Abstract
Hypotheses exploring the influence of dietary conditions on the life history trade-off between survival and reproductive success are extensively tested in female insects, but are rarely explored in males. Here, the impact of dietary quality and female access on age-specific reproduction and survival of male Mexican fruit flies, Anastrepha ludens Loew (Diptera: Tephritidae), are examined. There is a clear cost of female access for males with access to dietary protein, measurable as a decrease in life expectancy, which is further influenced by the age when females are introduced. A protein deficient diet reduces the lifespan benefit of virginity and masks the detrimental effect of female access on male life expectancy. Dietary protein is not necessary for reproductive success, but access to protein at eclosion improves the lifetime reproductive success of males compared to when it is delayed. Overall, reproductive success diminishes as the male flies age, regardless of the dietary conditions, providing evidence for reproductive senescence in males. Delaying the males’ access to a protein source fails to influence the negative effect of age on reproductive ability. Because age specific reproductive rates decline with age, regardless of diet, male fitness does not benefit from lifespan extension. Therefore, males can be expected to allocate available resources towards reproductive effort in favour of extended lifespan, regardless of mate and protein availability.
Keywords: Cost of mating, life history trade-offs, male ageing, male reproduction, resource allocation, senescence, Tephritidae
Introduction
When nutritional scarcity is encountered in an environment, reduced rates of senescence are often observed in animals, resulting in an extension in lifespan whilst reproductive effort is reduced or arrested (Weithoff, 2007; Carey et al., 2008). By avoiding the cost of reproduction and allocating available nutrients to somatic upkeep during unfavourable dietary conditions, organisms may improve their fitness by surviving until successful reproduction can begin or resume (Wiley, 1974; Tuljapurkar, 1990; Barrett et al., 2009). Although there is a deep literature on the physiological response to dearth periods, most research conducted on the factors affecting reproductive trade-offs in insects focuses primarily on females in response to dietary restriction and the timing of egg production (Awmack & Leather, 2002; Boggs & Freeman, 2005; Carey et al., 2008; Harwood et al., 2013). By contrast, studies investigating reproductive trade-offs in male insects are uncommon, with research primarily focused on the cost of reproduction in the absence of interactions with nutritional conditions (Partridge & Farquhar, 1981; Kotiaho & Simmons, 2003; Paukku & Kotiaho, 2005; Papadopoulos et al., 2010). Those studies that have tested the effects of dietary conditions on male reproductive trade-offs in insects find that poor nutritional conditions will cause a decline in reproductive effort , but the effects of the suboptimal diets on survival have been inconsistent (Hunt et al., 2004; Judge et al., 2008; Attisano et al., 2012).
In male insects, reproductive costs are linked to many of the sexual behaviours and physiological processes related to courtship, signalling, mating and insemination (Martin & Hosken, 2004; Kuijper et al., 2006; Papadopoulos, et al., 2010). Accordingly, male reproductive effort is expected to be postponed when the likelihood of successful reproduction is low, thereby preventing the cost of reproduction until the probability of reproductive success improves (Perrin & Sibly, 1993). Such a deferment in the cost of reproduction is well documented in the females of several species in the family Tephritidae (Carey, 2003). Specifically, female tephritids will enter a waiting period characterized by little or no reproductive effort and a lowered risk of mortality during unfavourable reproductive conditions. When conditions improve, the waiting period ends and the females experience an increase in age specific reproductive ability and a reduced risk of mortality (Carey et al., 1998).
In male tephritids, the lack of female access alone will not elicit a comparable waiting period, since males will still form leks and produce and release sexual attractant pheromones in the absence of females, each of which potentially contribute to the cost of reproduction (Liedo et al., 2002; Johansson et al., 2005; Zhang et al., 2006). A source of dietary protein appears to be essential for maximizing male tephritid reproductive ability, with copulation frequency, sexual signalling rate, pheromone production, and participation in leks all dependent on diet (Aluja et al., 2001; Yuval et al., 2002; Liedo et al., 2013). Therefore, the absence of certain dietary resources appears to prevent or reduce the reproductive effort of males. This reduced reproductive effort due to poor dietary conditions is expected to allow males to avoid the cost of reproduction and allow nutritional resources to be used for somatic upkeep, therefore improving life expectancy (Hunt et al., 2004; Attisano et al., 2012), especially when females are not available. Since an extended lifespan is assumed to only be adaptive if it results in greater reproductive success later in life (Bonduriansky et al., 2008), such an improvement in lifespan is expected to result in greater reproductive potential at advanced ages. Results contrary to this hypothesis have been presented for the males of some dipteran species, in which male reproductive rates and attractiveness have been observed to decrease with age (Bonduriansky & Brassil, 2005; Papanastasiou et al., 2011), so that lifespan extension cannot be expected to improve male fitness.
