Abstract
Rationale
Stress activates the hypothalamic-pituitary-adrenal (HPA) axis, and GABAergic neuroactive steroids contribute to homeostatic regulation of this circuitry. Acute forced swim stress (FSS) increases plasma, cortical, and hypothalamic (3α,5α)-3-hydroxy-pregnan-20-one (3α,5α-THP) levels in rats. However, there have not been systemic investigations of acute stress on changes in plasma and brain levels of 3α,5α-THP in mouse models.
Objectives
The present experiments aimed to assess circulating and local brain levels of 3α,5α-THP following acute FSS in C57BL/6J mice.
Methods
Mice were exposed to FSS (10 min), and 50 min later, blood and brains were collected. Circulating pregnenolone and 3α,5α-THP levels were assessed in serum. Free-floating brain sections (40 µm, four to five sections/region) were immunostained and analyzed in cortical and limbic brain structures.
Results
FSS decreased circulating 3α,5α-THP (−41.6± 10.4 %) and reduced 3α,5α-THP immunolabeling in the paraventricular nucleus of the hypothalamus (−15.2±5.7 %), lateral amygdala (LA, −31.1±13.4 %), and nucleus accumbens (NAcc) shell (−31.9±14.6). Within the LA, vesicular glutamate transporter 1 (VGLUT1) and vesicular GABA transporter were localized in 3α,5α-THP-positively stained cells, while in the NAcc shell, only VGLUT1 was localized in 3α,5α-THP-positively stained cells, suggesting that both glutamatergic and GABAergic cells within the LA are 3α,5α-THP-positive, while in the NAcc shell, 3α,5α-THP only localizes to glutamatergic cells.
Conclusions
The decrease in circulating and brain levels of 3α,5α-THP may be due to alterations in the biosynthesis/ metabolism or changes in the regulation of the HPA axis following FSS. Changes in GABAergic neuroactive steroids in response to stress likely mediate functional adaptations in neuronal activity. This may provide a potential targeted therapeutic avenue to address maladaptive stress responsivity.
Keywords: Stress; Allopregnanolone; Neuroactivesteroids; 3α,5α-THP
Introduction
Neuroactive steroids are endogenous modulators of neuronal activity that act as modulators of ion channels, including GABAA receptors. The 5α-reduced pregnane steroids, (3α,5α)-3-hydroxy-pregnan-20-one (3α,5α-THP; allopregnanolone) and (3α,5α)-3,21-dihydroxypregnan-20-one (3α,5α-THDOC, allotetrahydrodeoxycorticosterone), are two of the most potent positive allosteric modulators of GABAA receptors that enhance GABAA receptor activity at nanomolar concentrations (Morrow et al. 1987). GABAergic neuroactive steroids, including 3α,5α-THP, (3α,5β)-3-hydroxy-pregnan-20-one (3α,5β-THP; pregnanolone), and 3α,5α-THDOC, act at a known binding site within the α subunit on GABAergic receptors to enhance GABAergic activity (Hosie et al. 2006). 5α-reduced GABAergic neuroactive steroids are synthesized from cholesterol-derived progesterone in a two-step pathway requiring the enzyme 5α-reductase, which reduces progesterone to 5α-dehydroxyprogesterone (5α-DHP) and 3α-hydroxysteroid dehydrogenase (3α-HSD) which either converts 5α-DHP into 3α,5α-THP (reductive reaction) or converts 3α,5α-THP into 5α-DHP (oxidative reaction) (Compagnone and Mellon 2000; Guidotti and Costa 1998).
Plasma 3α,5α-THP levels are increased in response to stress in human subjects exposed to the Trier social stress test or exposed to CRF in the laboratory (Genazzani et al. 1998; Girdler and Klatzkin 2007). These effects are recapitulated in rat models, where acute forced swim stress (FSS) increases circulating, cerebral cortical, and hypothalamic levels of 3α,5α-THP and 3α,5α-THDOC, as well as the precursor pregnenolone (Barbaccia et al. 1996; Purdy et al. 1991; Vallee et al. 2000). FSS increases GABAA receptor-mediated chloride flux to increase GABAergic transmission (Schwartz et al. 1987), which may involve alterations in these GABAergic neuroactive steroids. Furthermore, acute ethanol stress also increases 3α,5α-THP in plasma and various brain regions (Cook et al. 2014; Porcu et al. 2010; VanDoren et al. 2000), but decreases 3α,5α-THP in the nucleus accumbens and the central nucleus of the amygdala (Cook et al. 2014). FSS also increases 5α-reductase, the primary biosynthetic enzyme that converts pregnenolone to progesterone, levels in prefrontal cortex (Sanchez et al. 2008). Together, these data indicate that acute stress alters plasma and brain levels of 3α,5α-THP in human and rat models, which may underlie the effects of stress on neurotransmission.
Swim stress can also induce anticonvulsant properties on seizure susceptibility in mouse models through its actions on neuroactive steroids in CBA, C3H J, and Swiss mice (Pericic et al. 2000, 2007; Reddy and Rogawski 2002) and rats (Reddy and Rogawski 2002). No change in seizure threshold following FSS was observed following inhibition of neuroactive steroid synthesis using finasteride, a potent inhibitor of 5α-reductase (Pericic et al. 2000; Reddy and Rogawski 2002). 3α,5α-THP, 3α,5β-THP, and progesterone administration enhanced seizure threshold in C3HJ, Swiss, and Swiss Webster mice (Gasior et al. 1997; Kokate et al. 1998, 1999; Pericic et al. 2007). 3α,5α-THP combined with swim stress potentiated the protection against seizures, indicating a synergistic interaction between swim stress and 3α,5α-THP administration (Pericic et al. 2007). Another neuroactive steroid, 3α,5α-THDOC, was also increased following swim stress and was associated with elevated seizure threshold against pentylenetetrazol-induced seizures (Reddy and Rogawski 2002). These data indicate that acute FSS can decrease seizure susceptibility through its effects on neuroactive steroids.
Very different effects of FSS have been observed in C57BL/6 mice. Whole brain levels of pregnenolone were decreased in C57BL/6 mice following forced swim (Phan et al. 2002). Acute ethanol administration, which can be considered an acute stressor, increased circulating pregnen-olone levels, but decreased circulating 3α,5α-THP levels and induced no change in cerebrocortical or hippocampal levels in C57BL/6J mice (Porcu et al. 2010, 2014). However, others have shown that acute injection of ethanol in C57BL/6 mice did not alter whole brain 3α,5α-THP levels (Finn et al. 2004).
The C57BL/6J mouse is one of the most commonly used mouse strains in research and serves as a wild-type strain for many genetic models. However, very little is known about the distribution of neuroactive steroids across the brain and how acute stress alters these levels. Furthermore, acute FSS shows different effects in the periphery and some brain regions of both rat and mouse models. Therefore, the present set of experiments aimed to identify circulating and local brain levels of 3α,5α-THP following acute FSS in C57BL/6J mice.
Materials and methods
Subjects
Adult male C57BL/6J mice (n=11–15/group) were obtained from Jackson Laboratories (Bar Harbor, ME) at 6–10 weeks of age. All mice were allowed to acclimate to the colony for at least 2 weeks and were between 11 and 13 weeks of age at the time of the experiment. Animals were group-housed five per cage with free access to food and water. All mice were maintained in a temperature (23±0.06°C) and humidity (40±10 %) controlled room with lights on from 0700 to 1900 hours. Animal care followed the National Institutes of Health Guidelines under the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee approved protocols.
