Abstract
We present the first quantitative characterization of electrodermal activity (EDA) patterns on the wrists of healthy adults during sleep using dry electrodes. We compare the new results on the wrist to prior findings on palmar or finger EDA by characterizing data measured from 80 nights of sleep consisting of 9 nights of wrist and palm EDA from 9 healthy adults sleeping at home, 56 nights of wrist and palm EDA from one healthy adult sleeping at home, and 15 nights of wrist EDA from 15 healthy adults in a sleep laboratory, with the latter compared to concurrent polysomnography. While high frequency patterns of EDA called “storms” were identified by eye in the 1960’s, we systematically compare thresholds for automatically detecting EDA peaks and establish criteria for EDA storms. We found that more than 80% of EDA peaks occurred in non-REM sleep, specifically during slow-wave sleep (SWS) and non-REM stage 2 sleep (NREM2). Also, EDA amplitude is higher in SWS than in other sleep stages. Longer EDA storms were more likely in the first two quarters of sleep and during SWS and NREM2. We also found from the home studies (65 nights) that EDA levels were higher and the skin conductance peaks were larger and more frequent when measured on the wrist than when measured on the palm. These EDA high frequency peaks and high amplitude were sometimes associated with higher skin temperature, but more work is needed looking at neurological and other EDA elicitors in order to elucidate their complete behavior.
Keywords: Electrodermal activity, Sleep, Galvanic skin response, Polysomnography, Qualitative Analysis, Skin conductance, Skin temperature
1. Introduction
Electrodermal activity (EDA) is widely used in psychophysiology and provides a measure of activity in the sympathetic nervous system, one of the main branches of the autonomic nervous system. Studies on EDA during sleep have shown that elevated levels of EDA, with high frequency “storm” patterns are more common during deep, slow wave sleep (SWS) (Koumans et al., 1968), while the frequency of EDA peaks is lower in the first cycle of the night (Freixa i Baqué et al., 1983) (Table 1). Classically, EDA has been measured as skin conductance level or skin conductance responses and involves attaching wired and gelled electrodes to the skin, usually on the fingers or palm (Boucsein, 1992; Fowles et al., 1981). However, several studies have shown valid measurement of EDA on other locations including the forearm (Table 2). Studies using dry electrodes on the forearm have demonstrated reliable long-term measures of EDA (Poh et al., 2010) and have also led to the discovery of correlations between EDA and significant neurological events measured from EEG (Poh et al. 2012).
Table 1.
Summary of previous sleep EDA studies
| Description | Location | |
|---|---|---|
| Asahina et al., 1964, N=20, | GSR high activity in stage 4 | galvanic skin response (measurement location unknown) |
| Broughton et al., 1965, N=unknown | Responses are rare in stage 4, and rare in REM sleep | electrodermal response on palm and dorsal forearm |
| Lester et al., 1967, N=53 | More GSR peaks in stage 4 | Galvanic skin response on finger |
| Koumans et al., 1968, N = unknown | Electrodermal fluctuations increase during SWS and decrease during REM | skin potential and response on palm and dorsal surface of forearm |
| Hori et al. 1970, N=15 | Skin potential response max: SWS, low: REM | skin potential activity on the palmar surface of finger and dorsal surface of hand |
| McDonald et al., 1976, N=46 | Storming in stage 3–4 | skin potential and resistance, unknown location |
| Freixa i Baqué et al., 1983, N=8 | Spontaneous skin potential responses increase during 2–4 sleep cycles | electrodermal activity on palm and dorsal surface of hand |
| Ware et al., 1984, N=12 | Storming occurs during NREM sleep | skin resistance response on hands |
| Burch, 1985, N=unknown, | GSR storms during sleep stage 4 | skin response (location unknown); |
| Liguori et al., 2000, N=53 | Spontaneous sympathetic skin responses was highest in stage 4 and lowest in REM sleep | Sympathetic skin response on hand |
| Kobayashi et al., 2003, N=8 | The GSR peaks and sweat rate were significantly less frequent during REM sleep than during NREM sleep. | Galvanic skin response on the dorsal side of hand; |
Table 2.
Summary of previous EDA studies
| Location | |
|---|---|
| Johnson and Lubin, 1966, N = 29 | Finger, GSR and SCR, sleep lab |
| Johns et al, 1969, N=31 | Finger, GSR, sleep lab |
| Liguori et al., 2000, N=5 | Hand, sympathetic skin response, sleep lab |
| Shiihara et al., 2000, N=5 | Finger, Skin conductance, Palm, Skin potential, sleep lab |
| Kobayashi et al., 2003, N=8 | Hand, galvanic skin response, sleep lab |
| Poh et al., 2010, N=26 | Finger and inner wrist, Electrodemal Activity, Physical, cognitive and emotional tasks |
| Poh et al. 2012, N=80 | Wrist, electrodermal acitivity, epilepsy patient admitted to the long-term video-EEG monitoring unit |
| van Dooren et al., 2012, N=17 | 16 positions (fingers, distal wrist, central wrist, vertical wrist, chest, foot (instep), calf, forehead, neck, shoulders, back, buttock, abdomen, armpit, upper arm, and thighbone), skin conductance, watch emotional film clips |
In this study, we used a wireless non-invasive EDA sensor worn as a wristband on the distal forearm, which made it easy for subjects to be monitored in the same manner in the sleep lab and at home. We collected and analyzed 80 nights of EDA data more than ever previously reported in a single study.
Our paper makes three main contributions: First, we compare wrist EDA (convenient for continuous long-term measurement) to palmar EDA (inconvenient). When we began this work, there was concern that the wrist measures would primarily reflect thermal sweating. Our work is the first to find significant EDA patterns in sleep from the forearm while simultaneously measuring skin temperature at the same position.