Accordingly, the effects of dietary conditions and female availability on the longevity and age specific insemination success of the male Mexican fruit fly (Anastrepha ludens Loew) are tested to determine if male reproductive effort can be delayed, thus extending male lifespan and slow reproductive senescence, if observed . The Mexican fruit fly, a significant economic pest (Dominguez et al., 2010), has been shown to be a useful model organism for understanding demographic trade-offs (Carey et al., 2008; Carey & Molleman, 2010). Much of what is known about the reproductive behaviour and life history of the male Mexican fruit fly is derived from studies focused on its effectiveness in the sterile insect technique (Shaw et al., 1967; Robacker & Garcia, 1993). These studies rarely focus on the trade-offs between reproduction and survival in males, and male reproductive ability has not been observed through the entire lifespan (Aluja et al., 2000; Aluja et al., 2008). Therefore, the data presented here, regarding male Mexican fruit fly life history characteristics, will be useful in controlling their populations and inform hypotheses regarding life history trade-offs in male insects.
Materials and methods
Husbandry
Flies were obtained from the Moscafrut mass-production facility at Metapa de Domínguez, Chiapas, Mexico, and reared using the techniques described by Dominguez et al. (2010). When these experiments were conducted the colony was approximately 170 generations old. Wild flies have been introduced into this colony periodically to maintain genetic diversity. All experiments were conducted under laboratory conditions (LD 12:12 photocycle at 23 ± 3° C and 60-75 % relative humidity). Adults were separated by sex at eclosion, and 450 males were each individually housed in 4 × 4 × 10 cm Plexiglas cages from eclosion until death. Treatments consisted of combinations of dietary conditions and female availability, with 50 males assigned to each treatment. All flies were provided daily access to water and to one of two ad libitum solid diets depending on the treatment type: 1) full diet, which included a carbohydrate and a yeast derived protein source (3:1 ratio mixture of sugar to yeast hydrolysate enzymatic; MP Biomedicals, LLC, Santa Ana, California) or 2) a diet consisting only of a carbohydrate (sugar only diet). The experimental control consisted of 50 males provided ad libitum solid full diet from eclosion, adult age 0 days, until death and never paired with females.
The difference in life expectancy of males with female access, as described in the following sections, compared to those in the control served as a proxy for the cost of reproduction. Cohorts of reproductive virgin females (age 10-15 days) were housed in large cages with several hundred same aged females and provided with constant access to water and full diet. Cohorts of virgin females were maintained for the duration of the study and resupplied with new females weekly to maintain the availability of 10-15 day olds. In the appropriate treatments, a single female was introduced into each of the males’ cage. After 24 hours of access, the female was removed and a new virgin female was presented to each male.
Diet and female access
Three treatments were used to establish the cost of reproduction and the influence of diet type, as outlined in the husbandry section, on male longevity: 1a) full diet from eclosion until death with constant female access, in which males received access to a new virgin female daily for their entire lifespan; 2a) sugar diet from eclosion until death with constant access to virgin females for the entire lifespan; or 3a) sugar-only diet with no female access. The cost of female access and the effect of diet quality on male lifespan were estimated by comparing the life expectancy of the males in each treatment with that of males in the experimental control described in the husbandry section (full diet from eclosion with no female access).
Truncated reproduction
To describe the influence of prior female access on male lifespan after females were no longer available, 50 males were assigned to two truncated access treatments: 1b) constant access to full diet for the entire lifespan, with virgin females only provided from eclosion until age 20 days, or 2b) sugar diet for the entire lifespan, with virgin females only provided until age 20 days. These two treatments were compared to the control (full diet with no female access) and those testing the effect of diet and female access described in the previous section.
Delayed reproduction
In an attempt to delay reproduction in males, access to virgin females, to full diet, and to both females and full diet were delayed for 20 days post eclosion. Fifty males were tested under each of the following delayed reproduction treatments: 1c) constant access to full diet from eclosion until death, while access to virgin females was delayed for 20 days, after which females were provided daily until death; 2c) sugar diet provided until age 20 days, after which full diet was provided until death, while daily virgin females were provided daily from eclosion until death; or 3c) both full diet and virgin females delayed until age 21 days, prior to which males were given sugar diet and not provided females. The treatment in which full diet and female access was constant for the entire lifespan (Treatment 1a from diet and female access) was included for a factorial design to determine if delayed diet or female access improved the males life expectancy compared to when reproduction was expected to be continuous.