Apparatus
Glass cylinders (Wholesale Flowers and Supplies; 20 cm diameter, 40 cm height) were used as the swim test apparatus. The glass cylinders were filled with water (23 to 25 °C) to approximately 20 cm in depth. Each cylinder was separated from another by a visual barrier.
Forced swim stress
Within 1 week of arrival to the animal vivarium, all mice were subjected to mild restraint for blood collection of approximately 50 µl from the submandibular space for assessment of baseline corticosterone levels. Specifically, mice were mildly restrained and poked in the cheek pouch with a lancet (Medipoint, Inc., Mineola, NY), 50 µl blood was collected, and mild pressure was applied to the wound area to promote blood clotting. No anesthesia is required for this method of blood collection. On the day of the experiment, all mice were transported to the procedure room to acclimate for a minimum of 60 min. Control animals were maintained in a room separate from the procedure room where mice were exposed to the forced swim test.
All swim stress sessions were conducted between 1300 and 1700 hours. At the beginning of the swim stress session, mice were removed from the home cage, weighed, and lowered into the apparatus for a 10-min swim session. Following the swim session, the mouse was removed from the swim tank and lightly dried, and blood was rapidly collected from the submandibular space. The animal was then returned to the home cage and placed on a warming pad for 15–20 min. After each swim session, the cylinders were emptied, cleaned, and replaced with fresh water for the next subject.
Experiment 1: Serum and adrenal analysis
For experiment 1, circulating levels of neuroactive steroids and biosynthetic enzymes were assessed following acute FSS (n=15 per group, nonstressed controls or stressed mice). Immediately upon removal from the swim tank, animals were dried, 50 µl of blood was collected, and mice were returned to the home cage for 50 min. At the 50-min time point, mice were decapitated and trunk blood was collected for the circulating pregnenolone and 3α,5α-THP serum analyses. Control mice were removed from the home cage and sacrificed concurrently. Brains and adrenal glands were harvested, frozen on dry ice, and stored at −80 °C until analysis.
Neuroactive steroid analysis
Blood samples were centrifuged at 1,730g for 15 min at 4 °C. Serum was collected and frozen at −80 °C until analysis. Pregnenolone and 3α,5α-THP levels were quantified in serum (200 µl) by gas chromatography/mass spectrometry (GC/MS) using previously established methods (Porcu et al. 2009).
Adrenal biosynthetic enzyme analysis
RNAwas extracted from the adrenals using the Direct-zol™ RNA MiniPrep (Zymo Research Corporation, Irvine, CA, USA), and RNA purity was verified using the OD260/280 ratio to be between 1.8 and 2.0. cDNA was produced by High Capacity RNA-to-cDNA™ Kit (Life Technologies, Grand Island, NY, USA). Quantitative real-time (qRT)-PCR for each sample was performed in triplicate 20 µl reactions using standard cycle conditions for 40 cycles on a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Warrington, UK). TaqMan primers for SRD5a1 (Mm00614213_m1), AKR1C14 (Mm00506338_m1), and GAPDH (Mm99999916_s1) and TaqMan probes and qRT-PCR mix were purchased from Life Technologies (Grand Island, NY, USA). All samples were analyzed and normalized with the expression level of GAPDH, and quantification of fold change was performed utilizing the 2−ΔΔCt method.
Experiment 2: Brain 3α,5α-THP analysis
For experiment 2, corticosterone analysis and immunohistochemistry (IHC) were performed for the analysis of 3α,5α-THP immunoreactivity following acute FSS (n=11 per group, nonstressed controls or stressed mice). All mice were treated identically as described in experiment 1, except that animals were transcardially perfused as indicated below.
Serum corticosterone
Serum was collected as described in experiment 1. Blood samples were centrifuged at l,730g- for 15 min at 4 °C. Serum was collected and frozen at −80 °C until analysis. Serum corticosterone levels were analyzed using a radioimmunoassay kit according to manufacturer’s instructions (MP Biomedicals, Orangeburg, NY, USA).
Tissue processing and immunohistochemistry
All mice for experiment 2 were euthanized with an overdose of pentobarbital sodium (150 mg/kg) and transcardially perfused. Mice were perfused at a flow rate of 3 ml/min with a total volume of 6 ml of 0.1M phosphate-buffered saline (PBS) followed by 12 ml of 4 % paraformaldehyde (PFA). Following perfusion, brains were extracted and postfixed in 4 % PFA for 24 hours at 4 °C and then stored in PBS until they were sectioned coronally at 40 µm on a vibrating microtome. Sections were stored at −30 °C until immunohistochemical analysis.
IHC was performed on free-floating sections (three to five sections/animal/brain region) using an affinity purified 3α,5α-THP sheep antibody that has been previously characterized by radioimmunoassay (Janis et al. 1998; VanDoren et al. 2000) and immunohistochemistry (Cook et al. 2014). All sections for 3,3′-diaminobenzidine (DAB, Sigma-Aldrich, St. Louis, MO, USA) were processed identically to recently published methods (Cook et al. 2014), except that sections were incubated in a rabbit-anti-sheep biotinylated secondary antibody (1:200, Vector Laboratories, Burlingame, CA, USA), that was preabsorbed in 2 % mouse serum (Sigma-Aldrich).
3α,5α-THP light microscopy analysis
Brain region immunoreactivity was visualized with an Olympus CX41 light microscope (Olympus America, Center Valley, PA, USA), and images were captured with a digital camera (Regita model, QImaging, Burnaby, BC) and analyzed using Bioquant Image Analysis Software (Nashville, TN, USA) to obtain linear integrated optical density for immunoreactivity assessment. The microscope, camera, and software were background corrected and normalized to preset light levels to ensure fidelity of data acquisition. Positive pixel count density of immunoreactivity was quantified from a circumscribed field, delineated as a brain region, divided by the area of the region in square millimeters, and expressed as pixels per square millimeter. Data from three to five sections were used per animal per brain region to average one value per mouse. The experimenter was blind to the condition of each animal when analyses were conducted. Matched sections were used for nonstressed and stressed mice for each brain region.
Brain regions analyzed included medial prefrontal cortex [mPFC (+1.98 to +1.70 Anterior:Posterior (AP))], nucleus accumbens [NAcc shell and core (+1.54 to +0.98 AP)], bed nucleus of the stria terminalis [BNST dorsal and ventral (+ 0.38 to +0.02 AP)], paraventricular hypothalamic nucleus [PVN (−0.58 to −0.94 AP)], paraventricular thalamic nucleus [PTN (−1.22 to −1.82 AP)], amygdala [lateral amygdala (LA), basolateral amygdala (BLA), and central nucleus of the amygdala (CeA) (−0.94 to −1.58 AP)], hippocampus [cornu ammonis 1 (CA1), cornu ammonis 3 (CA3), polymorph cell layer of the dentate gyrus (−1.34 to −1.82 AP)], and ventral tegmental area [VTA (−2.80 to −3.28 AP)]. All coordinates are expressed relative to bregma in the Mouse Brain Atlas in Stereotaxic Coordinates (Franklin and Paxinos 2007).
