradation rate was attributed to the addition of the nitro-functional group, as opposed to previously demonstrated results showing that dopamine-modified PEG-(Glu)4 degraded over a period of 2 months when incubated under the same conditions [8]. This extremely fast rate of degradation made it highly difficult to quantitatively track its degradation utilizing traditional approaches (e.g., determining the changes in mass with time).
Figure 2.
Photograph of PEG-(Glu-ND)4 hydrogel immediately after curing (A), and after 0 (B), 10 (C), and 23 (D) min incubation in PBS at 37 °C.
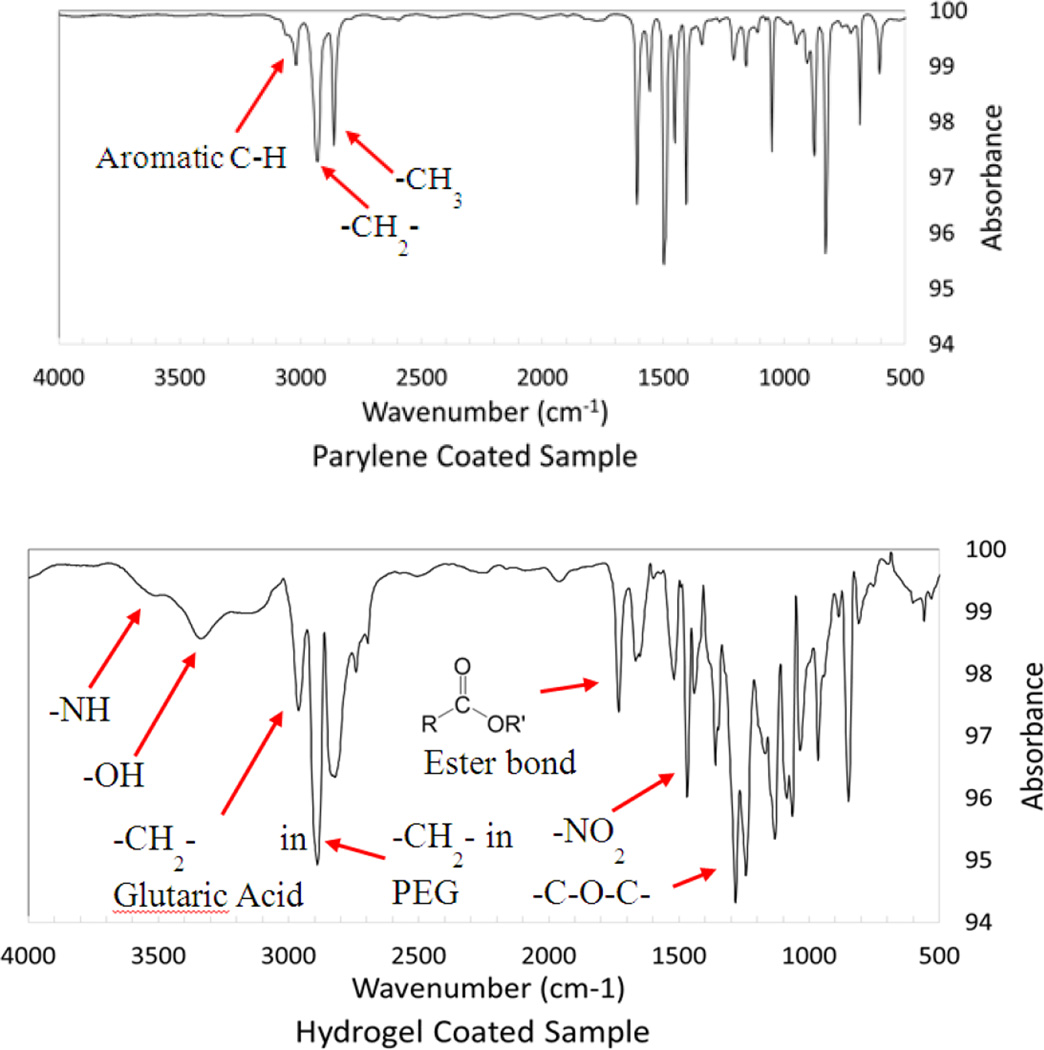
ME sensor strips were first coated with Parylene-C to create a moisture barrier to prevent the sensors from corrosion, which was necessary for testing adhesive degradation under physiological conditions. Parylene-C has been previously used to generate an inert surface for various implantable medical devices such as pacemakers and defibrillators [36]. Additionally, Parylene-C-coated ME sensor strips were previously demonstrated to remain functional both in culture and in vivo [18, 37]. To covalently link the PEG-(Glu-ND)4 network onto the ME sensors, samples were further coated with a polydopamine primer. When dopamine polymerizes in the presence of a surface (e.g., metals, metal oxides, semiconductors, ceramics, and polymers), the polymerized dopamine or polydopamine is coated on these substrates forming a thin layer (5–50 nm) of primer for further surface modification [38]. Finally, PEG-(Glu-ND)4 was formed in situ in the presence of a mild oxidant (NaIO4.). Oxidation of nitrodopamine