Second, we characterize EDA in natural sleep, proposing an automated method to extract features from the EDA, and using these features to create a taxonomy of EDA patterns during sleep. For 15 nights where we have concurrent synchronized polysomnography (PSG), we also characterize the EDA-PSG relationships and compare the new measures with results published in the 1960–70’s. PSG is currently the gold standard to evaluate and diagnose sleep patterns; however, the use of PSG requires scalp EEG electrodes and other sensors that tend to be uncomfortable and expensive, time-consuming to apply, and arguably interfere with the sleep they are measuring. Actigraphy is a much less invasive method often used to estimate daytime and sleep activity with a wrist-worn device; however, it does not measure neural activity such as stages of sleep. In this study, we measure both EDA and actigraphy to develop a quantitative characterization of EDA in natural sleep.
Lastly, we also compare EDA responses with skin temperature. It has long been recognized that thermoregulatory processes are suppressed during REM, while they persist during NREM (Adam et al., 1986). In a study of five healthy men, the largest sweating, averaged across multiple sites on the body, was recorded during SWS while the lowest was recorded during REM, although sweating was not completely blocked during REM (Sagot et al., 1987). But this occurred in the absence of significant changes in skin temperature across sleep stages. We provide the first characterization of the interaction between wrist/palm EDA, skin temperature, and sleep stages.
2. Methods
2.1 Measurement
Our studies examined EDA during sleep by monitoring skin conductance on the outer or inner wrist (dorsal or ventral forearm) or on the palmar surface, using the Affectiva Q™ sensor with 1cm diameter Ag-AgCl dry electrodes. The sensor logged EDA, actigraphy (3-axis accelerometer) and skin surface temperature at 32 Hz. The Massachusetts Institute of Technology Committee On the Use of Humans as Experimental Subjects (COUHES) approved both studies.
2.1.1 EDA at home from wrist and palm from healthy adult (65 nights)
Nine healthy adults (two females) wore the Q sensors on the right palm and wrist for one night each. A tenth person (healthy adult female) wore the Q sensors for 56 nights. Participants put the sensor on before going to bed, and took it off after waking.
2.1.2 EDA with concurrent PSG (15 nights)
Fifteen healthy university students (ages 18–22, 10 males) participated in a night of measurements in a sleep laboratory, wearing the Q sensor on the wrist. Sleep was simultaneously monitored with standard PSG and scored by standard criteria (Rechtschaffen and Kales, 1968).
2.2 Definition
We define the following terms:
EDA peak: Local EDA maximum that exceeds a defined threshold (see analysis below for details).
EDA-peak epoch: A 30 second section of EDA having at least one EDA peak
EDA storm: Consecutive EDA peak epochs. Thus, an EDA storm has a minimum duration of one minute, and has at least two peaks during that minute.
Burch storm: “A minimum of 5 galvanic skin response (GSR) peaks per minute for 10 consecutive minutes of sleep” (Burch N, 1965; Lester et al., 1967)
EDA event: A section of EDA data having one or more EDA peaks or storms (e.g., an EDA isolated peak, EDA peak epoch, EDA storm or Burch storm)
2.3. Analysis
In this work, we automate the processing of EDA data in order to remove noise and to extract features that are robust and meaningful for characterizing sleep, and in order to provide objective measures that can be used across nights, across participants, and across studies. In PSG, it is standard practice to label sleep stages in 30-second epochs; thus, we adopt the length of 30-second segments for our comparison analyses. The EDA data were processed in four steps.
Detection of sleep from actigraphy: Standard zero-crossing detection and Cole’s function were applied to the accelerometer data to discriminate between sleep and wake (Cole et al., 1992). Only EDA data that corresponded to the times scored as sleep were further processed. Thus, EDA data that might be associated during the night with getting out of bed and moving around were not included in the analyses below.
Pre-processing of EDA: All EDA data that corresponded to segments of sleep were subsequently low-pass filtered (cutoff frequency 0.4 Hz, 32nd order FIR filter).
EDA peaks: After EDA data were low-pass filtered, we computed the first derivative and determined where it exceeds a threshold. Part of our effort asked, “What is the optimal threshold that has meaning for sleep data?” We conduct in this paper tests varying the threshold over these values: 0.005, 0.01, 0.02, 0.03, 0.04 and 0.05 μS/s and describe below how dependent the results are on the particular value. In subsequent analyses comparing wrist and palm EDA, we used a threshold of 0.01 μS/s. We define EDA “peaks” as those whose rise phase exceeds the threshold. Peaks must be separated by at least one second or they will be counted as a single peak. Thus, this method can detect up to 30 peaks per epoch, although in sleep the most we have seen is 13 peaks in one epoch.
EDA storms: Our definition above is that an EDA storm must consist of at least two adjacent peak epochs. Thus, the slowest possible storm would have 2 peaks per minute. Often during sleep we see regions with much faster bursts of 5–8 peaks per minute (ppm), and once we saw 26 ppm. What should be the minimum number of peaks in a region to call a region a storm? During our analysis, we examined how robust the storm definition is by computing it multiple ways and seeing over which range of criteria the findings are robust relative to the sleep stages. Thus, for the analyses below, we compared definitions requiring 1, 2, 3, and 4 EDA peaks per epoch, before clustering the adjacent epochs into “storms.”