Double immunofluorescent labeling and confocal microscopy
Free floating sections (three to four sections/mouse) were rinsed, blocked in normal donkey serum, and incubated in primary antibody for vesicular transporter specific markers: for glutamate vesicles, vesicular glutamate transporter 1 (VGLUT1) [(1:1,000), Millipore Corporation, Billerica, MA, USA] and for GABA vesicles, vesicular GABA transporter (VGAT) [(1:500), Synaptic Systems, Goettingen, Germany] for 24 hours at 4 °C. Sections were then rinsed in PBS, blocked, and incubated with 3α,5α-THP primary antibody (1:500; purchased from Dr. R.H. Purdy) for 48 hours at 4 °C. Following 3a,5a-THP incubation, sections were rinsed and incubated with secondary antibody (Alexa Fluor 594, Life Technologies, Durham, NC, USA) for VGLUT1 and VGAT at 4 °C followed by rinsing and incubation with secondary antibody (Alexa Fluor 488) for 3a,5a-THP visualization. Immunofluorescence was visualized using a Leica SP2 laser scanning confocal microscope and computer software (Buffalo Grove, IL, USA). Vesicular transporter markers and 3α,5α-THP immunofluorescence were imaged sequentially to prevent fluorophore bleed-through. For image processing, average fluorescent intensity was calculated from 10 to 11 stacks per image using ImageJ software (National Institutes of Health, USA).
Statistical analysis
Data were analyzed using an unpaired t test to compare changes between nonstressed and stressed animals. Corticosterone data were analyzed using a two-way repeated measures ANOVA with Stress as a between-subjects variable and Day (basal, test day) as a repeated measure. The level of significance was set to an a level of ≤ 0.05.
Results
Serum neuroactive steroid and corticosterone levels
Acute FSS altered circulating neuroactive steroid and corticosterone levels (Table 1). Acute FSS increased serum corticosterone levels on the test day compared to nonstressed controls by 81.9±16.0 % (Day by Stress interaction [F(1, 24)=8.65, p<0.01], main effect of Stress [F(1, 24)=14.59, p<0.001]). Both nonstressed and stressed animals showed an increase in serum corticosterone levels on the test day compared to basal corticosterone levels (main effect of Day [F(1, 24)=67.63, p<0.0001]). The relative increase in corticosterone levels in stressed animals compared to nonstressed animals was blunted the later in the day the blood samples were collected (main effect of Time [F(2, 20)=4.44, p<0.05]; data not shown). Nonetheless, the overall increase in corticosterone levels in stressed mice compared to nonstressed controls was maintained, regardless of time of blood collection (main effect of Stress [F(1, 20)=9.8, p<0.01]).
Table 1.
Acute FSS decreases circulating 3α,5α-THP and corticosterone levels in C57BL/6J mice
| Pregnenolone | 3α,5α-THP | Corticosterone |
||
|---|---|---|---|---|
| Test | Test | Basal | Test | |
| No stress | 111.5±9.3 | 1,034.0±79.6 | 76.4±16.2 | 271.1±46.2+ |
| Stress | 140.7±11.8 | 607.6±75.1* | 81.6±14.7 | 493.2±43.3**# |
Data are presented as picograms per milliliter for pregnenolone and 3α,5α-THP and nanograms per milliliter for corticosterone in nonstressed and stressed mice (n=12–15).
p<0.001 (compared to nonstressed controls);
p<0.0001 (compared to nonstressed controls);
p<0.01 (compared to basal levels);
p<0.0001 (compared to basal levels)
There was a trend for an increase in circulating pregnenolone levels (t=1.88, p=0.08; Table 1). In contrast, FSS decreased circulating 3α,5α-THP levels by 41.6±10.4 % in serum (t=3.89, p<0.001; Table 1). There were no differences in serum pregnenolone or 3α,5α-THP levels across time of day (p>0.05).
Adrenal biosynthetic enzyme levels
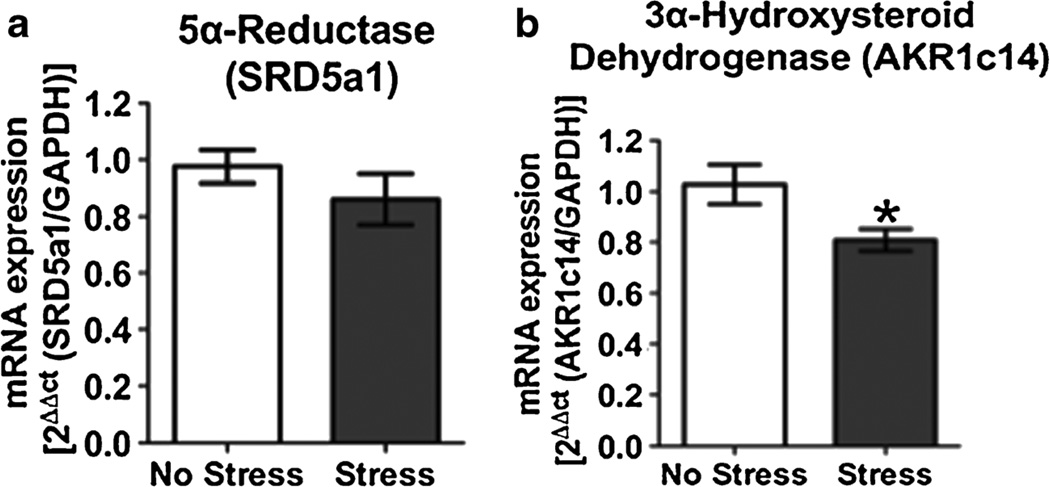
Adrenal mRNA levels of 5α-reductase (SRD5a1), the biosynthetic enzyme that converts progesterone to 5α-DHP, were not altered following acute exposure to FSS (Fig. 1a, p>0.05). In contrast, adrenal mRNA levels of 3α-hydroxysteroid dehydrogenase (AKR1c14), the biosynthetic enzyme that converts 5α-DHP to 3α,5α-THP, were decreased 21.3±8.8 % following acute swim stress (Fig. 1b, t=2.42, p<0.05).
Fig. 1.
Effects of acute forced swim stress (FSS) on 5α-reductase (SRD5a1) and 3α-hydroxysteroid dehydrogenase (AKR1c14) mRNA levels. Data depicted are mean mRNA expression relative to GAPDH±SEM. a SRD5a1 mRNA expression in nonstressed (clear bars) and stressed (gray bars) mice 50 min after acute FSS. b AKR1c14 mRNA expression in nonstressed (clear bars) and stressed (gray bars) mice 50 min after acute FSS. *p<0.05 compared to nonstressed controls (n=11–14)
Brain 3α,5α-THP immunoreactivity following acute swim stress
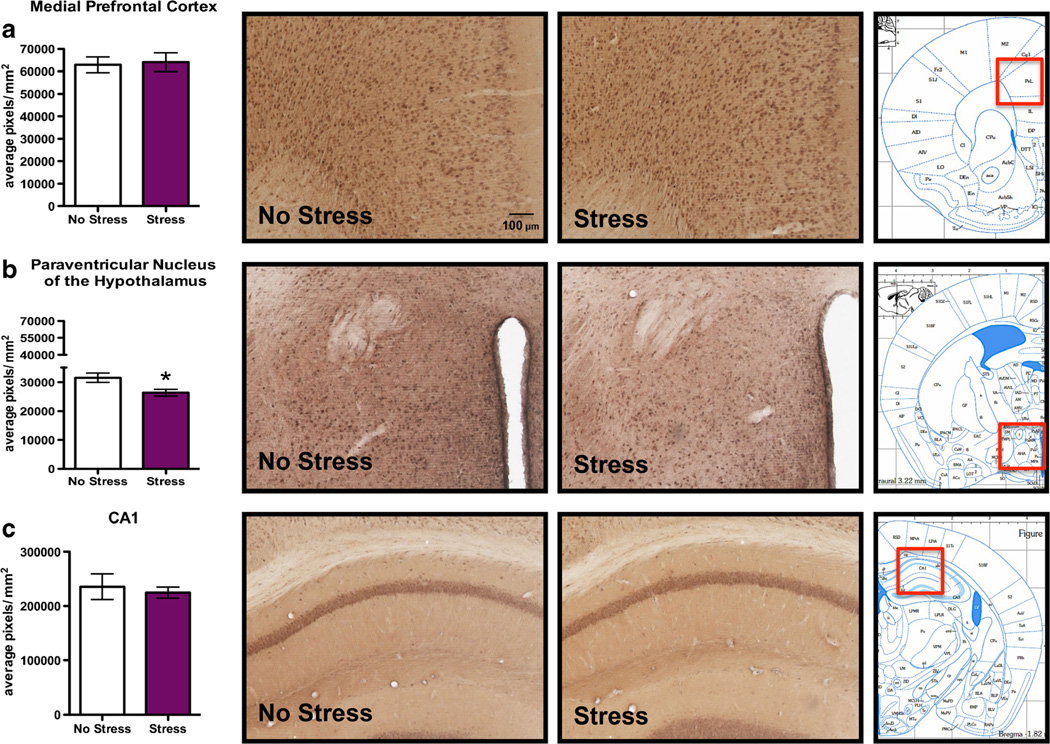
FSS produced no change in 3α,5α-THP immunoreactivity in the mPFC (Fig. 2a) or CA1 pyramidal cell layer of the hippocampus (Fig. 2c) following acute FSS compared to nonstressed control mice (p>0.05). Additionally, there were no stress-induced alterations in 3α,5α-THP immunoreactivity in the CA3 pyramidal cell layer of the hippocampus or the polymorph layer of the dentate gyrus (Table 2). In contrast, there was a 15.2±5.7 % decrease in 3α,5α-THP immunoreactivity in the PVN in stressed mice compared to nonstressed mice (Fig. 2b, t=2.67, p<0.05).