resulted in the solidification of PEG-(Glu-ND)4 and chemically linked the PEG-(Glu-ND)4 network to the polydopamine layer through catechol-catechol crosslinking [39]. The FTIR spectrum of PEG-(Glu-ND)4 coatings confirmed the presence of PEG ether bonds (-C-O-C- at 1240 cm−1), ester linkage (1710 cm−1), and nitrodopamine (-NO- and –OH at 1570 and 3350 cm−1, respectively) peaks (Figure 3). On average, 1.59 ± 0.47 mg of dried adhesive was coated onto the sensor strips. The addition of the PEG-(Glu-ND)4 reduced the resonant frequency of the sensor from 162.27 ± 1.04 kHz to 157.05 ± 1.14 kHz as a result of increased mass loading from the adhesive coating.
Figure 3.
FTIR of Parylene-C- (top) and hydrogel-coated (bottom) sensors.
The resonant frequency and amplitude of ME sensor strips coated with PEG-(Glu-ND)4 were monitored as the whole sensor was immersed in the test medium. When the adhesive degraded, the mass on the sensor decreased, increasing the resonant frequency. However, at the same time, water was absorbed into the adhesive, increasing the damping on the sensor and causing a slight decrease in the sensor’s resonant frequency. These two competing reactions affect the resonant frequency differently. Therefore, although the resonant frequency was shown to be accurate in tracking the mass loading of a dry coating, it was not suitable for monitoring a coating that was degrading and swelling at the same time. As a result, the degradation of the sensor was monitored by measuring the change in the sensor’s resonant amplitude, which consistently tracked the degradation of the coating over time.
