Abstract
In men, the association between poor physical performance and likelihood of incident vertebral fractures is unknown.
Using data from the MrOS study (N=5958), we describe the association between baseline physical performance [walking speed, grip strength, leg power, repeat chair stands, narrow walk (dynamic balance)] and incidence of radiographic and clinical vertebral fractures. At baseline and follow-up an average of 4.6 years later, radiographic vertebral fractures were assessed using semi-quantitative (SQ) scoring on lateral thoracic and lumbar radiographs. Logistic regression modeled the association between physical performance and incident radiographic vertebral fractures (change in SQ grade≥1 from baseline to follow-up). Every four months after baseline, participants self-reported fractures; clinical vertebral fractures were confirmed by centralized radiologist review of the baseline study radiograph and community acquired spine images. Proportional hazards regression modeled the association between physical performance with incident clinical vertebral fractures. Multivariate models were adjusted for age, BMD (by DXA), clinical center, race, smoking, height, weight, history of falls, activity level and co-morbid medical conditions; physical performance was analyzed as quartiles.
Of 4332 men with baseline and repeat radiographs, 192 (4.4%) had an incident radiographic vertebral fracture. With the exception of walking speed, poorer performance on repeat chair stands, leg power, narrow walk and grip strength were each associated in a graded manner with an increased risk of incident radiographic vertebral fracture (p for trend across quartiles <0.001). In addition, men with performance in the worst quartile on three or more exams had an increased risk of radiographic fracture (OR: 1.81, 9% CI: 1.33, 2.45) compared to men with better performance on all exams. Clinical vertebral fracture (N=149 of 5,813, 2.6%) was not consistently associated with physical performance.
We conclude that poorer physical performance is associated with an increased risk of incident radiographic (but not clinical) vertebral fracture in older men.
Keywords: epidemiology, hip fracture, strength, physical performance, walking
INTRODUCTION
Poor physical performance, particularly slow walking speed, is increasingly being seen as a “vital sign” in older adults. Slow walking speed and poor function as assessed by integrative measures such as the Short Physical Performance Battery (SPPB), have been associated with increased risk of mortality, disability, and hospitalization.1–4
Osteoporosis and fracture are multi-factorial events.5 Most hip fractures are the direct result of a fall,6 and we previously showed that older men with poor physical performance have an increased risk of hip and non-spine fractures and lower bone mineral density (BMD).7 Well established risk factors for vertebral fracture in men are older age, decreased bone mineral density and a history of height loss, while other factors less extensively evaluated include body mass index, smoking, glucocorticoid use and grip strength.8 In particular, the relation between physical performance and risk of vertebral fractures has not been investigated in men. We evaluated vertebral fractures identified by two distinct methods: 1) fractures identified by review of paired radiographs obtained at study visits (radiographic vertebral fractures); and 2) fractures identified by participant report and subsequent review of clinically acquired images (clinical vertebral fractures). We included both radiographic and clinically identified vertebral fractures as study outcomes because these outcomes differ in method of ascertainment and likely in severity.
The primary aim of these analyses was to describe the association between physical performance and the incident radiographic and clinical vertebral fractures in the Osteoporotic Fractures in Men (MrOS) study, a cohort of older community dwelling men.
MATERIALS AND METHODS
Study description
As previously described,9 5,994 men aged ≥65 years living in six communities in the United States were recruited to participate in the MrOS study. Eligible men must have been ambulatory (able to walk without assistance of another person or aide); must not have had bilateral hip replacements; and must have provided written informed consent. Participants completed a battery of clinical exams and a self-administered questionnaire during the baseline visit between March 2000 and April 2002. Institutional review boards at all clinic centers and the San Francisco Coordinating Center (University of California, San Francisco and California Pacific Medical Center Research Institute) approved this study. An average of 4.6±0.4SD years after baseline, 4,530 men returned to the clinical center for repeat x-rays and physical assessment and 699 provided questionnaire-based data only.
Physical performance
Physical performance was assessed during the single baseline examination as previously described.7 Rigorous centralized training, examiner certification, and periodic protocol review during the course of the study were used to ensure consistency in the measures of physical performance. Briefly, walking speed (m/s) at usual pace was determined over 6 meters. Dynamic balance was assessed on narrow walking path (20 cm) over 6 meters. Leg power (watts) was ascertained using the Nottingham Power Rig (Nottingham University, Nottingham, England).10 We also assessed ability and time to complete five repeated chair stands. Grip strength was measured using Jamar dynamometers (Sammons Preston Rolyan, Bolingbrook, IL, USA).11 To determine concurrent poor performance in several physical performance tests, a summary score for the physical performance measures was created as previously reported.7 The possible values of the summary score ranged from 0 to 5, with 0 indicating the ability to perform all tests and 5 indicating poor performance on all 5 tests. For each test with poor performance (defined as in the worst performance quartile or unable to complete the measure), one point was added to the score. To ensure a reasonable number of events in each strata, the Summary Physical Performance Score was analyzed as 0, 1–2 and 3 or more.