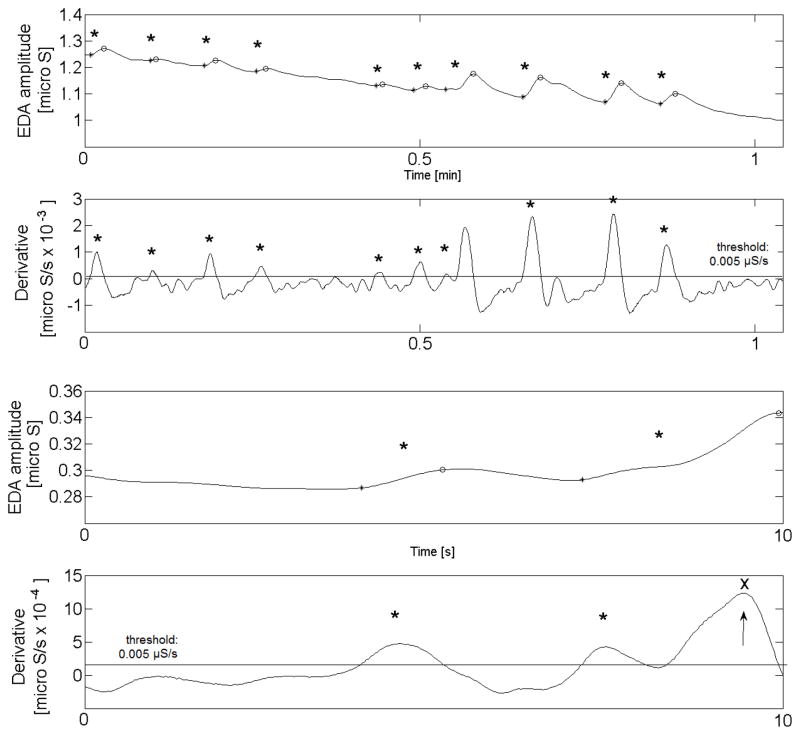
The EDA peak detector we developed is fully automated and has been quantitatively and qualitatively validated for accuracy. Figure 1 shows 10 seconds and 1 minute of EDA raw data and its derivative. Peaks shown here (black dots) are automatically detected when the derivative exceeds the threshold of 0.005 μS (red line). An asterisk marks the location of the rising edge of the peak. All peaks during sleep that meet the criteria are detected except when 2 peaks occur less than 1 second apart. When 2 peaks are less than 1 second apart then it marks only the first of the two peaks. The third peak in the bottom of Fig. 1 (x and arrow) is not detected as two peaks occur within a second.
Fig. 1.
EDA peak detection (EDA amplitude and derivatives). The black asterisks show detected peaks and x shows a peak which is detected within 1 s after the previous one and counted as one peak.
Figure 2 displays one night of filtered EDA data, the number of EDA peaks for each 30-second epoch, along with a 4-min segment of the filtered EDA data and the detection of EDA peaks for the 4-min segment using the most sensitive threshold of 0.005 μS/s.
Fig. 2.
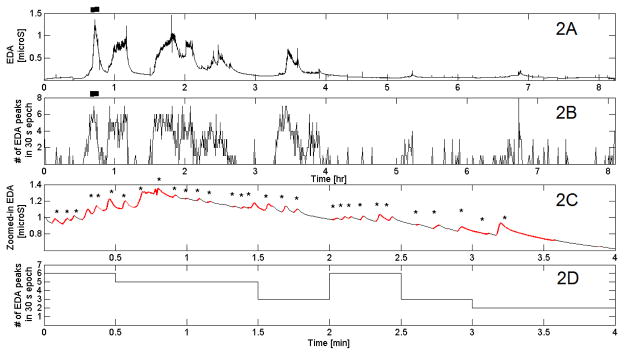
2A: Filtered EDA data for one night in a healthy adult. 2B: detected EDA peaks in 30-s epochs. 2C: Zoom of region marked with a bar on 2A. 2D: # of EDA peaks in each 30-s epoch
Our analysis, below, has three main parts:
Compare EDA amplitude (skin conductance level) and the number of peaks for wrist and palm recordings.
Compare wrist EDA amplitude and the number of peaks in sleep stages and during the four quarters of the night (ANOVA and post hoc t-test); also characterize storm durations.
Compare EDA and skin-surface temperature at the EDA electrodes (correlation analysis)
3. Results
3.1 Wrist vs. Palmar EDA
Most prior studies of EDA during sleep have looked at palmar skin conductance as a measure of EDA, e.g. Doberenz et al collected one night of palmar data from each of 48 subjects (Doberenz et al., 2011). We found that EDA measured on the wrist usually gives a larger signal than that measured on the palm, although otherwise the two signals are usually reasonably correlated during sleep (e.g., Fig. 3). To quantify this, we analyzed the difference between the wrist and palm EDA data (after filtering as above) from 9 healthy adults using 0.03 μS as tolerance (epsilon). Across participants, the palmar skin conductance measured during sleep was at least 0.03 μS lower than the inner wrist skin conductance during 74% of samples. Despite this difference, the palm and the inner wrist EDA show the same number of EDA peaks for 71% of 30-sec sleep epochs, with more EDA peaks on the wrist tend to be seen during 21% of sleep epochs.
Fig. 3.
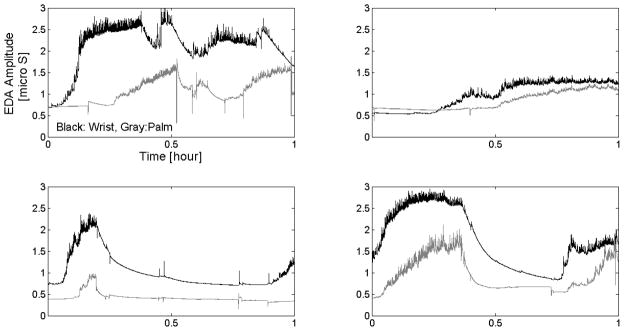
Examples of wrist and palm EDA during sleep
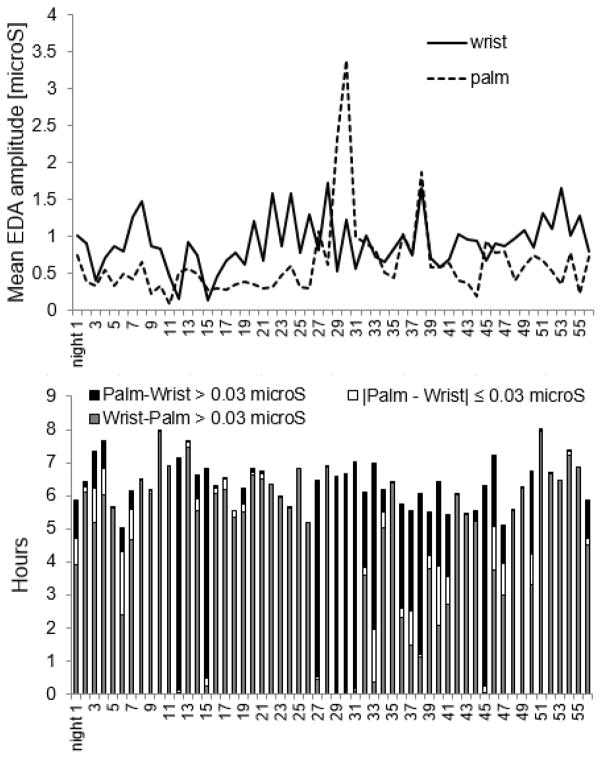
We also analyzed the difference between the wrist and palm EDA data for 56 nights (longitudinal case study) because, increasingly, long-term measurement is important in understanding intra-individual differences as well as in treatment and intervention studies, and we wish to compare a set of individual results to the group results. As shown in Fig. 4, on 48 of the 56 nights (86%), the average skin conductance level measured from the inside of the wrist was higher than the palmar level during sleep (both measured on the right side of the body). On the remaining 8 nights, the palmar skin conductance had larger amplitude than the wrist. When analyzed by hour of sleep, the wrist EDA was higher than the palmar EDA 71% of the time (255 hours of sleep), while 23% of the time (84 hrs of sleep) the palmar EDA exceeded the wrist EDA, and 5% of the time (18 hrs of sleep), the difference between wrist and palmar EDA was less than 0.03 μS.