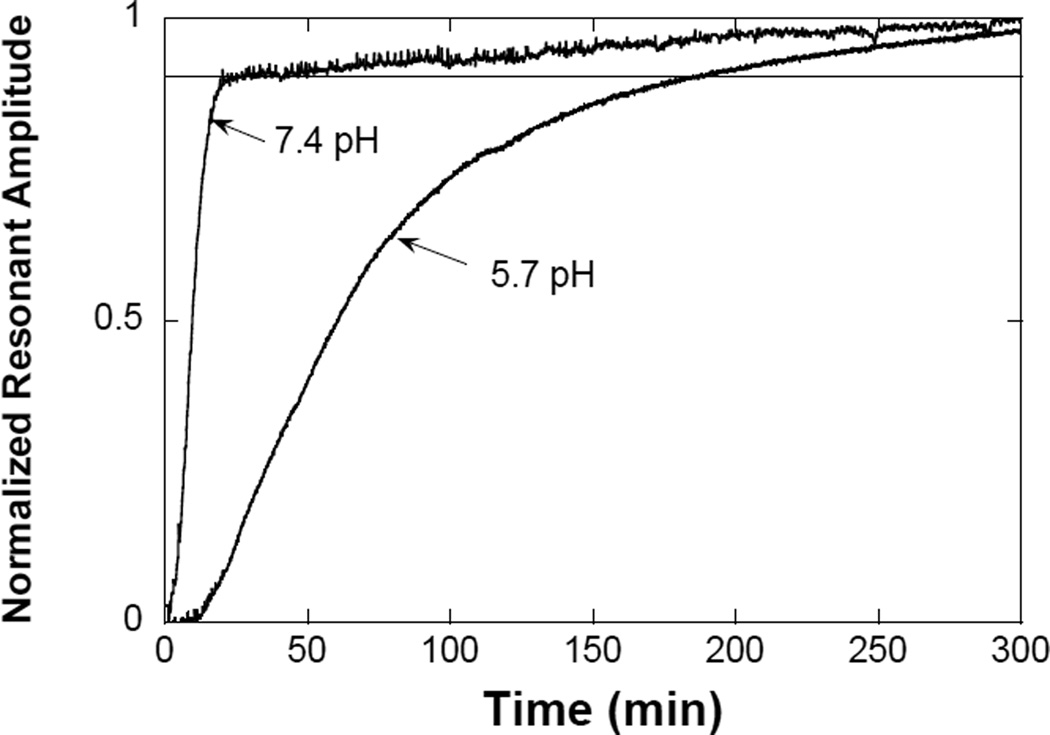
Figure 4 plots the change of the resonant amplitude for the adhesive-coated sensors as they degraded in pH 7.4 and 5.7. As indicated in the figure, the degradation process of the adhesive was faster at pH 7.4, where the sensor response decreased sharply over the period of 22 minutes. This indicated that most adhesive on the sensor has been degraded at 22 minutes after the sample was immersed in pH 7.4. This finding is in agreement with the qualitative degradation test of the bulk adhesive hydrogel (Figure 2). The slow increase in resonant amplitude after 22 minutes may be caused by the remaining small quantity of adhesive on the sensor surface, which was slowly dispersed into the medium over time. When the adhesive-coated sensor was incubated in a mildly acid pH (5.7), the degradation process was slower and more gradual, with curve indicating the adhesive took over 3 hours to degrade. This observation is expected as hydrolysis of ester bond is faster in a basic pH [40, 41].
Figure 4.
Change in the normalized resonant amplitudes of magnetoelastic sensors when coated hydrogel degraded at pH 5.7 and 7.4. The curves represent the average value of 3 samples.
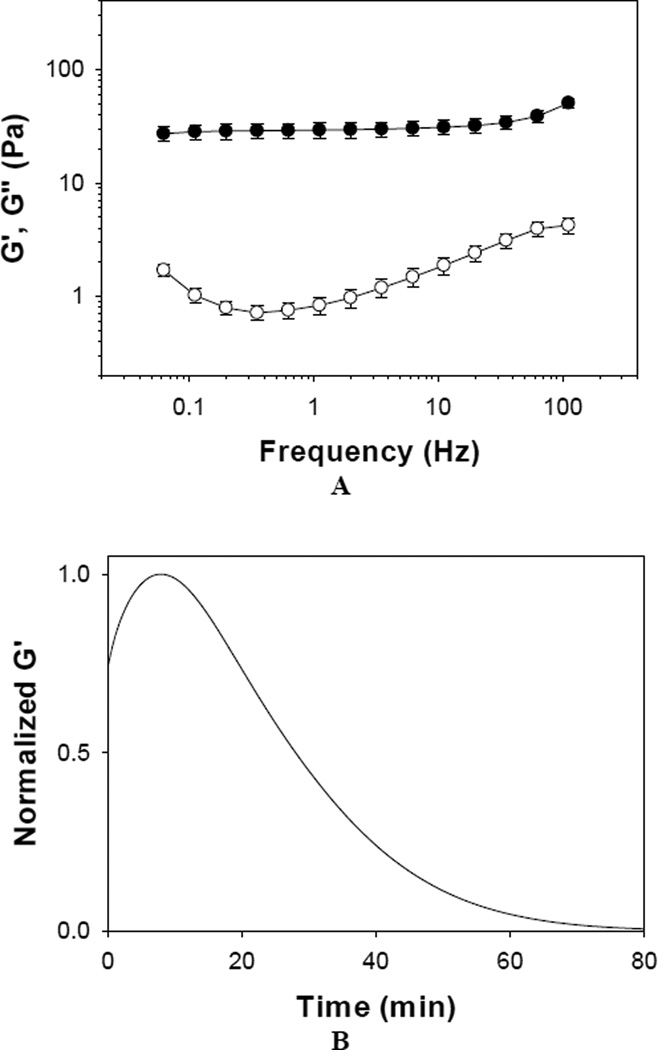
To further confirm the formation and degradation of PEG-(Glu-ND)4 adhesive, samples were monitored using oscillatory rheometry (Figure 5). The adhesive demonstrated storage modulus (G’) values that were independent of frequency and G’ was significantly greater than the loss modulus (G”) within the frequency range tested (Figure 5A). These observations indicated that PEG-(Glu-ND)4 formed an elastic and covalently crosslinked polymeric network [42, 43]. Degradation of PEG-(Glu-ND)4 was followed by tracking the change in G’ with time while incubating the adhesive in an heated bath (37 °C) of PBS (pH 7.4) (Figure 5B). G’ initially increased over the first 7–8 minutes indicating the solidification of the adhesive through polymerization of the catechol group [42]. After which, G’ values decreased with time and became less than 1% of the maximum G’ value after 60 min. The reduction in G’ indicated a decrease in crosslinking density in the polymer network as PEG-(Glu- ND)4 underwent degradation.
Figure 5.