Fig. 2.
Effects of acute forced swim stress (FSS) on 3α,5α-THP immunoreactivity in the mPFC, PVN, and pyramidal cell layer of CA1. Data depicted are mean positive pixels per square millimeter±SEM of nonstressed or stressed mice (n=10–11). a 3α,5α-THP immunoreactivity between nonstressed (clear bars) and stressed mice (purple bars) in mPFC. b 3α,5α-THP immunoreactivity between nonstressed (clear bars) and stressed mice (purple bars) in the PVN. c 3α,5α-THP immunoreactivity between nonstressed (clear bars) or stressed mice (purple bars) in CA1. Representative photomicrographs (10X) of 3α,5α-THP immunoreactivity after FSS (n=10–11). The red box indicates the location of representative photomicrographs. *p<0.05 compared to nonstressed controls. CA1, cornu ammonis area 1
Table 2.
Acute FSS does not alter neuronal 3α,5α-THP levels across various brain structures
| No stress | Stress | |
|---|---|---|
| CA3 | 75,851±6,446 | 72,273±4,270 |
| Polymorph layer of the dentate gyrus | 19,813±1,374 | 21,437±2,536 |
| BNST | 11,398±1,895 | 16,147±2,173 |
| PTN | 40,018±4,613 | 36,333±2,696 |
Data are presented as mean positive pixels per square millimeter±SEM of nonstressed and stressed mice in the CA3 pyramidal cell layer of the hippocampus, polymorph layer of the dentate gyrus, bed nucleus of the stria terminalis (BNST), and paraventricular thalamic nucleus (PTN) in nonstressed and stressed mice (n=10–11)
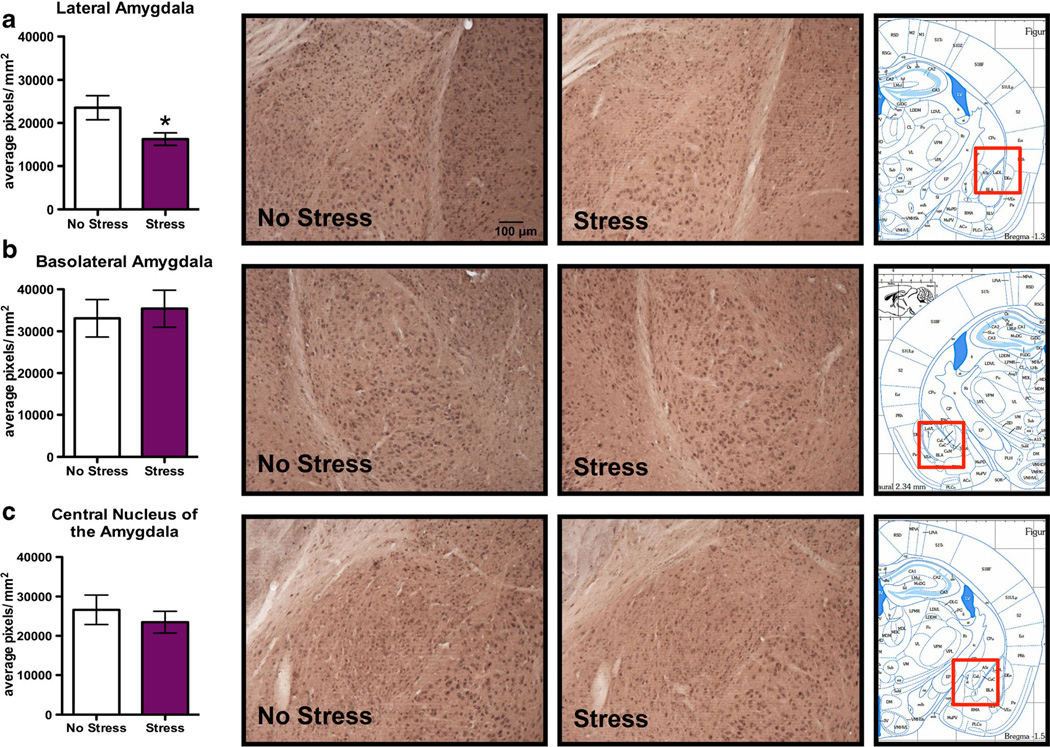
Subregional specificity in the effects of FSS on 3α,5α-THP immunoreactivity was observed in amygdalar structures. Specifically, there was a 31.1±13.4 % decrease in 3α,5α-THP immunoreactivity in the LA following FSS (Fig. 3a, t=2.30, p<0.05). However, there were no differences in 3α,5α-THP immunoreactivity in the BLA (Fig. 3b) or the CeA (Fig. 3c) following swim stress.
Fig. 3.