Fig. 4.
EDA amplitude comparison between palm and wrist (56 nights, 357 hours)
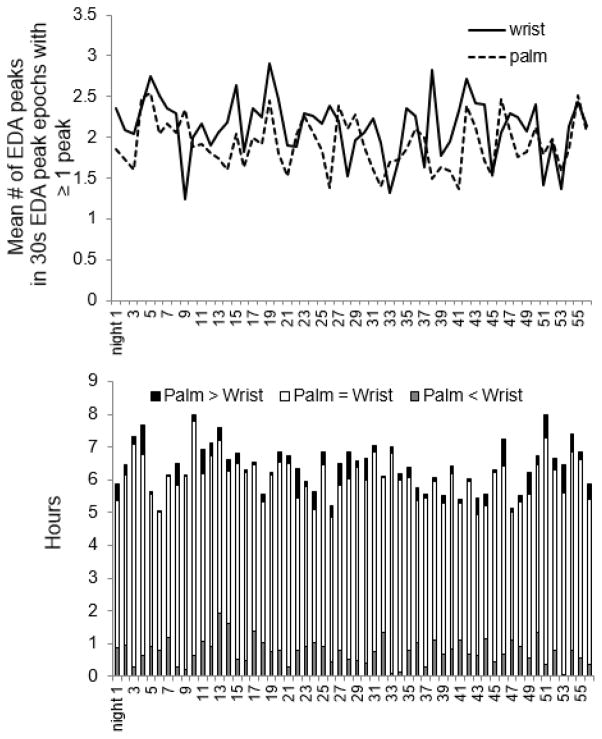
Our software detected EDA peaks during sleep both for palm and wrist on all 56 nights. As seen in Fig. 5, on 42 of the 56 nights, more EDA peaks were detected on the inner wrist. Of 357 hours of sleep, the wrist and palmar EDA-peak counts per epoch were equal 83% of the time (296 hours of sleep); 12% of the time the wrist EDA showed more peaks (42 hrs of sleep), and 5% of the time the palmer EDA had more peaks (19 hrs of sleep). Thus, overall the wrist appears to be a more sensitive location for capturing EDA events during sleep. Moreover, these results were consistent both across individuals and long-term within an individual.
Fig. 5.
# of EDA peaks comparison between palm and wrist (56 nights, 287 hours) Y-axis of the top figure: mean number of EDA peaks per 30s epoch containing ≥ 1 peak, not for all epochs.
3.2 Characteristics of EDA
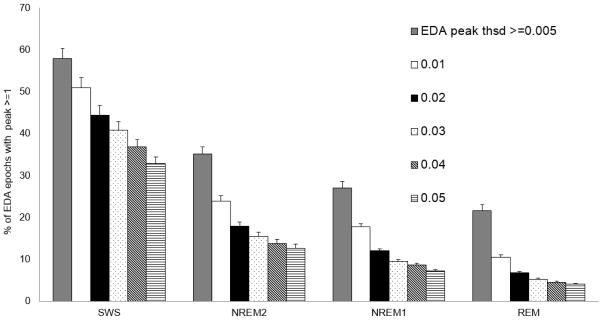
We wish to characterize EDA peaks and their relation to sleep stages. First, we examine the sensitivity of the peak-detection parameters for our automated algorithm. We computed the distribution of the number of EDA peaks per 30s epoch for thresholds from .005 to .05 μS/sec (n = 15 in the laboratory) (Fig. S2). Over the fifteen nights, more than 60% of the 30-s epochs did not show any peaks, regardless of peak threshold. As expected, a lower threshold for EDA peaks showed more peaks.
We then analyzed how the peaks that occurred are distributed across the sleep stages Most of the night was spent in NREM2 (Fig. S1), and indeed we see most of the peaks (55 ± 4%) occurred in NREM2 (Fig. S3). The next highest are 25±4% in SWS, 12±1% in REM and 4±0% in NREM1. This relative ordering of NREM2 > SWS > REM > NREM1 holds regardless of the threshold that we used for detecting peaks. Thus, this finding is robust over a large range of parameter values. However, the relative number found in each stage varied: the ratio of EDA peaks in REM compared to SWS varies systematically from 39% at the highest threshold to 77% at the lowest.
Figure 6 shows that SWS has the highest percentage of epochs with EDA peaks during sleep. The percentage of sleep epochs containing EDA peaks varied significantly across sleep stages (repeated measures ANOVA, F=12.70, df=3, p< 4.82E-06). Overall, EDA peaks were more than 1.5 times more frequent in SWS than in NREM2 and more than 3 times more frequent in SWS than in REM (post hoc t-test, p=0.05). While the exact percentages of peaks decrease as the threshold gets higher, the main findings relating EDA to sleep stages are consistent for thresholds from .005 to .05 μS. Thus, the EDA peaks measured on the wrist with dry electrodes show robust properties related to sleep stages. Figure 7 shows the distribution of EDA peak epochs over the night. Most of the EDA peak epochs occurred in the first half of the night.