Diet and age specific reproduction
To quantify the effect of age and dietary conditions on male lifetime reproductive ability, the insemination success of twenty males from the treatments when 1a) full diet and female access were constant, 2a) sugar only diet and female access were constant, and 2c) full diet was delayed for 20 days while female access was constant, were monitored every fifth day beginning at eclosion. The 60 females (20 from each of the 3 treatments) associated with these males on the observation days were removed after being paired and then held for 15 days in individual cages with an egg laying substrate (silicon coated mesh) and access to water and full diet. Eggs were collected, placed inside a plastic petri dish on a moist sponge wrapped in black satin, and observed for larval hatch over an additional 15 day period. The presence of larvae indicated successful insemination for the male at the age when it was paired with the female, while a lack of larval hatch was interpreted as failed insemination. Daily insemination success was recorded as the proportion of males associated with larval production at each observation period.
Statistical analysis
The effect of diet and female access on male life expectancy was tested with two linear regressions. The first assumed the control (full diet from eclosion until death, with no female access) as the baseline for estimating the effects of constant diet, constant female access, and truncated female access on the male lifespan. The second assessed the effects of delaying female access, delaying full diet, and delaying both on lifespan compared to when both female access and full diet were constant (treatment 1a). This treatment (1a) was selected as the baseline to determine if delaying access to females and/or full diet would lead to lifespan extension compared to when reproduction was assumed to be constant. Additional pairwise comparisons were made among treatments with two-tailed t-tests. A final linear regression model analysed the effects and interaction of diet type and age, on male insemination success, with treatment 1a (full diet and female access are constant) selected as the intercept and age was analysed as a continuous independent variable.
Results
Diet and female access
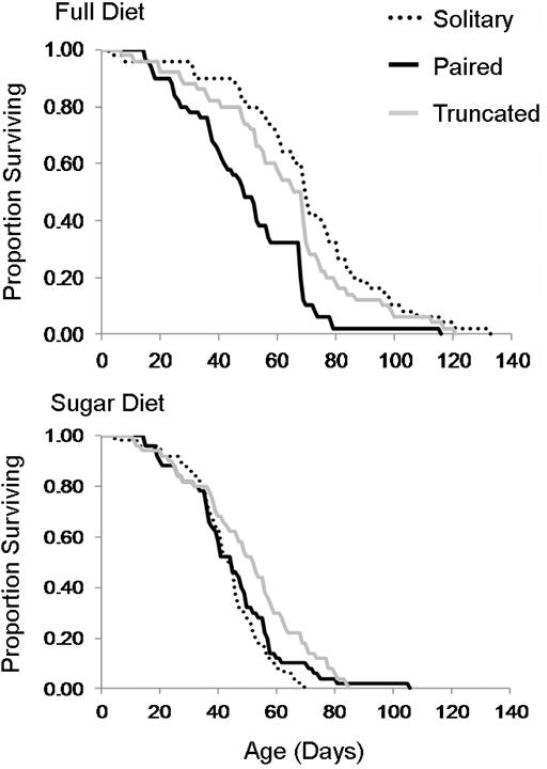
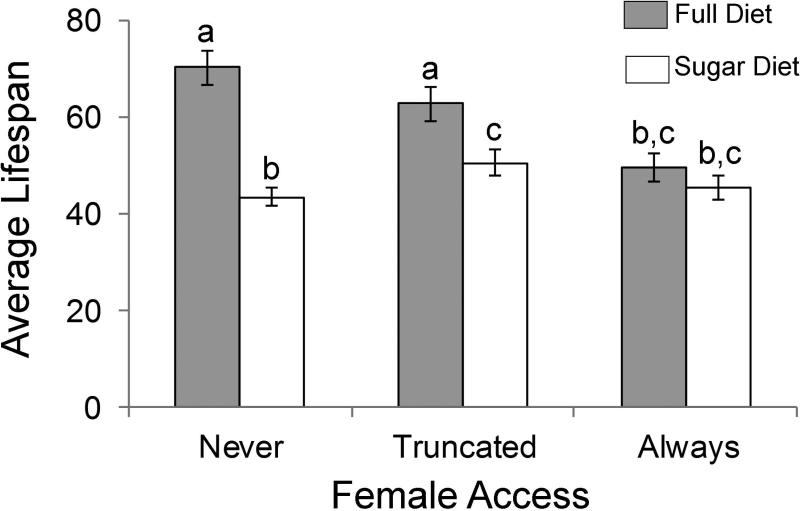
The life expectancy of males fed full diet and never paired with females was 70.32 days. Female access reduced the life expectancy of males fed full diet by 20.74 days (t = −5.11, P < 0.001), but female access was not observed to reduce the probability of survival until after age 20 days (Fig. 1 and 2). The life expectancy of males on sugar diet without female access was reduced by 26.94 days compared to when full diet was provided (t = −6.64, P < 0.001; Fig. 2). When sugar diet was combined with constant female access the male life expectancy was 25.04 days less than that of control flies (t = −3.95, P < 0.001), but was not significantly different from the life expectancy of males supplied full diet and constant female access (t = −1.31, P =0.20; Fig. 2). There was no significant difference in the life expectancies of sugar fed males with and without female access (t = −0.61, P =0.54; Fig. 2), and age specific survival was similar in both of these treatments (Fig. 1).