Radiographic vertebral fracture assessment
Vertebral fracture assessment in MrOS has been described in detail elsewhere.37 Lateral thoracic and lumbar spine radiographs were acquired according to study protocol.
The general process for review of spine images in MrOS was as follows. First, all spine images were assessed for quality and underwent a “triage process” by trained technicians, the purpose of which was to eliminate grossly normal images from semi-quantitative (SQ) scoring, thereby reducing the number of images that needed to be read by the physician reader (JS). Once triage was complete, all films from participants with a possible fracture or other abnormality (“triage positive”) were evaluated by a physician reader using the SQ method of Genant;12 triage negative films were assumed to be fracture free and the SQ score was set to 0 for all levels. The triage process had few false negatives (that is, a high sensitivity: 96.8%) and the SQ scoring had excellent reproducibility as kappa scores ranged from 0.79–0.91 on a series of quality assurance readings.
Clinical vertebral facture assessment
Every four months after the baseline exam, participants completed a mailed questionnaire that asked about falls and fractures during the previous four-month period. If a participant reported a fracture, he was contacted by study staff to receive permission to obtain medical records and record the circumstances of the fracture. Verification of “clinical vertebral fractures” was completed by the central study radiologist by comparing the clinically acquired image (including any x-rays, CT and MRI studies) obtained from the community health setting with baseline study thoracic and lumbar radiographs. In order to compare results to the study assessed radiographic vertebral fractures, only clinically identified vertebral fractures identified in T4-T12 and L1-L4 were included in the analyses. The mean (SD) follow-up time for incident clinical vertebral fractures was 9.8 ± 3.0 years.
Other measures
Participants completed questionnaires that included information about age, race, smoking status, alcohol use, history of falls in the previous year, self-rated health, depressed mood, all fractures since age 50, vertebral fractures at any age, and alcohol use (classified as none, 1–14 drinks per week, and >14 drinks/week). Height and weight were measured to calculate body mass index (BMI) as weight (kg) / height2 (m2). Activity level was determined from the Physical Activity Scale for the Elderly (PASE)13; a higher score indicated a higher activity level. Participants also reported a history of a physician diagnosis of the selected medical conditions (see Table 1 footnote). Total hip, femoral neck bone and lumbar spine bone mineral density (BMD) were measured using Hologic 4500 dual energy x-ray absorptiometry (DXA) machines (Hologic, Inc., Bedford, MA) as previously described.14 At baseline, participants were asked to bring all prescription medications they had been taking for at least 1 month and medication use was coded using standard study procedures.15