Fig. 6.
Mean percentages of sleep stage epochs containing EDA peaks. (N=15, Error bars: s.e.m.)
Fig. 7.
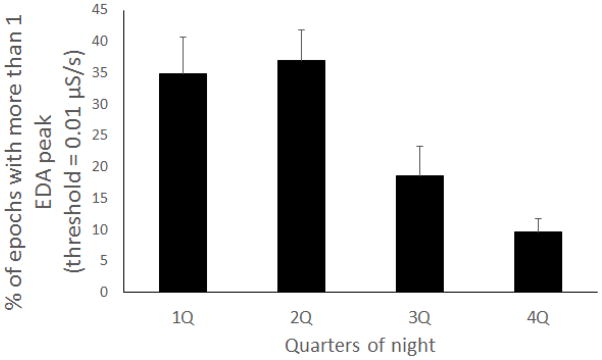
Percentage of epochs with more than 1 EDA peak (threshold = 0.01 μS/s)
Next, we analyze the basic properties of EDA amplitude, peaks and storms. Median EDA-amplitude (Averaged median across participants) was 0.44, 0.26, 0.18, and 0.26 in SWS, NREM2, NREM1 and REM. The median EDA amplitude in SWS was significantly higher than in the other sleep stages. (ANOVA and post hoc t-test, p < 0.05). (We computed the median because the distribution of EDA amplitude is far from Gaussian.) Thus, the wrist EDA median amplitude varies with sleep stages. We also compared the EDA amplitude between epochs with EDA peaks and those without EDA peaks. In twelve out of 15 participants, median EDA amplitude was higher in epochs with EDA peaks. The EDA-peak frequency (peaks per epoch) was also significantly higher in SWS than in NREM2, NREM1 and REM (ANOVA and post hoc t-test, p=0.05).
We also validated the robustness of the new automated criteria for detecting EDA storms: the number of EDA peaks required per epoch (Fig. S4). We again found that the relative distribution of storms is robust across the criteria: About 85% of storms lasted under 5 minutes regardless of the amplitude gain threshold for EDA peaks (0.005–0.05 μS) and regardless of the peaks-per-epoch threshold for EDA storms (1–4 peaks/epoch).
Burch was the original scientist identifying EDA storms, which he and his colleague did visually after measuring GSR on the left middle finger with Ag-AgCl electrodes and a sodium-chloride paste (Lester et al., 1967). We wanted to compare today’s sensor data and automated algorithm to their original hand counted values. Among all EDA events in our data, identified from wrist EDA, only 11% of EDA events met Burch’s criteria (≥5 EDA peaks/min, and duration ≥ 10 minutes). Of these Burch storms, 95% occurred during NREM2 and SWS, compared to 89% of isolated EDA peaks and non-Burch storms. Similarly, 77% of Burch storms occurred during the first half of the night, compared to only 43% of the other peaks and storms. Thus, we have qualitative similarities between our automated and objective measures and Burch’s hand-count observations in EDA peaks and storm occurrences in NREM2 and SWS, but difference in the distribution across the night.
3.3 EDA vs skin temperature
The purpose of the analysis here is to determine whether skin surface temperature is the cause of the EDA changes we see during sleep. Note that skin surface temperature is not the same as core body temperature; core body temperature drop is usually preceded by wrist temperature increase (Sarabia et al., 2008). We have also found that skin temperature tends to climb for most of our participants during sleep, which is consistent with the previous finding (Martinez-Nicolas, 2013). We do not have measures of ambient temperature or of whether or not the person’s wrist was under a blanket, which is likely to make the skin warmer; nonetheless, it is still interesting to examine correlations between the skin surface temperature and the EDA, both measured at the position of the same pair of electrodes. We first examine the correlation between skin temperature and EDA overall as well as during each sleep stage. Out of 15 participants, 12 participants showed significant positive correlations between 30s epoch averaged skin conductance level (SCL) and 30s epoch averaged skin temperature level. Also, 9 of the 15 participants showed significant positive correlation between the number of EDA peaks and skin temperature per epoch. However, 13 out of 15 participants also showed higher wrist temperature in SWS than in REM generally, making causal links unclear. While EDA amplitude and peaks do have a statistical relationship with skin temperature in our 30-sec data, the correlation breaks down at a finer time scale. Examples can be found (e.g., Fig. 8), where EDA and skin temperature are completely dissociated. Thus, increases in EDA amplitude and peaks are not simply the immediate consequence of changes in skin surface temperature.
Fig. 8.
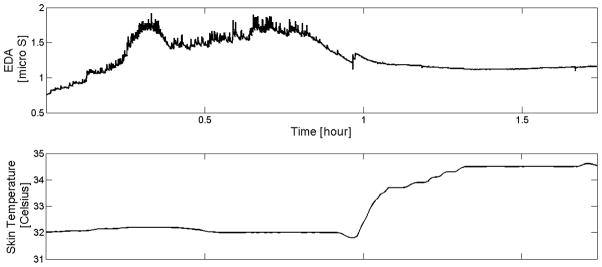
Example showing that changes in EDA are not always caused by changes in temperature (Skin temperature on the wrist was flat when EDA showed storms, and there are no storms when temperature climbs)
Both the wrist and the palm contain eccrine sweat glands, which have a primary function of thermoregulation, and which are denser on the palm than on the wrist (Dawson et al, 2007). We examined if wrist or palm differed in how their EDA responded to temperature during sleep, comparing wrist and palm temperature when there were and were not EDA peaks. On the wrist, 6 out of 9 participants showed higher temperature during epochs without peaks than with peaks; thus, the EDA peaks were not simply associated with warmer skin on the wrist. In contrast, on the palm, 7 out of 9 showed higher temperature during epochs with EDA peaks than without (wrist vs. palm, χ2 = 3.6, p = 0.058). Thus, there may be a slight tendency for higher temperature on the palms to lead to more peaks on the palms (binomial, p = .089). All 9 participants showed higher mean temperature on the wrist than on the palm during EDA peak epochs. Also, 7 out of 9 showed higher mean wrist temperature than palm during non-storm epochs. When the wrist temperature was higher than the palm temperature, then the wrist EDA was almost always higher than the palm EDA (95% of these epochs).