Fig. 1.
Age specific survival of adult male Mexican fruit flies, Anastrepha ludens when fed either a full diet (3:1 sugar to yeast protein) or sugar only diet, when female access was prevented (solitary), constant from eclosion until death (paired), or only provided until age 20 days (truncated).
Fig. 2.
The average lifespan (days) ± SE of male Mexican fruit flies, Anastrepha ludens provided either a full diet (3:1 sugar to yeast protein) or a sugar only diet, while female access was prevented (never), only provided until age 20 days (truncated), or constant from eclosion until death (always). Averages with different letters represent significant differences (P < 0.05) among treatments, as determined through a linear regression and subsequent t-tests.
Truncated female access
Truncated female access combined with full diet did not reduce the life expectancy of males relative to the control (t = −1.84, P = 0.07), and extended the lifespan relative to those that had constant female access by 13.10 days (t = 3.27, P = 0.001; Fig. 2). In combination with sugar diet, the truncated female access resulted in a significant loss of life expectancy compared to the control (t = 1.75, P = 0.01), but lessened the loss of life expectancy associated with the sugar diet by 7.30 days (t = −2.15, P = 0.03), and appeared to improve age specific survival after age 40 days compared to the other sugar diet treatments (Fig. 1). The life expectancy of males with truncated access to females was not significantly different from those sugar fed males with constant female access (t = −1.40, P = 0.16; Fig. 2).
Delayed reproduction
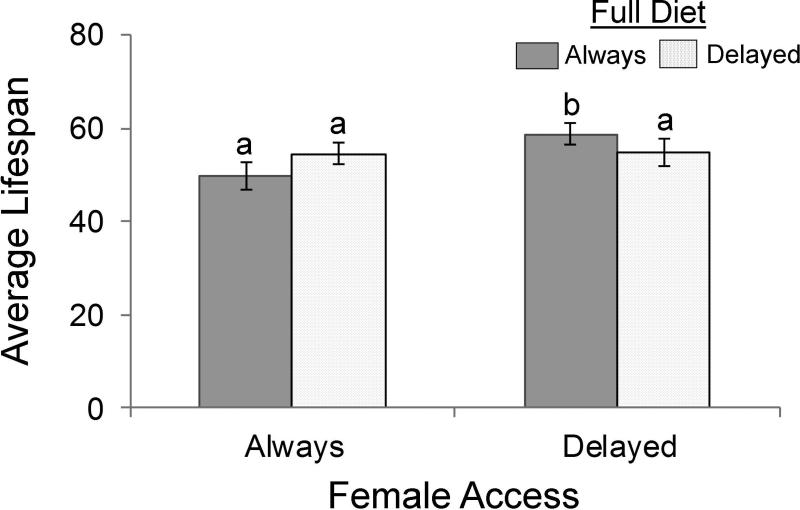
Relative to the life expectancy of males with constant access to full diet and females, delaying female access for 20 days when access to full diet was contestant improved the life expectancy of males by 9.20 days (t = 2.38, P = 0.020; Fig. 3), while delaying only full diet when female access was constant and delaying access to both full diet and females for 20 days did not significantly affect the life expectancy of males (t =1.30, P = 0.199 and t = −1.96, P = 0.107, respectively; Fig. 3).
Fig. 3.
The average lifespan (days) ± SE of male Mexican fruit flies, Anastrepha ludens provided full diet (3:1 sugar to protein derived yeast) at eclosion (always), or at age 21 days (delayed), prior to which only sugar was provided, when females were provided beginning at eclosion (always), or beginning at age 21 days (delayed). Averages with different letters represent significant differences (P < 0.05) among treatments, as determined through a linear regression and subsequent t-tests.