Table 1.
Characteristics [mean±SD, or N(%)] of MrOS participants by prevalent vertebral fracture status
| Characteristic | No Fracture (SQ=0,1) N= 5397 | Fracture (SQ≥2) N= 431 | p-value |
|---|---|---|---|
| Age, y | 73.4±5.8 | 75.0±6.3 | <.001 |
| White | 4814 (89.2) | 401 (93.0) | 0.012 |
| Body mass index, kg/m2 | 27.4 ± 3.8 | 27.2 ± 3.8 | 0.466 |
| Height, m | 1.74 ± 0.07 | 1.73 ± 0.07 | <.001 |
| Weight, kg | 83.3 ± 13.2 | 81.3 ± 13.1 | 0.003 |
| PASE score | 148.2 ± 68.0 | 140.2 ± 69.2 | 0.019 |
| Smoking Never | 2040 (37.8) | 139 (32.3) | 0.05 |
| Past | 3168 (58.7) | 279 (64.7) | |
| Current | 188 (3.5) | 13 (3.0) | |
| Alcohol use, drink/week 0–2 | 3218 (59.7) | 250 (58.0) | 0.152 |
| 3 to 13 | 1552 (28.8) | 118 (27.4) | |
| 14+ | 620 (11.5) | 63 (14.6) | |
| Self-reported vertebral fracture at any age | 116 (2.2) | 118 (27.4) | <.001 |
| Fell during the past 12 months | 1096 (20.3) | 115 (26.7) | 0.002 |
| One of more medical conditions** | 2698 (50.0) | 244 (56.6) | 0.008 |
| Current use of bisphosphonates | 55 (1.1) | 41 (10.0) | <.001 |
| Total hip BMD, g/cm2 | 0.964 ± 0.138 | 0.887 ± 0.141 | <.001 |
| Lumbar spine BMD, g/cm2 | 1.077 ± 0.184 | 0.986 ± 0.185 | <.001 |
| Unable to complete 5 chairs stands | 117 (2.2) | 24 (5.6) | <.001 |
| Time to complete 5 chair stands, seconds | 11.0 ± 3.2 | 11.9 ± 4.5 | <.001 |
| Leg power, watts | 210.5 ± 62.5 | 191.0 ± 63.4 | <.001 |
| Unable to complete narrow walk | 397 (7.4) | 44 (10.2) | 0.031 |
| Time to complete narrow walk, seconds | 5.6 ± 1.9 | 6.0 ± 2.6 | 0.007 |
| Walking speed, m/s | 1.21 ± 0.2 | 1.15 ± 0.2 | <.001 |
| Unable to perform grip strength | 85 (1.57) | 8 (1.8) | 0.654 |
| Grip strength, kg | 41.9 ± 8.4 | 39.5 ± 8.7 | <.001 |
| Clinical incident vertebral fracture | 114 (2.1%) | 35 (8.1%) | <.001 |
Medical conditions include a history of stroke, diabetes, hyperthyroidism, hypothyroidism, Parkinson’s disease, heart attack, congestive heart failure, COPD and non-skin cancer.
Analytic Sample
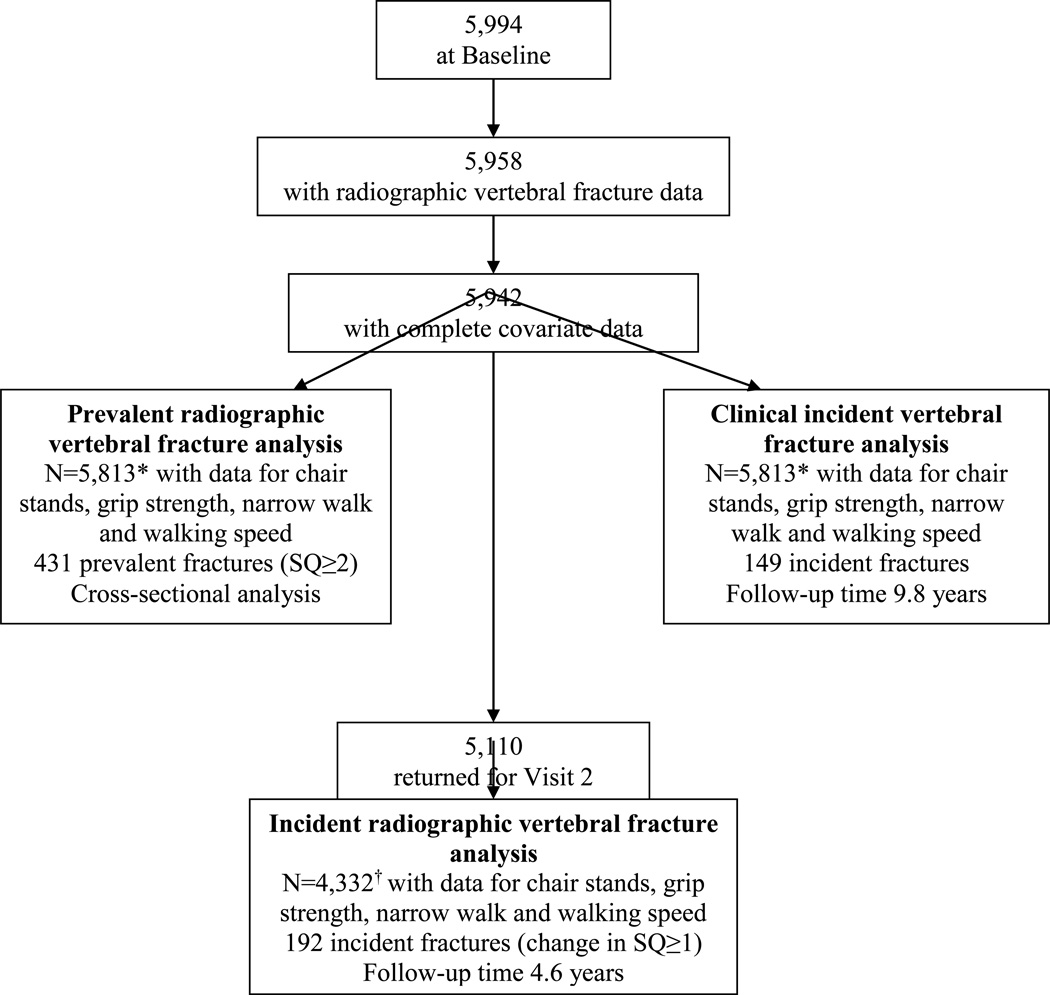
At baseline, thoracic and lumbar radiographs were acquired for 5994 men, of whom 5958 had technically adequate images for SQ scoring (Figure 1). Of these men, 145 were missing data for walking speed, narrow walk, chair stands, or grip strength, or covariates, leaving 5813 in the main cross-sectional analyses for prevalent radiographic vertebral fracture. Due to machine failures, an additional 492 men were missing leg power data, leaving 5321 in the main cross-sectional analyses for leg power.