4. Discussion
This EDA study, with 80 nights of data, examined and characterized basic EDA properties during sleep. Our study includes the first longitudinal characterization (56 nights) as well as 15 nights with synchronized PSG and nine additional nights of healthy adults at home. Consistent with previous studies, our data showed that the mean EDA amplitude in SWS is significantly larger than in other sleep stages. Consistent with these prior studies, we also observed a decreased number of peaks in EDA during REM sleep. These common findings are noteworthy because ours is the first significant sleep study to use a convenient-to-wear dry-electrode EDA skin conductance sensor on the wrist, while most prior studies measured the EDA on the palmar surface or fingers with wired gelled electrodes. We also developed the first fully automated EDA sleep peak detection algorithm providing objective measures across a range of thresholds, and showed that the findings were robust across these thresholds. We will further discuss comparisons of forearm vs. palmar EDA below, but these significant findings serve to validate both the occurrence of EDA peaks and the sleep-stage dependence of the EDA peaks for this alternate convenient location of wearing a sensor.
In our study, EDA peaks were not distributed uniformly over the night, but were more likely to be located in the first half of the night. This can be because more SWS occurs in the earlier half of the night. However, some nights showed no EDA peaks in the first SWS cycle. It is important to note that EDA peaks and storms did not happen in all cycles of SWS and NREM2; thus the EDA peaks provide different information than that normally obtained from PSG. In fact on some nights, some participants have no EDA storms, while on other nights they may have many. Meanwhile, when EDA storming does happen, it is most likely to appear during SWS and NREM2.
We found that the largest number of peaks per epoch occurred in SWS and NREM2. Freixa i Baqué et al. (1983), Johnson et al. (1968) and Hori et al. (1970) also found more peaks in SWS, and McDonald showed a decrease in the EDA storm rate in stages 1 and 2 sleep (1976), all of which are consistent with our results. Liguori et al. (2000) showed that the frequency of spontaneous sympathetic skin conductance peaks in stage 4 was 5–9 per minute. This result is slightly different but consistent with our tendency (the most frequent in SWS, 2–26 per minute). One earlier finding that did not match ours is that of Freixa i Baqué et al. (1983) who found that spontaneous EDA activity showed a smaller number of EDA peaks per minute (i.e., 60%) during the first sleep cycle (the different EEG stages from sleep onset appearance of alpha rhythm (NREM1) until the end of the first REM) compared to the subsequent three sleep cycles (defined as different EEG stages between the ends of two REM periods) (Freixa i Baqué, 1983, N=8). In our data, the first and second quarters of the night showed a larger number of EDA peaks per 30-second epoch than the latter two. Hori et al. also found that EDA peak frequency was less frequent in the latter half of sleep, especially after the third full REM cycle (Hori et al., 1970, N=15), consistent with our findings.
Most of our EDA storms lasted under 5 minutes. Of all EDA peaks detected over the 80 nights, only 11% were in EDA storms that met Burch’s storm criteria. Nevertheless, we found more storms per night than the 2–3 storm nightly average reported by Freixa i Baqué et al. (Freixa i Baqué, 1983); this may in part be due to the stronger EDA signal obtained when measured on the wrist. In addition, our result showed that longer storms, with a larger number of EDA peaks, are more likely to occur in the earlier part of the night and in SWS and NREM2.
We measured EDA on the forearm using a wristband, while previous studies examined EDA mostly on the palmar surface or fingers. Our results showed that the EDA amplitude and storm patterns during sleep are usually more pronounced on the forearm than on the palm, and thus peaks are more likely to be detected when measured with a wristband. These observations are the opposite from activities during daytime awake tasks (Van Dooren et al., 2012) where peaks tend to be more pronounced on the palm. The stronger signal we observed on the wrist during sleep may explain why we found more EDA peaks than earlier studies, not only during SWS but also during NREM2 as well. This sensitivity on the wrist was found even using dry electrodes, which avoids the problem of a gel breaking down over long-term wear and interfering with signal level over time.
Our findings of a higher mean skin temperature during SWS may appear to contradict those of Sagot et al. who showed no statistical relationship between skin temperature and sleep stages; however, they averaged skin temperature from 10 different points on the body, including distal and proximal skin temperature, while our findings were specific to the wrists.
Warmer wrists help explain the higher SCL and larger number of peaks found on the wrists overall. That said, we cannot say that the higher SCL and peaking are always associated with skin surface temperature changes: There are instances, such as Fig. 8, where SCL on the palm is higher than on the wrist, while the skin temperature is higher on the wrist than on the palm. An overall correlation is present, but the relatively rapid changes we see in EDA do not appear to be caused only by changes in skin surface temperature.
When we began these studies, we were initially perplexed by this discrepancy: During sleep, we would expect low emotional arousal and low EDA responsivity; however, we found higher EDA responsivity, even after removal of sleep-motion artifacts, and even at times when skin surface temperature was dropping. Since that surprise, we have learned about key neurological findings showing, for example, that the amygdala and hippocampus, when directly stimulated with depth electrodes, elicit large skin conductance responses (Mangina et al., 1996). The amygdala and hippocampus regions of the brain are known for being involved in memory and emotion. In fact, in recent work we have found that automatically computed features of the skin conductance over a night’s sleep are more accurate predictors of improvement in a learning task (learned before sleep, tested after sleep) than are classic features measured from EEG or from PSG (Sano et al., 2013). It is thus possible that neurological memory-related processes may also be contributing to the patterns of EDA responsivity we measure during sleep.