Diet and age specific reproduction
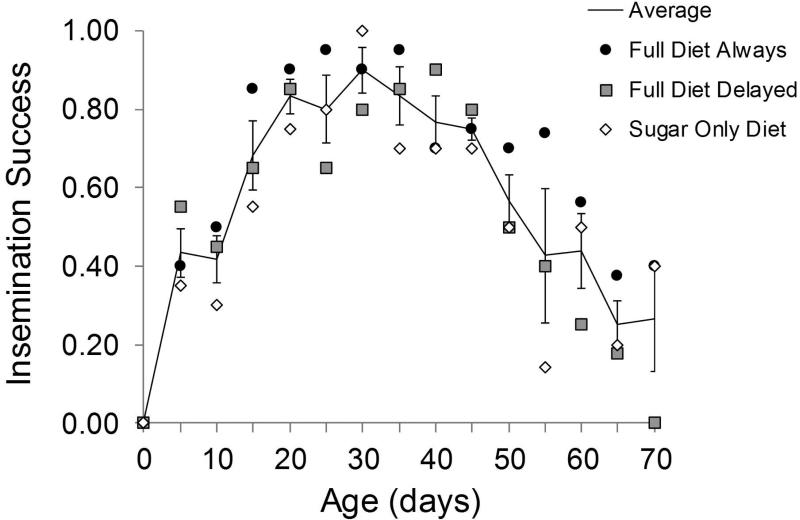
Male insemination success was dependent on age and described to behave as a quadratic function (t = −10.86, P < 0.001; Table 1). Fig. 4 demonstrates how insemination success first increased with age and then decreased after approximately day 30. A two-piece linear function with a change point at day 30 was also tested, but both models resulted in the same conclusion for the treatment effects (diet and female access), so only the quadratic fitting is presented here. The diet treatments were found to have a significant effect on average insemination success (F2, 40 = 4.07, P = 0.024), with both delayed full diet and sugar diets reducing average insemination success compared with constant full diet (t = −2.31, P = 0.025; t = −2.60, P = 0.012 respectively; Table 1). However, there was no difference in the insemination success of males provided the sugar diet and those that experienced a 20 day delay in access to full diet (t = 0.04, P = 0.966). The interaction of age and diet was not significant, so that the effect of age on reproductive ability was not influenced by the dietary conditions.
Table 1.
The estimated effect of each diet factor (level) and the covariate Age on the average (± SE) proportion of male Mexican fruit flies Anastrepha ludens that successfully inseminated females. The estimate shows how the diet affects insemination success relative to the intercept (full diet), with the t-values and P-values calculated from two tail t-tests. Age Squared shows the quadratic effect of age on insemination success (See Fig. 4).
| Estimate | t value | P | |
|---|---|---|---|
| Full Diet (Intercept) | 0.242 ±0.065 | 3.705 | 0.001 |
| Diet (level) | |||
| Sugar Only | −0.123 ±0.053 | −2.313 | 0.026 |
| Delayed Full Diet | −0.139 ±0.053 | −2.604 | 0.013 |
| Covariates | |||
| Age | 0.039 ±0.004 | 10.255 | < 0.001 |
| Age Squared | −0.001 ±0.000 | −10.863 | < 0.001 |
Fig. 4.
Age specific insemination success of male Mexican fruit flies, Anastrepha ludens measured as the proportion of females to lay eggs that produced larvae, when full diet was provided from eclsoion until death (full diet always), when it was delayed until age 21 days, prior to which sugar diet was provided (full diet delayed), and when only sugar diet was provided (sugar diet). The line is the average proportion of males to exhibit successful insemination across all diets at each age with error bars representing the standard error about the mean at each age.
Discussion
Female access reduces by 30% the life expectancy of male Mexican fruit flies, Anastrepha ludens, fed a full diet, a result consistent with the cost of male reproduction reported in other insects (Kotiaho & Simmons, 2003; Martin & Hosken, 2004; Papadopoulos, et al., 2010). This reproductive cost is affected by the timing and duration that females are available, so truncated and delayed female access improves male life expectancy compared to when female access is constant. On average, Mexican fruit fly males are not expected to be sexually mature until age 10 days (Pereira et al. 2013) and reproductive success does not appear to peak until after age 20 days. Therefore, truncated female access likely improves the life expectancy of males by reducing their opportunities to mate. Similarly, delaying female access lessens its cost on male life expectancy because reproduction is postponed until ages when reproductive ability appears to be declining. Therefore, delaying reproduction limits the males’ opportunity to mate until they are older and less likely to copulate.
Sugar diet is detrimental to the lifespan of male Mexican fruit flies, reducing the life expectancy by 40% compared to unpaired males fed full diet. Surprisingly, the life expectancies of paired and unpaired males fed sugar diet are not different. Initially this result appears to suggest that males are avoiding the cost of female access when the poor quality diet is available, supporting the assumptions of optimal resource allocation models (Perrin & Sibly, 1993). However, sugar fed males still mate when provided females, albeit at reduced success. If sugar diet prevents the cost of female access, the life expectancy of males with truncated female access should be the same as those of paired and unpaired males. Instead, truncated female access increases the life expectancy of sugar fed males. It is unclear why truncated female access extends the lifespan of the sugar fed males, but the prior female exposure may reduce male reproductive effort later in life, as observed in the tephritid, Ceratitis capitata (Liedo, et al., 2002). It is unlikely that sugar fed males are avoiding the cost of reproduction. They still inseminate females and their lifespan appears affected by prior female access. Instead, sugar diet acts as a limiting factor on survival, as observed in some other insect species (Judge et al., 2008; Harwood et al., 2013). Therefore, dietary conditions have a stronger influence on male Mexican fruit fly survival than female access.