Storage (●) and loss (○) moduli of PEG-(Glu-ND)4 hydrogel during oscillatory frequency sweep (0.1–100 Hz, strain = 0.1, n = 4) experiment (A). Change in the normalized storage modulus with time under constant frequency (1 Hz) and strain (0.1) performed in PBS (pH 7.4) at 37 °C. The curve represents the average value of 3 samples (B).
Oscillatory rheometry confirmed the formation and degradation of rapidly degrading PEG-(Glu-ND)4. However, it took significantly longer for PEG-(Glu-ND)4 to degrade in a rheometer setup as compared to samples coated on a ME sensor (Figure 4) or a bulk gel submerged in a buffer (Figure 2). As PEG-(Glu-ND)4 undergoes degradation, it releases acidic gluatric acid. The gap in the cone and plate setup has a significantly lower surface area for exchanging solute between the gel network and the surrounding media (PBS at pH 7.4). The buildup of the degradation product (e.g., glutaric acid) likely reduced pH locally and drastically decreased the hydrolysis rate of ester bonds. As such, it may be difficult to realistically simulate physiological conditions for degrading adhesives using a rheometer. Additionally, the rheometry required significantly higher amount of sample for testing (~40 times higher compared to coating used on ME sensors).
Collectively, ME sensor provides a useful tool to characterize the degrdation behavior of tissue adhesive in real time. Sensors were submerged in aqueous buffers under physiological conditions (i.e., saline at body temperature) at varying pH levels. Although the pH of oxygenated blood and internal tissues ranges from 7.2 to 7.45 [44, 45], the pH levels of skin (pH = 4–6) [46], subcutaneous tissues (pH = 6.7–7.1) 47], tumor tissues (pH < 6.9) [48], and internal tissues after prolonged hemorrhage (pH < 7) [49, 50] are more acidic. Therefore, it is necessary to characterize the degradation behaviors of tissue adhesives under conditions that better mimic various physiological environments. However, traditional degradation tests require a large amount of sample, which makes it prohibitive to conduct a comprehensive study to evaluate various factors on the degradation behavior of bioadhesives. Additionally, ME sensor accurately tracked the mass loss of rapidly degrading PEG-(Glu-ND)4, which is not feasible using traditional approaches. ME sensors have previously been utilitzed to characterize biointerfacial events in vivo [18, 19]. This indicates that this remotely sensing technology can potentially be used to monitored adhesive degradations in vivo.