Effects of acute forced swim stress (FSS) on 3α,5α-THP immunoreactivity in the amygdala. Data depicted are mean positive pixels per square millimeter±SEM of nonstressed or stressed mice (n=10–11). a 3α,5α-THP immunoreactivity between nonstressed (clear bars) or stressed mice (purple bars) in the LA. b 3α,5α-THP immunoreactivity between nonstressed (clear bars) and stressed mice (purple bars) in the BLA. c 3α,5α-THP immunoreactivity between nonstressed (clear bars) and stressed mice (purple bars) in the CeA. Representative photomicrographs (10X) of 3α,5α-THP immunoreactivity after FSS (n=10–11). The red box indicates the location of representative photomicrographs. *p<0.05 compared to nonstressed controls
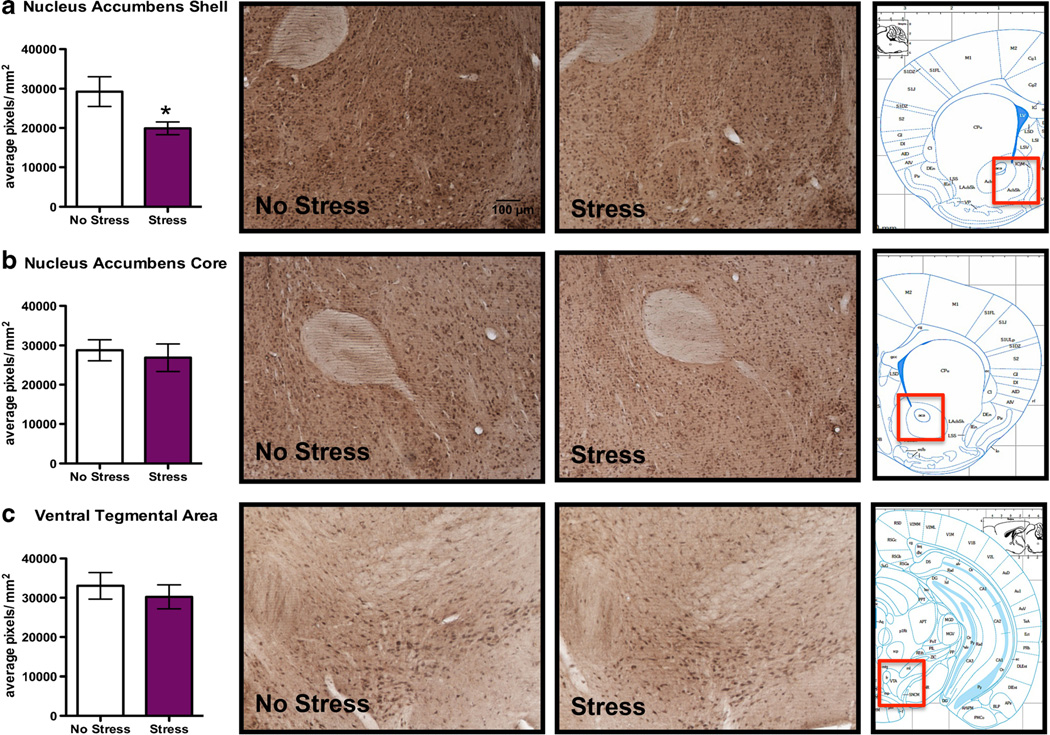
Subregional differences in 3α,5α-THP immunoreactivity following swim stress were also observed in the NAcc. Specifically, there was a 31.9±14.6 % decrease in 3α,5α-THP immunoreactivity in the NAcc shell (Fig. 4a, t=2.19, p<0.05), but not in the NAcc core (Fig. 4b). Similarly, there was no alteration in 3α,5α-THP immunoreactivity in the VTA (Fig. 4c), BNST or PTN (Table 2) following FSS.
Fig. 4.
Effects of acute forced swim stress (FSS) on 3α,5α-THP immunoreactivity in the NAcc and VTA. Data depicted are mean positive pixels per square millimeter±SEM of nonstressed or stressed mice (n= 10–11). a 3α,5α-THP immunoreactivity between nonstressed (clear bars) and stressed mice (purple bars) in the NAcc shell. b 3α,5α-THP immunoreactivity between nonstressed (clear bars) and stressed mice (purple bars) in the NAcc core. c 3α,5α-THP immunoreactivity between nonstressed (clear bars) and stressed mice (purple bars) in VTA. Representative photomicrographs (10X) of 3α,5α-THP immunoreactivity after FSS (n=10–11). The red box indicates the location of representative photomicrographs. *p<0.05 compared to nonstressed controls
3α,5α-THP localization with VGAT and VGLUT1
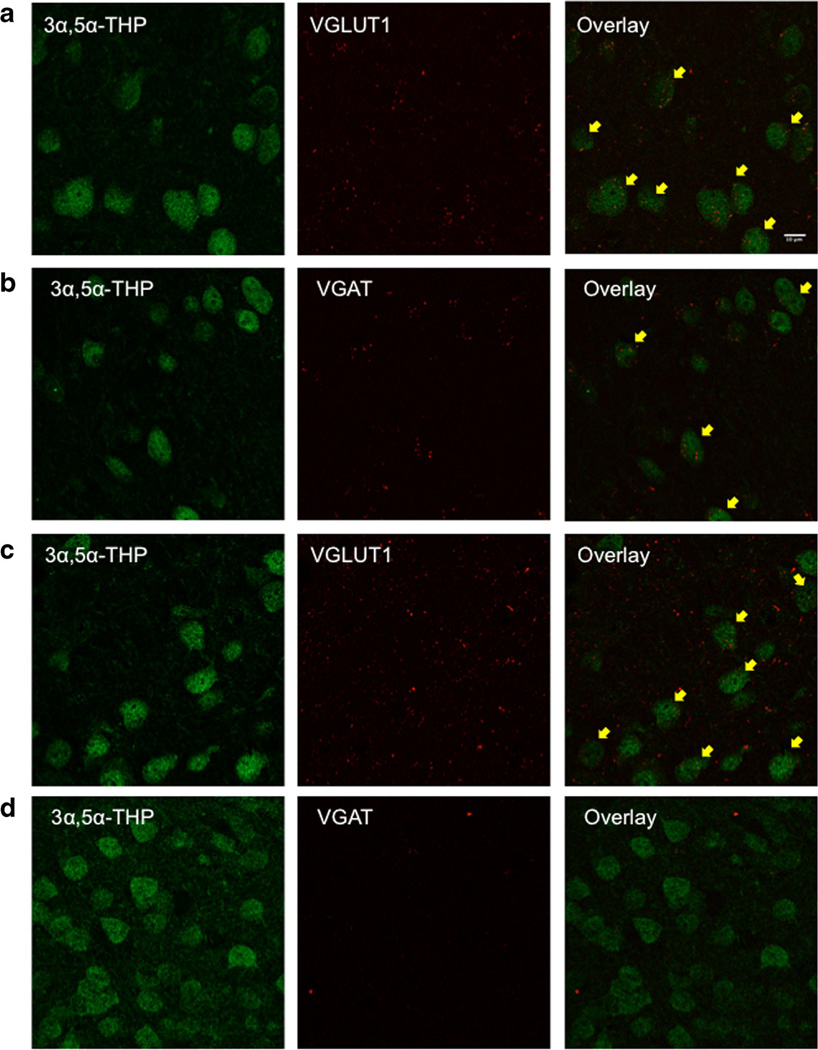
Since the greatest reductions in 3α,5α-THP immunoreactivity were observed in the LA and NAcc shell, we were interested in determining the cell types that synthesize 3α,5α-THP in these regions. Since pyramidal neurons are the primary neuron type in the LA and GABAergic medium spiny neurons are the primary neuron type in the NAcc, we used scanning laser confocal microscopy to identify if VGAT and VGLUT1 localize with 3α,5α-THP in these regions. We found abundant labeling of 3α,5α-THP in both subregions (Fig. 5). Both VGLUT1 (Fig. 5a) and VGAT (Fig. 5b) localized in 3α,5α-THP-positively stained cells in the LA, based on visual and Z-stack analysis. In contrast, we observed VGLUT1 (Fig. 5c) localized in 3α,5α-THP-positively stained cells, but not VGAT (Fig. 5d) in the NAcc shell.
Fig. 5.