Figure 1.
*N=5,321 with data for leg power in prevalent vertebral fracture analysis and clinical incident vertebral fracture analyses
†N=4002 with data for leg power in incident radiographic vertebral fracture analysis
Of those 5,813 men with data available for the cross-sectional analysis, 5,110 returned for visit 2. Of these, 4,332 men had data for incident radiographic vertebral fracture analyses. Due to machine failures, an additional 330 men were missing leg power data, leaving 4,002 in the main prospective radiographic vertebral fracture analyses using leg power as a predictor.
All 5,813 men with data for the predictors and covariates at baseline had data available for incident clinical vertebral fracture analyses.
Statistical Analyses
Participant characteristics were compared by prevalent vertebral fracture status (SQ≥2 versus SQ≤1) with t-tests for continuous normally distributed variables, Wilcoxon rank sum tests for continuous skewed variables and chi-square tests for categorical variables.
In the cross-sectional analyses, prevalent vertebral fractures were defined as moderate or worse (SQ≥2) compared to mild or no fracture (SQ≤1).
In the prospective analyses, incident vertebral fractures were primarily defined as a new or worsening fracture at follow-up, defined as a change in SQ score of 1 or more without adjustment for baseline fracture status. In secondary analyses, we adjusted the primary models for presence of a baseline vertebral fracture (SQ≥1 at baseline). We also excluded participants with a prevalent fracture (SQ≥1 at baseline) and estimated the likelihood of developing a new vertebral fracture (SQ≥1) at follow-up.
Grip strength, narrow walk, and chair stands performance were analyzed as quartiles, with an additional category for those unable to complete the measure. Walking pace and leg power were analyzed as quartiles; inability to complete a measure was not assessed for leg power and was not applicable to walking speed, as ability to walk without assistance was an entrance criterion for the study. All individual physical performance measures were also analyzed as continuous variables with the odds ratio reported per SD; those unable to complete the measure were excluded from such models. As results from these continuous models were similar to the quartiles analyses, only the quartiles analyses are presented.
Logistic regression models were used to estimate the likelihood of prevalent or incident radiographic vertebral fractures. Cox proportional hazards models were used to estimate the risk of incident clinical vertebral fractures. Multivariable models were adjusted for age, clinical center, total lumbar spine BMD, smoking status (current/past/never), race (white vs. non-white), height, weight, history of falls, activity level, and one or more co-morbid conditions (see footnote, Table 1). Models for new or worsening incident radiographic vertebral fractures were also further adjusted for prevalent vertebral fracture (SQ≥1 at baseline). Models for incident clinical vertebral fractures were also further adjusted for history of self-reported vertebral fracture.
RESULTS
Cross-sectional analyses: characteristics by prevalent radiographic vertebral fractures
At baseline, 230 men (4.0%) had a maximum SQ=1; 306 men (5.3%) had a maximum SQ=2; 125 men (2.1%) had a maximum SQ=3; and 5167 men (88.7%) had a maximum SQ=0. Thus, 431 (7.4%) had a moderate or severe prevalent fracture (SQ≥2) and 661 (11.3%) had a mild or worse prevalent fracture (SQ≥1). Characteristics of men by prevalent vertebral fracture are shown in Table 1. In general, men with prevalent fracture were older and less healthy although not all associations were significant.
Cross-sectional analyses: physical performance and prevalent vertebral fracture
Compared to those with SQ≤1, men with SQ≥2 took longer to rise from a chair and were less likely to be able to complete this test. They also had lower leg power and were less likely to be able to complete the narrow walk and took longer to complete the narrow walk. They walked more slowly and had lower grip strength, although ability to complete the grip strength test did not differ by baseline fracture status (Table 1).