4. Conclusions
Fast degrading, biomimetic PEG-(Glu-ND)4 was coated onto ME sensor strips and the degradation behavior of the adhesive was monitored by tracking the resonant amplitude of the sensor. The resonant amplitude increased overtime, corresponding to the mass loss of the adhesive. Additionally, degradation behavior observed using ME sensors matched the qualitative degradation of the bulk adhesive. Oscillatory rheometry was used to confirm the formation and degradation of PEG-(Glu-ND)4. However, the rheometry setup was not ideal in simulating degradation under physiological condition due to the slow removal of acidic degradation product that lengthened the degradation rate of the adhesive hydrogel. This sensing technology was demonstrated as a potential useful tool for evaluating the degradation rate of bioadhesives.
Acknowledgements
This project was supported by NIH (GM104846). MM was support in part by Summer Undergraduate Research Fellowship provided by Michigan Technological University MTU). The HR-2 rheometer was supported in part by Biotechnology Research Center (MTU) and Research Excellence Fund (MTU).
References
- 1.Hidalgo M, Castillo MJ, Eymar JL, Hidalgo A. Hernia. 2005;9:242. doi: 10.1007/s10029-005-0334-x. [DOI] [PubMed] [Google Scholar]
- 2.Koniger J, Redecke J, Butters M. Langenbecks Arch. Surg. 2004;389:361. doi: 10.1007/s00423-004-0496-5. [DOI] [PubMed] [Google Scholar]
- 3.Stark E, Oestreich K, Wendl K, Rumstadt B, Hagmüller E. Surg. Endosc. 1999;13:878. doi: 10.1007/s004649901124. [DOI] [PubMed] [Google Scholar]
- 4.Berndsen FH, Petersson U, Arvidsson D, Leijonmarck CE, Rudberg C, Smedberg S, Montgomery A. Hernia. 2008;12:445. doi: 10.1007/s10029-007-0214-7. [DOI] [PubMed] [Google Scholar]
- 5.Liu CD, Glantz GJ, Livingston EH. Obes. Surg. 2003;13:45. doi: 10.1381/096089203321136575. [DOI] [PubMed] [Google Scholar]
- 6.Ikada Y. In: Wound Closure Biomaterials and Devices. Chu CC, von Fraunhofer JA, Greisler HP, editors. Boca Raton, Florida: CRC Press, Inc.; 1997. p. 317. [Google Scholar]
- 7.Mehdizadeh M, Yang J. Macromol. Biosci. 2013;13:271. doi: 10.1002/mabi.201200332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Zhan H, Skelton S, Lee BP. MRS Proc. 2013;1569:mrss13. [Google Scholar]
- 9.Murphy JL, Vollenweider L, Xu F, Lee BP. Biomacromolecules. 2010;11:2976. doi: 10.1021/bm1007794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernando A, Vazquez M, Barandiaran J. J. Phys. E: Sci. Instrum. 1988;21:1129. [Google Scholar]
- 11.Modzelewski C, Savage H, Kabacoff L, Clark A. IEEE Trans. Magn. 1981;17:2837. [Google Scholar]
- 12.O'handley RC. Modern Magnetic Materials: Principles and Applications. Wiley-VCH; 1999. p. 768. [Google Scholar]
- 13.Grimes CA, Mungle CS, Zeng K, Jain MK, Dreschel WR, Paulose M, Ong KG. Sensors. 2002;2:294. [Google Scholar]