Confocal scanning microscopy of 3α,5α-THP-labeled cells that localize with VGLUT1 and VGAT in the LA and NAcc shell. a 3α,5α-THP (green) localizes with VGLUT1 (red) in the LA. b 3α,5α-THP (green) localizes with VGAT (red) in the LA. c 3α,5α-THP (green) localizes with VGLUT1 (red) in the NAcc shell. d 3α,5α-THP (green) does not localize with VGAT (red) in the NAcc shell. Yellow arrows indicate localization of VGLUT1 or VGAT in 3α,5α-THP-positive cells
Discussion
Overall, FSS produced a decrease in 3α,5α-THP levels in the brain and in the periphery in C57BL/6J mice. These results contrast with previous data obtained in Sprague-Dawley rats, where increased 3α,5α-THP levels were observed in the plasma, cerebral cortex, and hypothalamus (Purdy et al. 1991; Vallee et al. 2000). FSS also increases the precursors pregnenolone and progesterone in the rat (Purdy et al. 1991; Vallee et al. 2000), but pregnenolone was not significantly increased in C57BL/6J mice. Therefore, the lack of increase in precursor may contribute to the differential effects of FSS on 3α,5α-THP levels in these mice compared to those in rats. Basal and stress-induced changes in pregnenolone and 3α,5α-THP were very similar to those of the previously published work assessed using GC/MS (Porcu et al. 2010). There was no variation in circulating pregnenolone and 3α,5α-THP levels across time of day, and the decrease in 3α,5α-THP between stressed and nonstressed mice was maintained across time of day. Decreased conversion of progesterone to 3α,5α-THP could also be responsible for the decreased 3α,5α-THP levels obtained in serum following acute swim stress in the present set of experiments since decreased 3α-HSD levels were observed in the adrenals of mice exposed to acute FSS. However, further studies are needed to examine this possibility. These findings may be limited to potent stressors, such as FSS, and may be specific to 3α,5α-THP and not generalize to other neuroactive steroids, including 3α,5α-THDOC.
Test-day levels of corticosterone showed elevations in both nonstressed controls and stressed mice, compared to basal levels. However, the relative increase in corticosterone levels was greater in stressed mice compared to nonstressed controls. Indeed, simple procedural factors, including bleeding order, can increase corticosterone levels up to threefold within 4–8 min in C57BL/6 mice (Kugler et al. 1988). Similar to recent work (Auvinen et al. 2012), corticosterone levels increased across time of day in nonstressed animals, where animals were removed from the vivarium to the novel procedure room. However, the FSS-induced increases in corticosterone were maintained at a constant level, regardless of time of day the mice were stressed.
It is unknown if acute stress alters brain levels of 5α-reductase and 3α-HSD of C57BL/6J mice. Acute FSS increased 5α-reductase levels in the rat prefrontal cortex (Sanchez et al. 2008). In contrast, chronic social isolation stress decreased 5α-reductase levels in several Swiss-Webster mouse brain regions and decreased 3α-HSD levels in the dentate gyrus of the hippocampus (Agis-Balboa et al. 2007). Chronic social isolation decreased 3α,5α-THP levels in frontal cortex, which was associated with decreased 5α-reductase mRNA and protein levels and no change in 3α-HSD mRNA levels (Dong et al. 2001). However, there have been no systematic investigations of 5α-reductase and 3α-HSD levels across mouse brain following acute stress. Therefore, it is not clear how acute swim stress would alter 5α-reductase and 3α-HSD in discrete brain regions following FSS.
Similar differences in 3α,5α-THP biosynthesis in C57BL/6 mice vs rats have been observed in other studies. Ethanol increased cortical and serum 3α,5α-THP levels in rats (Porcu et al. 2010; VanDoren et al. 2000). Similarly, Δ9-tetrahydrocannabinol administration increased 3α,5α-THP levels in the cortex of Sprague-Dawley rats and in the NAcc of Wistar rats (Grobin et al. 2005; Vallee et al. 2014). In contrast, decreased serum 3α,5α-THP and no change in cortical or hippocampal 3α,5α-THP levels were observed in C57BL/6J mice (Porcu et al. 2010, 2014). Similarly, Δ9-tetrahydrocannabinol administration did not change 3α,5α-THP levels in the NAcc of C57BL/6N mice (Vallee et al. 2014). Biosynthetic enzyme activity may be impaired following acute ethanol administration in the C57BL/6J mouse, but not in the rat, which may to lead to divergent changes in 3α,5α-THP levels (Porcu et al. 2010). Furthermore, precursors to the 5α-reduced GABAergic neuroactive steroids, 3α,5α-THP and 3α,5α-THDOC, may be metabolized to corticosterone in plasma and brain following acute ethanol injection in the C57BL/6J mouse (Porcu et al. 2014). Therefore, following acute FSS, pregnenolone and/or progesterone may be metabolized to 3α,5α-THP in rats but may be metabolized to corticosterone in plasma and brain in the C57BL/6J mouse. Together, these data indicate that there are different mechanisms at work in mouse and rat models following acute stress (i.e., acute FSS or acute drug administration) to mediate the differential 3α,5α-THP response.
It has been suggested that increased neurosteroid levels may act to inhibit activity of the hypothalamic-pituitary-adrenal (HPA) axis by increasing GABAergic inhibitory tone in the PVN [for review, see Paul and Purdy 1992]. Many rat studies suggest that decreased 3α,5α-THP is associated with hyperexcitability of the HPA axis (Evans et al. 2012; Genazzani et al. 1996; Owens et al. 1992; Patchev et al. 1994). Additionally, in a Wistar rat model, chronic variable stress was found to alter the GABAergic inputs onto corticotropin-releasing hormone (CRH) neurons within the PVN, with an increase in dendritic contacts and a decrease in soma contacts, which was associated with hyperactivity of the HPA axis (Miklos and Kovacs 2012). However, recent studies in C57BL/6 mice demonstrate that 3α,5α-THDOC administration following acute restraint stress induces a collapse of the chloride gradient, changing the activity of GABA from inhibitory to excitatory in CRH neurons in the PVN (Sarkar et al. 2011). Additionally, early life stress was associated with a loss of 3α,5α-THP suppression of neuronal firing, resulting in hypothalamic hyperexcitability (Gunn et al. 2013). It is possible that the effect of acute stress in C57BL/ 6J mice is similar to the effects of chronic stress in rat models. These effects appear to be associated with decreased neuroactive steroid levels in the PVN and excitatory GABAergic tone following stress in this region.
We observed decreases in 3α,5α-THP immunoreactivity in the PVN, LA, and NAcc shell following FSS, with the greatest effects in the LA (−31.1±13.4 %) and NAcc shell (−31.9± 14.6 %). The purpose of the dual-label experiments was to identify if 3α,5α-THP localized with VGAT or VGLUT1 vesicular markers in these regions and to assess if we could attribute the reductions in 3α,5α-THP immunoreactivity with one specific cell type. Therefore, we conducted a detailed analysis of the primary types of neurons present in these regions, glutamatergic pyramidal neurons in the LA and GABAergic medium spiny neurons in the NAcc, that localized with 3α,5α-THP-positive staining using laser scanning confocal microscopy. In the LA, we observed localization of VGLUT1 and VGAT within the cells that were positively stained with 3α,5α-THP. This indicates that both glutamatergic and GABAergic cells within the LA are 3α,5α-THP-positive and may suggest decreased 3α,5α-THP activity within these cells or the targets of these cells. In contrast, 3α,5α-THP-positively stained cells localized only with VGLUT1 in the NAcc shell, likely indicating that 3α,5α-THP localizes to glutamatergic cells in this subregion. Further studies are needed to determine how FSS specifically alters 3α,5α-THP in these cells in these important subregions of the amygdala and accumbal circuitry.