This study has several limitations. Several factors can influence an individual’s EDA. For example, thermal regulation influences sweating and we did not measure core body temperature or environmental temperature, nor did we videotape to track the position of participants’ wrists. Only the temperature on the skin location of the EDA electrodes was measured. Core body temperature is usually higher earlier in sleep (when there is usually more SWS) and tapers down over the course of sleep. Sleep stages such as SWS and NREM2 have been associated with higher core body temperature on average than REM (Sagot et al., 1987). Core body temperature behaves in ways different from distal skin surface temperature (Krauchi, 2002); thus, thermoregulation remains a potential driver of some of our findings, even when there is no strong correlation between temperature at the electrode location and the skin conductance measured at the same position. Another mystery is that some nights had no EDA responses, despite that we might still expect that core body temperature dropped over the night. One possible explanation for the women in the study is that they have reduced sweating during the luteal phase (latter half) of their menstrual cycle, and this could cause a reduction in measured EDA storm peaks (Mackinnon, 1954). Future sleep studies should examine the timing of the measurements made relative to female participants’ menstrual cycles. Our longitudinal study of one subject, who was female, showed quite a bit of variation from night to night in the EDA patterns. Future work is needed to characterize inter- and intra-individual differences in long-term EDA features.
5. Conclusion
This work presents the first systematic taxonomy of autonomic activity patterns measured in healthy adults based on forearm skin conductance and actigraphy during sleep. Our analyses focused on the automated detection of EDA peaks and on regions of continuous peaks called “storms,” and their comparison with concurrent PSG as well as with skin surface temperature.
Most of the EDA data in this study were measured from the wrist and on most nights the results showed greater activity at this location than at the traditional palmar location in terms of both amplitude and the number of peaks; thus, the wrist is a viable location to get long-term data about EDA patterns during sleep.
About 80% of wrist EDA peaks are observed in SWS and NREM2 sleep, and mostly in the first half of the night. This property is robust over different thresholds to detect EDA peaks. Only 11% of all EDA peak epochs were contained in Burch’s EDA storms (classically defined as more than 5 peaks per minute and durations longer than 10 minutes), and these occurred mostly in the first half of the night. EDA amplitude was also on average higher during EDA-peak epochs.
We analyzed the relationship between EDA and skin temperature, where we found a higher frequency of EDA peaks and a higher average skin conductance level in SWS, measured on the wrist, tending to co-occur with higher temperature on the wrist, although not always in association with higher temperature. While we know that thermoregulation influences EDA, the temperature on the surface of the skin does not fully account for the EDA patterns measured at that location.
Overall, our work has characterized strong patterns in EDA that can be measured at home or in the lab, using automated methods that are robust to different parameter settings. Our findings characterize consistent EDA patterns related to sleep stages derived from gold standard PSG. Future work is needed to elucidate the many neurological, environmental, and thermoregulatory influences contributing to the rise of these EDA patterns.
Supplementary Material
Fig. S1. Total time of each sleep stage over night and objective sleep efficiency (N=15) If kept, this should include a bar at the top which is the average across subjects.
Fig. S2. Distribution of the number of EDA peaks in 30-s epoch (N=15)
Fig. S3. Mean percentage of epochs with EDA peaks across sleep stage (N=15, Error Bars: s.e.m)
Fig. S4. Histogram of EDA storm duration (N=15) shows similar distributions across thresholds. (The left most sets of bars show the percentage of EDA storms lasting less than 5 minutes.)
Highlights.
We present the first characterization of sleep electrodermal activity with dry electrodes.
We compare thresholds for detecting EDA peaks and establish criteria for EDA storms.
More than 80% of EDA peaks occurred during slow-wave and non-REM stage 2 sleep.
Amplitude is higher in SWS than other sleep stage.
EDA levels and peaks were more pronounced on the wrist than those on the palm.
Acknowledgments
This research was supported by the MIT Media Lab Consortium with a generous donation by Samsung and NIH grant 1R01GM105018-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Akane Sano, Massachusetts Institute of Technology, Media Lab, Affective Computing Group.
Rosalind W. Picard, Massachusetts Institute of Technology, Media Lab, Affective Computing Group
Robert Stickgold, Harvard Medical School, Beth Israel Deaconess Medical Center, Center for Sleep and Cognition.
References
- Adam K, Tomeny M, Oswald I. Physiological and psychological differences between good and poor sleepers. J Psychiatr Res. 1986;20:301–316. doi: 10.1016/0022-3956(86)90033-6. [DOI] [PubMed] [Google Scholar]
- Asahina K, Omura K. Phenomenological study of paradoxical phase and reverse of sleep. Jpn J Physiol. 1964;14:365–72. doi: 10.2170/jjphysiol.14.365. [DOI] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal Activity. Springer; 1992. [Google Scholar]
- Broughton RJ, Poire R, Tassinari Ca. the Electrodermogram (Tarchanoff Effect) During Sleep. Electroencephalogr Clin Neurophysiol. 1965;18:691–708. doi: 10.1016/0013-4694(65)90113-6. [DOI] [PubMed] [Google Scholar]
- Burch N. Data Processing of psychophysiological recordings. Symposium on the Analysis of Central Nervous System and Cardiovascular Data Using Computer Methods; 1965. pp. 165–180. [Google Scholar]
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo T, LGT, GGB, editors. Handbook of Psychophysiology. Cambridge University Press; 2007. pp. 159–181. [Google Scholar]