- 14.Zourob M, Ong KG, Zeng K, Mouffouk F, Grimes CA. Analyst. 2007;132:338. doi: 10.1039/b616035b. [DOI] [PubMed] [Google Scholar]
- 15.Ong KG, Zeng K, Yang X, Shankar K, Ruan C, Grimes CA. IEEE Sens. J. 2006;6:514. [Google Scholar]
- 16.Roy SC, Ong KG, Zeng K, Grimes CA. Sens. Lett. 2007;5:432. [Google Scholar]
- 17.Ong KG, Leland JM, Zeng K, Barrett G, Zourob M, Grimes CA. Biosens. Bioelectron. 2006;21:2270. doi: 10.1016/j.bios.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Holmes HR, Tan EL, Ong KG, Rajachar RM. Biosensors. 2012;2:57. doi: 10.3390/bios2010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlaisavljevich E, Holmes HR, Tan EL, Qian Z, Trierweiler S, Ong KG, Rajachar RM, Mater J. Sci.- Mater. Med. 2013;24:1093. doi: 10.1007/s10856-013-4854-0. [DOI] [PubMed] [Google Scholar]
- 20.Waite JH. Int. J. Adhes. Adhes. 1987;7:9. [Google Scholar]
- 21.Waite JH. Ann. Ny Acad. Sci. 1999;875:301. doi: 10.1111/j.1749-6632.1999.tb08513.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee BP, Messersmith PB, Israelachvili JN, Waite JH. Annu. Rev. Mater. Res. 2011;41:99. doi: 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilic G, Brubaker C, Messersmith PB, Mallik AS, Quinn TM, Haller C, Done E, Gucciardo L, Zeisberger SM, Zimmermann R, Deprest J, Zisch AH. Am. J. Obstet. Gynecol. 2010;202:85. doi: 10.1016/j.ajog.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haller CM, Buerzle W, Kivelio A, Perrini M, Brubaker CE, Gubeli RJ, Mallik AS, Weber W, Messersmith PB, Mazza E, Ochsenbein-Koelble N, Zimmermann R, Ehrbar M. Acta Biomater. 2012;8:4365. doi: 10.1016/j.actbio.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 25.Brodie M, Vollenweider L, Murphy JL, Xu F, Lyman A, Lew WD, Lee BP. Biomed. Mater. 2011;6:015014. doi: 10.1088/1748-6041/6/1/015014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehdizadeh M, Weng H, Gyawali D, Tang L, Yang J. Biomaterials. 2012;33:7972. doi: 10.1016/j.biomaterials.2012.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brubaker CE, Kissler H, Wang L-J, Kaufman DB, Messersmith PB. Biomaterials. 2010;31:420. doi: 10.1016/j.biomaterials.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong S, Yang K, Kang B, Lee C, Song IT, Byun E, Park KI, Cho S-W, Lee H. Adv. Funct. Mater. 2013;23:1774. [Google Scholar]
- 29.Kastrup CJ, Nahrendorf M, Figueiredo JL, Lee H, Kambhampati S, Lee T, Cho S-W, Gorbatov R, Iwamoto Y, Dang TT, Dutta P, Yeon JH, Cheng H, Pritchard CD, Vegas AJ, Siegel CD, MacDougall S, Okonkwo M, Thai A, Stone JR, Coury AJ, Weissleder R, Langer R, Anderson DG. Proc. Natl. Acad. Sci. U. S. A. 2012;109:21444. doi: 10.1073/pnas.1217972110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amstad E, Gehring AU, Fischer H, Nagaiyanallur VV, Hahner G, Textor M, Reimhult E. J. Phys. Chem. C. 2011;115:683. [Google Scholar]