There is an abundant intracellular accumulation of GABAergic neuroactive steroids (Akk et al. 2005). In the Sprague-Dawley rat hippocampal slice, ethanol or ammonia exposure increased neurosteroid immunofluorescent staining in pyramidal neurons in the CA1 region of the hippocampus while decreasing LTP in this same region (Izumi et al. 2013; Tokuda et al. 2011). When neurosteroidogenesis was blocked with finasteride, a 5α-reductase inhibitor, LTP was restored in CA1 pyramidal cells (Tokuda et al. 2011). Extrapolating the change in neurosteroid levels with the change in neuronal firing (Izumi et al. 2013; Tokuda et al. 2011) and applying this concept to the present results, the observed decrease in 3α,5α-THP levels in pyramidal neurons in the LA would be predicted to facilitate neuronal firing of these neurons following FSS. The results from the present set of experiments were observed using immunohistochemical techniques, which target intracellular changes in 3α,5α-THP.
GABAergic amygdalar projections synapse onto GABAergic inputs to the PVN, resulting in an overall excitation of the PVN through this disinhibitory circuitry (Herman et al. 2005). Lesions of the amygdala reduce HPA axis activity induced by stress (Herman et al. 2004). The role of 3α,5α-THP within the PVN is to maintain a homeostatic state (Gunn et al. 2011). The decreased 3α,5α-THP levels observed in the LA following acute stress, coupled with the collapse of the Cl− gradient within the PVN following acute stress (Sarkar et al. 2011), could potentially explain the hyperexcitable state of the PVN within this circuitry.
Within the NAcc, the role of 3α,5α-THP is not well defined in the literature. Acute restraint stress produced an inhibition of dopamine (DA) release in the NAcc in C57BL/6 mice (Ventura et al. 2001). Intracerebroventricularly administered 3α,5α-THP increased DA release in the NAcc of male Sprague-Dawley rats (Rouge-Pont et al. 2002). In the present experiment, we observed a decrease in 3α,5α-THP immunoreactivity within the NAcc shell, which may be associated with decreased DA release in the NAcc. We observed localization of VGLUT1 in 3α,5α-THP-positively stained cells within this region using laser scanning confocal microscopy. However, we did not observe localization of VGAT in cells positively stained for 3α,5α-THP. Therefore, it is likely that FSS exerts effects indirectly through alterations in 3α,5α-THP and its interaction in glutamatergic cells in this brain region.
Recently, it was shown that acute systemic ethanol injection did not alter cerebral cortex or hippocampal levels of 3α,5α-THP in C57BL/6J mice; however, there were significant increases in brain corticosterone levels in these same brain regions (Porcu et al. 2014). 3α,5α-THP has been shown to potentiate GABA-induced Cl− flux in the cortex and hippocampus of rats (Morrow 2007; Stromberg et al. 2006; Wilson and Biscardi 1997). In Swiss mice, significant increases in progesterone levels were observed in the hippocampus, but no change in the cortex or plasma in response to FSS (Urani et al. 2001). In the present set of experiments, we did not observe changes in 3α,5α-THP following acute FSS in the mPFC or the hippocampus. Therefore, it is quite possible that the lack of change in the brain levels of 3α,5α-THP in the mPFC and hippocampus following acute stress observed in the present experiment in C57BL/6J mice could be due to a change in the biosynthetic pathway, whereby pregnenolone is metabolized to deoxycorticosterone and corticosterone (Morrow 2007), rather than progesterone and 3α,5α-THP. Together, these data support the notion that the C57BL/6J mouse exhibits a unique response to stress that is very different from Sprague-Dawley rats and other mouse models.
The general consensus is that 3α,5α-THP serves to maintain homeostasis following acute stress within the brain, particularly within the PVN (Genazzani et al. 1996; Gunn et al. 2011; Owens et al. 1992; Patchev et al. 1994; Paul and Purdy 1992). In the present work, acute FSS decreased circulating and local levels of 3α,5α-THP in the PVN, LA, and NAcc shell. The mechanism by which decreased circulating 3α,5α-THP levels occur appears to involve decreased 3α-HSD levels in the adrenals of stressed mice. The likely mechanism for deceased 3α,5α-THP levels within the brain may be due to a change in the biosynthetic pathway, with increased deoxycorticosterone and corticosterone levels produced in the C57BL/6J mouse (Morrow 2007; Porcu et al. 2014). Together, these data indicate that the C57BL/6J mouse exhibits a dysregulated stress response and HPA axis activity following acute stress. This mouse model could serves as a useful experimental model for a subpopulation of individuals who show dysregulation in their acute stress responses.
Acknowledgments
This research was supported by the NIAAA INIA UO1-AA020935 (ALM). AMD was supported by UNC Curriculum in Toxicology NIEHS Training Grant T32 ES007126.
Abbreviations
- 3α,5α-THP
(3α,5α)-3-Hydroxy-pregnan-20-one
- 3α,5α-THDOC
(3α,5α)-3,21-Dihydroxypregnan-20-one
- 3α,5β-THP
(3α,5β)-3-Hydroxy-pregnan-20-one
- 5α-DHP
5α-Dehydroxyprogesterone
- 3α-HSD
3α-Hydroxysteroid dehydrogenase
- BLA
Basolateral amygdale
- BNST
Bed nucleusofthe stria terminalis
- CeA
Central nucleusofthe amygdale
- CA1
Cornu ammonis 1
- CA3
Cornu ammonis 3
- CRF
Corticotropin-releasing factor
- DA
Dopamine
- FSS
Forced swim stress
- GC/MS
Gas chromatography/mass spectrometry
- HPA
Hypothalamic-pituitary-adrenal
- IHC
Immunohistochemistry
- LA
Lateral amygdale
- mPFC
Medial prefrontal cortex
- NAcc
Nucleus accumbens
- PVN
Paraventricular hypothalamic nucleus
- PTN
Paraventricular thalamic nucleus
- PBS
Phosphate-buffered saline
- PFA
Paraformaldehyde
- VTA
Ventral tegmental area
- VGLUT1
Vesicular glutamate transporter 1
- VGAT
Vesicular GABA transporter
Footnotes
Conflict of interest The authors declare no conflict of interest.