- Doberenz S, Roth W, Wollburg E. Methodological considerations in ambulatory skin conductance monitoring. Int J. 2011;80:87–95. doi: 10.1016/j.ijpsycho.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, Venables PH. Committee report. Publication recommendations for electrodermal measurements. Psychophysiology. 1981;18:232–9. doi: 10.1111/j.1469-8986.1981.tb03024.x. [DOI] [PubMed] [Google Scholar]
- Freixa i Baqué E. Reliability of spontaneous electrodermal activity in humans as a function of sleep stages. Biol Psychol. 1983;17:137–43. doi: 10.1016/0301-0511(83)90014-5. [DOI] [PubMed] [Google Scholar]
- Freixa i Baqué E, Chevalier B, Grubar JC, Lambert C, Lancry A, Leconte P, Mériaux H, Spreux F. Spontaneous electrodermal activity during sleep in man: an intranight study. Sleep. 1983;6:77–81. doi: 10.1093/sleep/6.1.77. [DOI] [PubMed] [Google Scholar]
- Hori T, Miyasita A, Niimi Y. Skin potential activities and their regional differences during normal sleep in humans. Jpn J Physiol. 1970;20:657–71. doi: 10.2170/jjphysiol.20.657. [DOI] [PubMed] [Google Scholar]
- Johns MW, Cornell BA, Masterton JP. Monitoring sleep of hospital patients by measurement of electrical resistance of skin. J Appl Physiol. 1969;27:898–901. doi: 10.1152/jappl.1969.27.6.898. [DOI] [PubMed] [Google Scholar]
- Johnson LC, Lubin A. Spontaneous electrodermal activity during waking and sleeping. Psychophysiology. 1966;3:8–17. doi: 10.1111/j.1469-8986.1966.tb02673.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi R, Koike Y, Hirayama M, Ito H, Sobue G. Skin sympathetic nerve function during sleep—a study with effector responses. Auton Neurosci. 2003;103:121–126. doi: 10.1016/s1566-0702(02)00261-8. [DOI] [PubMed] [Google Scholar]
- Koumans AJ, Tursky B, Solomon P. Electrodermal levels and fluctuations during normal sleep. Psychophysiology. 1968;5:300–6. doi: 10.1111/j.1469-8986.1968.tb02826.x. [DOI] [PubMed] [Google Scholar]
- Krauchi K. How is the circadian rhythm of core body temperature regulated? Clin Auton Res. 2002;12:147–149. doi: 10.1007/s10286-002-0043-9. [DOI] [PubMed] [Google Scholar]
- Lester BK, Burch NR, Dossett RC. Nocturnal EEG-GSR profiles: the influence of presleep states. Psychophysiology. 1967;3:238–48. doi: 10.1111/j.1469-8986.1967.tb02701.x. [DOI] [PubMed] [Google Scholar]
- Liguori R, Donadio V, Foschini E, Di Stasi V, Plazzi G, Lugaresi E, Montagna P. Sleep stage-related changes in sympathetic sudomotor and vasomotor skin responses in man. Clin Neurophysiol. 2000;111:434–439. doi: 10.1016/s1388-2457(99)00294-1. [DOI] [PubMed] [Google Scholar]
- Mackinnon PC. Variations in the number of active palmar digital sweat glands during the human menstrual cycle. J Obstet Gynaecol Br Emp. 1954;61:390–3. doi: 10.1111/j.1471-0528.1954.tb07501.x. [DOI] [PubMed] [Google Scholar]
- Mangina CA, Beuzeron-Mangina JH. Direct electrical stimulation of specific human brain structures and bilateral electrodermal activity. Int J Psychophysiol. 1996;22:1–8. doi: 10.1016/0167-8760(96)00022-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Nicolas A, Ortiz-Tudela E, Rol MA, Madrid JA. Uncovering different masking factors on wrist skin temperature rhythm in free-living subjects. PLoS One. 2013;8:e61142. doi: 10.1371/journal.pone.0061142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DG, Shallenberger HD, Koresko RL, Kinzy BG. Studies of spontaneous electrodermal responses in sleep. Psychophysiology. 1976;13:128–34. doi: 10.1111/j.1469-8986.1976.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Poh MZ, Loddenkemper T, Reinsberger C, Swenson NC, Goyal S, Madsen JR, Picard RW. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology. 2012;78:1868–76. doi: 10.1212/WNL.0b013e318258f7f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh MZ, Swenson NC, Picard RW. A wearable sensor for unobtrusive, long-term assessment of electrodermal activity. IEEE Trans Biomed Eng. 2010;57:1243–52. doi: 10.1109/TBME.2009.2038487. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen AK. A Manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. 1968. [DOI] [PubMed] [Google Scholar]
- Sagot JC, Amoros C, Candas V, Libert JP. Sweating responses and body temperatures during nocturnal sleep in humans. Am J Physiol. 1987;252:R462–70. doi: 10.1152/ajpregu.1987.252.3.R462. [DOI] [PubMed] [Google Scholar]
- Sano A, Picard RW. Recognition of sleep dependent memory consolidation with multi-modal sensor data. 2013 IEEE International Conference on Body Sensor Networks; IEEE; 2013. pp. 1–4. [Google Scholar]
- Sarabia JA, Rol MA, Mendiola P, Madrid JA. Circadian rhythm of wrist temperature in normal-living subjects A candidate of new index of the circadian system. Physiol Behav. 2008;95:570–80. doi: 10.1016/j.physbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Shiihara Y, Umezawa A, Sakai Y, Kamitamari N, Kodama M. Continuous recordings of skin conductance change during sleep. Psychiatry Clin Neurosci. 2000;54:268–9. doi: 10.1046/j.1440-1819.2000.00672.x. [DOI] [PubMed] [Google Scholar]
- Van Dooren M, de Vries JJGGJ, Janssen JH. Emotional sweating across the body: comparing 16 different skin conductance measurement locations. Physiol Behav. 2012;106:298–304. doi: 10.1016/j.physbeh.2012.01.020. [DOI] [PubMed] [Google Scholar]
- Van Dooren M, de Vries JJGGJ, Janssen JH. Emotional sweating across the body: comparing 16 different skin conductance measurement locations. Physiol Behav. 2012;106:298–304. doi: 10.1016/j.physbeh.2012.01.020. [DOI] [PubMed] [Google Scholar]
- Ware JC, Karacan I, Salis PJ, Thornby J, Hirshkowitz M. Sleep-related electrodermal activity patterns in impotent patients. Sleep. 1984;7:247–54. doi: 10.1093/sleep/7.3.247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Total time of each sleep stage over night and objective sleep efficiency (N=15) If kept, this should include a bar at the top which is the average across subjects.
Fig. S2. Distribution of the number of EDA peaks in 30-s epoch (N=15)
Fig. S3. Mean percentage of epochs with EDA peaks across sleep stage (N=15, Error Bars: s.e.m)
Fig. S4. Histogram of EDA storm duration (N=15) shows similar distributions across thresholds. (The left most sets of bars show the percentage of EDA storms lasting less than 5 minutes.)