- 31.Amstad E, Gillich T, Bilecka I, Textor M, Reimhult E. Nano Lett. 2009;9:4042. doi: 10.1021/nl902212q. [DOI] [PubMed] [Google Scholar]
- 32.Napolitano A, Dischia M, Costantini C, Prota G. Tetrahedron. 1992;48:8515. [Google Scholar]
- 33.Dalsin JL, Lee BP, Vollenweider L, Silvary S, Murphy JL, Xu F, Spitz A, Lyman A. Multi-armed catechol compound blends. 8, 119,742. US Patent. 2012
- 34.Shafiq Z, Cui J, Pastor-Pérez L, San Miguel V, Gropeanu RA, Serrano C, del Campo A. Angew. Chem. Int. Ed. 2012;124:4408. doi: 10.1002/anie.201108629. [DOI] [PubMed] [Google Scholar]
- 35.Menyo MS, Hawker CJ, Waite JH. Soft Matter. 2013;9:10314. doi: 10.1039/C3SM51824H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz JA, Contescu Cristian I, Putyera Karol. Dekker Encyclopedia of Nanoscience and Nanotechnology. New York: CRC Press; 2004. [Google Scholar]
- 37.Vlaisavljevich E, Janka L, Ong K, Rajachar R, Med J. Devices. 2009;3:027528. [Google Scholar]
- 38.Lee H, Dellatore SM, Miller WM, Messersmith PB. Science. 2007;318:426. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafiq Z, Cui J, Pastor-Pérez L, San Miguel V, Gropeanu RA, Serrano C, del Campo A. Angew. Chem., Angew. Chem. Int. Ed. 2012;51:4332. doi: 10.1002/anie.201108629. [DOI] [PubMed] [Google Scholar]
- 40.Chao GT, Qian ZY, Huang MJ, Kan B, Gu YC, Gong CY, Yang JL, Wang K, Dai M, Li XY, Gou ML, Tu MJ, Wei YQ, Biomed J. Mater. Res. A. 2008;85:36. doi: 10.1002/jbm.a.31362. [DOI] [PubMed] [Google Scholar]
- 41.van Dijk-Wolthuis WNE, van Steenbergen MJ, M. Underberg WJ, Hennink WE. J. Pharm. Sci. 1997;86:413. doi: 10.1021/js9604220. [DOI] [PubMed] [Google Scholar]
- 42.Lee BP, Dalsin JL, Messersmith PB. Biomacromolecules. 2002;3:1038. doi: 10.1021/bm025546n. [DOI] [PubMed] [Google Scholar]
- 43.Skelton S, Bostwick M, O'Connor K, Konst S, Casey S, Lee BP. Soft Matter. 2013;9:3825. [Google Scholar]
- 44.Waugh A, Grant A. Anatomy ans Physiology in Health and Illness. Elsevier: Churchill Livingstone; 2010. [Google Scholar]
- 45.Soller BR, Zhang S. Proc. SPIE. 1998;3259:122. [Google Scholar]
- 46.Ohman H, Vahlquist A. Acta Derm-venereol. 1994;74:375. doi: 10.2340/0001555574375379. [DOI] [PubMed] [Google Scholar]
- 47.Soller BR, Micheels RH, Coen J, Parikh B, Chu L, Hsi C, Clin J. Monit. Comput. 1996;12:387. doi: 10.1007/BF02077636. [DOI] [PubMed] [Google Scholar]
- 48.Tannock IF, Rotin D. Cancer Res. 1989;49:4373. [PubMed] [Google Scholar]
- 49.Sims C, Seigne P, Menconi M, Monarca J, Barlow C, Pettit J, Puyana JC, Trauma J. Inj. Infect. Crit. Care. 2001;51:1137. doi: 10.1097/00005373-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 50.Soller BR, Khan T, Favreau J, Hsi C, Puyana JC, Heard SO. J. Surg. Res. 2003;114:195. doi: 10.1016/s0022-4804(03)00251-8. [DOI] [PubMed] [Google Scholar]