Contrary to the effect of dietary timing in female tephritids (Carey et al., 1998), delaying access to full diet does not improve the life expectancy of male Mexican fruit flies. Additionally, there was no improvement in male life expectancy when both female access and full diet are delayed. Consequently, sugar diet at eclosion nullifies the improvement in life expectancy associated with delaying female access.
As observed previously, the reproductive ability of male Mexican fruit flies is influenced by diet (Liedo et al., 2013; Pereira et al., 2013). While the nutrients in the full diet are not required for successful insemination, they are required to maximise lifetime copulation success. Any period of sugar only diet reduces the overall insemination success of males. This reduction in insemination success may result from a decrease in pheromone production, as reported by Liedo et al. (2013), which are needed to initiate copulation. Additionally, the sugar diet may weaken the males in some way, making them less likely to copulate. The loss of reproductive success due to sugar diet cannot be reversed when full diet is provided at age 20 days. Therefore, the lack of appropriate nutrients early in life constrains the male's lifetime mating ability.
The effect of age on reproductive ability does not vary among diet types. Regardless of dietary treatment, males demonstrate three distinct age specific reproductive periods corresponding to those observed in female insects (Novoseltsev et al., 2003; Novoseltsev et al., 2004). First is the period of reproductive onset, from age 0-20 days, when the males began to mature. Next, reproductive maturity is reached between ages 25 and 30 days, as demonstrated by the plateau of reproductive success when the highest levels of insemination success are reached. Finally, the period of maturity ends with the onset of reproductive senescence at 30 days, when insemination success begins to decline. Physiologically controlled reproductive senescence is well documented in females from various species (Austad, 2010; Haaga et al., 2010), but sexual selection theory and experimental data suggest that male reproductive performance should increase with age (Williams, 1966; Bonduriansky et al., 2008; Maklakov et al., 2009). Contrary to these predictions, reproductive senescence in the male Mexican fruit fly began when nearly 80% of the males were still alive. Male tephritids are reported to decrease reproductive effort in a response to prior reproductive success and age (Liedo et al., 2002; Lopez-Guillen et al., 2008), and the willingness of the female tephritid, C. capitata, to mate with males is observed to be negatively correlated with the male age (Papanastasiou et al., 2011). Nevertheless, these changes in behaviour may be linked to a physiological decline in reproductive ability due to senescence, and would therefore be mutually affecting. Further investigation is required to isolate the effects of each on age specific reproduction.
Based on these results, male Mexican fruit flies do not gain a reproductive advantage by avoiding reproductive effort when dietary protein and females are unavailable. The males are still able to reproduce successfully in the absence of a protein source, even though the poor dietary conditions shorten their life expectancy and constrain life time reproductive ability. Additionally, any delay in reproductive effort is expected to result in a loss of reproductive opportunity associated with ageing, so that the males cannot gain a fitness benefit through delaying reproduction and extending the lifespan. Therefore, it can be expected that male Mexican fruit flies in their natural environment will attempt to attract and copulate with females regardless of the nutritional conditions encountered, since delaying reproduction decreases their fitness. Moreover, the possibility of male reproductive senescence, in which male reproductive ability is affected by both natural lifespan and the onset of senescence, challenges the hypotheses of the male disposable soma theory, in which male reproductive effort should increase with age (Williams, 1966).
Acknowledgements
We thank R.E. Bustamente, S. E. Salgado, E. De Leon, R. Rincon, A. Oropeza S. L. Rodriguez, and G. Rodas for logistical support and assistance in conducting experiments and J.M. Hash, N.T. Papadopoulos, F. G. Zalom, and P. Lower for comments on earlier drafts and editorial assistance. This research was funded through the NIH/NIA PO1 programme, the Biodemographic Determinants of Life Span (P01 AG022500-01 and P01 AG08761-10).