Contributor Information
Antoniette M. Maldonado-Devincci, Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, 3027 Thurston Bowles Building, CB 7178, Chapel Hill, NC 27599, USA Curriculum in Toxicology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
Matthew C. Beattie, Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, 3027 Thurston Bowles Building, CB 7178, Chapel Hill, NC 27599, USA
Danielle H. Morrow, Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, 3027 Thurston Bowles Building, CB 7178, Chapel Hill, NC 27599, USA
Raechel E. McKinley, Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, 3027 Thurston Bowles Building, CB 7178, Chapel Hill, NC 27599, USA
Jason B. Cook, Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, 3027 Thurston Bowles Building, CB 7178, Chapel Hill, NC 27599, USA Curriculum in Neurobiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
Todd K. O’Buckley, Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, 3027 Thurston Bowles Building, CB 7178, Chapel Hill, NC 27599, USA
A. Leslie Morrow, Email: morrow@med.unc.edu, Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, 3027 Thurston Bowles Building, CB 7178, Chapel Hill, NC 27599, USA; Curriculum in Toxicology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Curriculum in Neurobiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Department of Pharmacology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
References
- Agis-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci U S A. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvinen HE, Romijn JA, Biermasz NR, Pijl H, Havekes LM, Smit JW, Rensen PC, Pereira AM. The effects of high fat diet on the basal activity of the hypothalamus-pituitary-adrenal axis in mice. J Endocrinol. 2012;214:191–197. doi: 10.1530/JOE-12-0056. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Cook JB, Dumitru AM, O’Buckley TK, Morrow AL. Ethanol administration produces divergent changes in GABAergic neuroac-tive steroid immunohistochemistry in the rat brain. Alcohol Clin Exp Res. 2014;38:90–99. doi: 10.1111/acer.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci U S A. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Sun Y, McGregor A, Connor B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology. 2012;63:1315–1326. doi: 10.1016/j.neuropharm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd edn. San Diego: Elsevier; 2007. [Google Scholar]
- Gasior MJ, Carter RB, Goldberg SR, Witkin JM. Anticonvulsant and behavioral effects of neuroactive steroids alone and in conjunction with diazepam. J Pharmacol Exp Ther. 1997;282:543–553. [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Salvestroni C, Guo A-L, Palumbo M, Cela V, Casarisa E, Luisi M, Genazzani AD, Petraglia F. Neurosteroids and regulation of neuroendocrine function. In: Genazzani AR, Petraglia F, Purdy RH, editors. The brain: source and target for sex steroid hormones. New York: Parthenon Publishing Group; 1996. pp. 83–92. [Google Scholar]
- Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther. 2007;116:125–139. doi: 10.1016/j.pharmthera.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin AC, VanDoren MJ, Porrino LJ, Morrow AL. Cortical 3α-hydroxy-5α-pregnan-20-one levels after acute administration of Δ9-tetrahydrocannabinol, cocaine and morphine. Psychopharmacology (Berlin) 2005;179:544–550. doi: 10.1007/s00213-004-2084-3. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Costa E. Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3α,5α-tetrahydroprogesterone (allopregnanolone) availability? Biol. Psychiatry. 1998;44:865–873. doi: 10.1016/s0006-3223(98)00070-5. [DOI] [PubMed] [Google Scholar]
- Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABA(A) receptor interactions: a focus on stress. Front Neurosci. 2011;5:131. doi: 10.3389/fnins.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BG, Cunningham L, Cooper MA, Corteen NL, Seifi M, Swinny JD, Lambert JJ, Belelli D. Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: relevance to neurosteroids and programming of the stress response. J Neurosci Off J Soc Neurosci. 2013;33:19534–19554. doi: 10.1523/JNEUROSCI.1337-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, Figueiredo H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann N Y Acad Sci. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Svrakic N, O’Dell K, Zorumski CF. Ammonia inhibits long-term potentiation via neurosteroid synthesis in hippocampal pyramidal neurons. Neuroscience. 2013;233:166–173. doi: 10.1016/j.neuroscience.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22:2055–2061. [PubMed] [Google Scholar]
- Kokate TG, Banks MK, Magoo T, Yamaguchi S-I, Rogawski MA. Finasteride, a 5α-reductase inhibitor, blocks the anticonvulsant activity of progesterone in mice. J Pharmacol Exp Ther. 1999;288:679–684. [PubMed] [Google Scholar]
- Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, Rogawski MA. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther. 1998;287:553–558. [PubMed] [Google Scholar]
- Kugler J, Lange KW, Kalveram KT. Influence of bleeding order on plasma corticosterone concentration in the mouse. Exp Clin Endocrinol. 1988;91:241–243. doi: 10.1055/s-0029-1210754. [DOI] [PubMed] [Google Scholar]
- Miklos IH, Kovacs KJ. Reorganization of synaptic inputs to the hypothalamic paraventricular nucleus during chronic psychogenic stress in rats. Biol Psychiatry. 2012;71:301–308. doi: 10.1016/j.biopsych.2011.10.027. [DOI] [PubMed] [Google Scholar]
- Morrow AL. Recent developments in the significance and therapeutic relevance of neuroactive steroids—introduction to the special issue. Pharmacol Ther. 2007;116:1–6. doi: 10.1016/j.pharmthera.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Ritchie JC, Nemeroff CB. 5α-Pregnane-3α,21-diol-20-one (THDOC) attenuates mild stress-induced increases in plasma corticosterone via a non-glucocorticoid mechanism: comparison with alprazolam. Brain Res. 1992;573:353–355. doi: 10.1016/0006-8993(92)90788-b. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OFX. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–271. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pericic D, Strac DS, Vlainic J. Interaction of diazepam and swim stress. Brain Res. 2007;1184:81–87. doi: 10.1016/j.brainres.2007.09.039. [DOI] [PubMed] [Google Scholar]
- Pericic D, Svob D, Jazvinscak M, Mirkovic K. Anticonvulsive effect of swim stress in mice. Pharmacol Biochem Behav. 2000;66:879–886. doi: 10.1016/s0091-3057(00)00267-7. [DOI] [PubMed] [Google Scholar]
- Phan VL, Urani A, Romieu P, Maurice T. Strain differences in sigma(1) receptor-mediated behaviours are related to neurosteroid levels. Eur J Neurosci. 2002;15:1523–1534. doi: 10.1046/j.1460-9568.2002.01989.x. [DOI] [PubMed] [Google Scholar]
- Porcu P, Locci A, Santoru F, Beretti R, Morrow AL, Concas A. Failure of acute ethanol administration to alter cerebrocortical and hippocampal allopregnanolone levels in C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 2014;153:340–350. doi: 10.1111/acer.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL. Simultaneous quantification of GABAergic 3α, 5α/3α,5β neuroactive steroids in human and rat serum. Steroids. 2009;74:463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, Morrow AL. Differential effects of ethanol on serum GABAergic 3α,5α/3α,5β neuroactive steroids in mice, rats, cynomolgus monkeys and humans. Alcohol Clin Exp Res. 2010;34:432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAA receptor function and seizure susceptibility. J Neurosci. 2002;22:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouge-Pont F, Mayo W, Marinelli M, Gingras M, Le Moal M, Piazza PV. The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens. Eur J Neurosci. 2002;16:169–173. doi: 10.1046/j.1460-9568.2002.02084.x. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Torres JM, Gavete P, Ortega E. Effects of swim stress on mRNA and protein levels of steroid 5alpha-reductase isozymes in prefrontal cortex of adult male rats. Neurochem Int. 2008;52:426–431. doi: 10.1016/j.neuint.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci. 2011;31:18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RD, Wess MJ, Labarca R, Skolnick P, Paul SM. Acute stress enhances the activity of the GABA receptor-gated chloride ion channel in brain. Brain Res. 1987;411:151–155. doi: 10.1016/0006-8993(87)90692-5. [DOI] [PubMed] [Google Scholar]
- Stromberg J, Haage D, Taube M, Backstrom T, Lundgren P. Neurosteroid modulation of allopregnanolone and GABA effect on the GABA-A receptor. Neuroscience. 2006;143:73–81. doi: 10.1016/j.neuroscience.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J Neurosci. 2011;31:9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urani A, Roman FJ, Phan VL, Su TP, Maurice T. The antidepressant-like effect induced by sigma(1)-receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. J Pharmacol Exp Ther. 2001;298:1269–1279. [PubMed] [Google Scholar]
- Vallee M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal Biochem. 2000;287:153–166. doi: 10.1006/abio.2000.4841. [DOI] [PubMed] [Google Scholar]
- Vallee M, Vitiello S, Bellocchio L, Hebert-Chatelain E, Monlezun S, Martin-Garcia E, Kasanetz F, Baillie GL, Panin F, Cathala A, Roullot-Lacarriere V, Fabre S, Hurst DP, Lynch DL, Shore DM, Deroche-Gamonet V, Spampinato U, Revest JM, Maldonado R, Reggio PH, Ross RA, Marsicano G, Piazza PV. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343:94–98. doi: 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Cabib S, Puglisi-Allegra S. Opposite genotype-dependent mesocorticolimbic dopamine response to stress. Neuroscience. 2001;104:627–631. doi: 10.1016/s0306-4522(01)00160-9. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Biscardi R. Influence of gender and brain region on neurosteroid modulation of GABA responses in rats. Life Sci. 1997;60:1679–1691. doi: 10.1016/s0024-3205(97)00110-0. [DOI] [PubMed] [Google Scholar]