References
- Aluja M, Jacome I, Macias-Ordonez R. Effect of adult nutrition on male sexual performance in four neotropical fruit fly species of the genus Anastrepha (Diptera : Tephritidae). Journal of Insect Behavior. 2001;14:759–775. [Google Scholar]
- Aluja M, Perez-Staples D, Sivinski J, et al. Effects of male condition on fitness in two tropical tephritid flies with contrasting life histories. Animal Behaviour. 2008;76:1997–2009. [Google Scholar]
- Aluja M, Pinero J, Jacome I, et al. Behavior of flies in the genus Anastrepha (Trypetinae: Toxotrypanini). In: Aluja M, Norrbom AL, editors. Fruit flies (Tephritidae): Phylogeny and Evolution of Behavior. CRC Press; London: 2000. pp. 375–408. [Google Scholar]
- Attisano A, Moore AJ, Moore PJ. Reproduction-longevity trade-offs reflect diet, not adaptation. Journal of Evolutionary Biology. 2012;25:873–880. doi: 10.1111/j.1420-9101.2012.02476.x. [DOI] [PubMed] [Google Scholar]
- Austad SN. Animal models of reproductive aging: what can they tell us? Annals of the New York Academy of Sciences. 2010;1204:123–126. doi: 10.1111/j.1749-6632.2010.05609.x. [DOI] [PubMed] [Google Scholar]
- Awmack CS, Leather SR. Host plant quality and fecundity in herbivorous insects. Annual Review of Entomology. 2002;47:817–844. doi: 10.1146/annurev.ento.47.091201.145300. [DOI] [PubMed] [Google Scholar]
- Barrett ELB, Moore AJ, Moore PJ. Diet and social conditions during sexual maturation have unpredictable influences on female life history trade-offs. Journal of Evolutionary Biology. 2009;22:571–581. doi: 10.1111/j.1420-9101.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Boggs C, Freeman K. Larval food limitation in butterflies: effects on adult resource allocation and fitness. Oecologia. 2005;144:353–361. doi: 10.1007/s00442-005-0076-6. [DOI] [PubMed] [Google Scholar]
- Bonduriansky R, Brassil CE. Reproductive ageing and sexual selection on male body size in a wild population of antler flies (Protopiophila litigata). Journal of Evolutionary Biology. 2005;18:1332–1340. doi: 10.1111/j.1420-9101.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- Bonduriansky R, Maklakov A, Zajitschek F, et al. Sexual selection, sexual conflict and the evolution of ageing and life span. Functional Ecology. 2008;22:443–453. [Google Scholar]
- Carey JR. Longevity: The Biology and Demography of Life Span. Princeton University Press; Princeton, New Jersey: 2003. [Google Scholar]
- Carey JR, Harshman LG, Liedo P, et al. Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell. 2008;7:470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Muller HG, et al. Dual modes of aging in Mediterranean fruit fly females. Science. 1998;281:996–998. doi: 10.1126/science.281.5379.996. [DOI] [PubMed] [Google Scholar]
- Carey JR, Molleman F. Reproductive aging in tephritid fruit flies. Annals of the New York Academy of Sciences. 2010;1204:139–148. doi: 10.1111/j.1749-6632.2010.05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez J, Artiaga T, Solís E, et al. Métodos de colonización y cría masiva. In: Montoya P, Toledo J, Hernandez E, editors. Moscas de la Frutas: Fundamentos y Procedimientos Para su Manejo. S y G Editores; Distrito Federal México, México: 2010. pp. 259–276. [Google Scholar]
- Haaga J, O'connor K, Weinstein M, et al. Reproductive aging: theoretical perspectives, mechanisms, nonhuman models, and health correlates. Annals of the New York Academy of Sciences. 2010;1204:1–10. doi: 10.1111/j.1749-6632.2010.05700.x. [DOI] [PubMed] [Google Scholar]
- Harwood JF, Chen K, Müller H-G, et al. Effects of diet and host access on fecundity and lifespan in two fruit fly species with different life-history patterns. Physiological Entomology. 2013;38:81–88. doi: 10.1111/phen.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J, Brooks R, Jennions MD, et al. High quality males field crickets invest heavily in sexual display but die young. Nature. 2004;432:1024–1027. doi: 10.1038/nature03084. [DOI] [PubMed] [Google Scholar]
- Johansson BG, Jones TM, Widemo F. Cost of pheromone production in a lekking Drosophila. Animal Behaviour. 2005;69:851–858. [Google Scholar]
- Judge KA, Ting JJ, Gwynne DT. Condition dependence of male life span and calling effort in a field cricket. Evolution. 2008;62:868–878. doi: 10.1111/j.1558-5646.2008.00318.x. [DOI] [PubMed] [Google Scholar]
- Kotiaho JS, Simmons LW. Longevity cost of reproduction for males but no longevity cost of mating or courtship for females in the male-dimorphic dung beetle Onthophagus binodis. Journal of Insect Physiology. 2003;49:817–822. doi: 10.1016/S0022-1910(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Kuijper B, Stewart AD, Rice WR. The cost of mating rises nonlinearly with copulation frequency in a laboratory population of Drosophila melanogaster. Journal of Evolutionary Biology. 2006;19:1795–1802. doi: 10.1111/j.1420-9101.2006.01186.x. [DOI] [PubMed] [Google Scholar]
- Liedo P, De Leon E, Barrios MI, et al. Effect of age on the mating propensity of the Mediterranean fruit fly (Diptera: Tephritidae). Florida Entomologist. 2002;85:94–101. [Google Scholar]
- Liedo P, Orozco D, Cruz-Lopez L, et al. Effect of post-teneral diets on the performance of sterile Anastrepha ludens and Anastrepha obliqua fruit flies. Journal of Applied Entomology. 2013;137:49–60. [Google Scholar]
- Lopez-Guillen G, Cruz-Lopez L, Malo EA, et al. Factors influencing the release of volatiles in Anastrepha obliqua males (Diptera : Tephritidae). Environmental Entomology. 2008;37:876–882. doi: 10.1603/0046-225x(2008)37[876:fitrov]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Maklakov AA, Hall MD, Simpson SJ, et al. Sex differences in nutrient-dependent reproductive ageing. Aging Cell. 2009;8:324–330. doi: 10.1111/j.1474-9726.2009.00479.x. [DOI] [PubMed] [Google Scholar]
- Martin OY, Hosken DJ. Copulation reduces male but not female longevity in Saltella sphondylli (Diptera : Sepsidae). Journal of Evolutionary Biology. 2004;17:357–362. doi: 10.1046/j.1420-9101.2003.00668.x. [DOI] [PubMed] [Google Scholar]
- Novoseltsev VN, Carey RJ, Novoseltseva JA, et al. Systemic mechanisms of individual reproductive life history in female medflies. Mechanisms of Ageing and Development. 2004;125:77–87. doi: 10.1016/j.mad.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Novoseltsev VN, Novoseltseva JA, Boyko SI, et al. What fecundity patterns indicate about aging and longevity: insights from Drosophila studies. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2003;58:484–494. doi: 10.1093/gerona/58.6.b484. [DOI] [PubMed] [Google Scholar]
- Papadopoulos NT, Liedo P, Muller HG, et al. Cost of reproduction in male medflies: the primacy of sexual courting in extreme longevity reduction. Journal of Insect Physiology. 2010;56:283–287. doi: 10.1016/j.jinsphys.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanastasiou SA, Diamantidis AD, Nakas CT, et al. Dual reproductive cost of aging in male medflies: dramatic decrease in mating competitiveness and gradual reduction in mating performance. Journal of Insect Physiology. 2011;57:1368–1374. doi: 10.1016/j.jinsphys.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Farquhar M. Sexual-activity reduces lifespan of male fruitflies. Nature. 1981;294:580–582. [Google Scholar]
- Paukku S, Kotiaho JS. Cost of reproduction in Callosobruchus maculatus: effects of mating on male longevity and the effect of male mating status on female longevity. Journal of Insect Physiology. 2005;51:1220–1226. doi: 10.1016/j.jinsphys.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Pereira R, Teal PEA, Conway H, et al. Influence of methoprene and dietary protein on maturation and sexual performance of sterile Anastrepha ludens (Diptera:Tephritidae). Journal of Applied Entomology. 2013;137:49–60. [Google Scholar]
- Perrin N, Sibly RM. Dynamic models of energy allocation and investment. Annual Review of Ecology and Systematics. 1993;24:379–410. [Google Scholar]
- Robacker DC, Garcia JA. Effects of age, time of day, feeding history, and gamma irradiation on attraction of Mexican fruit flies (Diptera: Tephritidae), to bacterial odor in laboratory experiments. Environmental Entomology. 1993;22:1367–1374. [Google Scholar]
- Shaw JG, Sanchezr M, Spishako LM, et al. Dispersal and migration of tepa-sterilized Mexican fruit flies. Journal of Economic Entomology. 1967;60:992–994. [Google Scholar]
- Tuljapurkar S. Delayed reproduction and fitness in variable environments. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:1139–1143. doi: 10.1073/pnas.87.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weithoff G. Dietary restriction in two rotifer species: the effect of the length of food deprivation on life span and reproduction. Oecologia. 2007;153:303–308. doi: 10.1007/s00442-007-0739-6. [DOI] [PubMed] [Google Scholar]
- Wiley RH. Effects of delayed reproduction on survival, fecundity, and rate of population increase. American Naturalist. 1974;108:705–709. [Google Scholar]
- Williams GC. Natural selection, the costs of reproduction, and a refinement of Lack's principle. The American Naturalist. 1966;100:687–690. [Google Scholar]
- Yuval B, Kaspi R, Field SA, et al. Effects of post-teneral nutrition on reproductive success of male Mediterranean fruit flies (Diptera: Tephritidae). Florida Entomologist. 2002;85:165–170. [Google Scholar]
- Zhang Y, Müller H-G, Carey JR, et al. Behavioral trajectories as predictors in event history analysis: male calling behavior forecasts medfly longevity. Mechanisms of Ageing and Development. 2006;127:680–686. doi: 10.1016/j.mad.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]