In multivariate models, inability to complete the chair stands test and poor performance on this test were associated in a graded manner with an increased likelihood of prevalent fracture (SQ grade ≥2) (Supplemental Table 1). For example, men unable to complete the chair stand test were 2.5 times more likely to have a prevalent vertebral fracture (SQ≥2) than men with the best performance on this test (time to complete <8.9 seconds). Slower walking speed was also associated with increased likelihood of prevalent vertebral fracture: men in the slowest quartile of walking speed (<1.06 m/s) were 64% more likely to have a vertebral fracture than men in the fastest quartile of walking speed (≥1.36 m/s). There was a borderline significant association between poor performance on the other tests of performance (narrow walk, grip strength and leg power) and prevalent vertebral fracture.
Men with concurrent poor performance on several tests (Summary Physical Performance Score≥3) were 81% more likely to have a vertebral fracture compared to men who did not have poor performance on any test (Summary Physical Performance Score=0). Men with intermediate performance (Summary Physical Performance Score=1 or 2) did not have an increased likelihood of vertebral fracture compared to men who did not have poor performance on any test.
Prospective analyses: physical performance and incident radiographic vertebral fractures
Of the 4,332 men included in the prospective analyses, 192 (4.4%) had a new or worsening vertebral fracture (change in SQ≥1). Of those without a prevalent vertebral fracture at baseline (baseline SQ=0, N=3864), 117 (3.0%) had a new vertebral fracture (SQ≥1) at follow-up.
In multivariate models, inability to rise from a chair, and poorer performance on this test, were associated with an increased risk of a new or worsening vertebral fracture (change in SQ ≥1, Table 2). Adjustment for prevalent vertebral fracture (baseline SQ≥1) and restricting the analyses to those without a fracture at baseline (that is, restricting to those with baseline SQ=0) did not substantially alter the results (Table 2 and Supplemental Table 2). Worse leg power was also associated with an increased risk of new or worsening vertebral fracture in both primary and secondary analyses. For example, men with the lowest leg power (quartile 1) had a 2–3 fold increased risk of fracture compared to men with the highest leg power (quartile 4). Inability to complete the narrow walk test was associated with new or worsening vertebral fracture, but longer time to complete this test among those able to do so was not associated with increased vertebral fracture risk. Adjustment for prevalent fracture yielded similar results, but the association between inability to complete the narrow walk and new fracture in those without a vertebral fracture at baseline was not significant (Table 2). Walking speed was not associated with risk of radiographic vertebral fracture in any model. Similarly, inability to complete the grip strength measure was not associated with incident fracture in any model, but lower grip strength increased the risk of new or worsening vertebral fracture in the primary analyses and analyses additionally adjusted for prevalent fracture. In analyses restricted to those without a fracture at baseline, those with lower grip strength had a borderline increased fracture risk compared to those with the highest strength (Supplemental Table 2).
Table 2.
Likelihood of incident radiographic vertebral fracture* by category of baseline physical performance in MrOS men
| Test of physical performance | N in group |
N (%) fractured |
Model 1† (OR, 95% CI) |
Model 2‡ (OR, 95% CI) |
|
|---|---|---|---|---|---|
| Repeated chair stands | Unable | 61 | 6 (9.8) | 2.88 (1.10, 7.53) | 2.69 (1.00, 7.22) |
| Quartile 4 (worst) ≥12.6 s | 932 | 61 (6.6) | 1.93 (1.22, 3.06) | 1.93 (1.21, 3.08) | |
| Quartile 3 ≥10.5 to <12.6 s | 1068 | 40 (3.8) | 1.21 (0.75, 1.95) | 1.25 (0.77, 2.04) | |
| Quartile 2 ≥9.0 to <10.5 s | 1132 | 51 (4.5) | 1.43 (0.91, 2.24) | 1.46 (0.92, 2.3) | |
| Quartile 1 (best) < 8.9 s | 1139 | 34 (3.0) | 1.00 (referent)‡ | 1.00 (referent)‡ | |
| Leg power | Quartile 1 (worst) <164.7 watts | 803 | 54 (6.7) | 3.00 (1.67, 5.39) | 2.07 (1.18, 3.60) |
| Quartile 2 ≥164.7 to < 207.4 watts | 1023 | 46 (4.5) | 2.05 (1.19, 3.56) | 1.96 (1.14, 3.34) | |
| Quartile 3 ≥207.4 to <247.8 watts | 1048 | 44 (4.2) | 1.95 (1.15, 3.31) | 1.04 (0.87, 1.23) | |
| Quartile 4 (best) ≥247.8 watts | 1128 | 23 (2.0) | 1.00 (referent)‡ | 1.00 (referent)‡ | |
| Narrow walk | Unable | 217 | 20 (9.2) | 1.99 (1.08, 3.68) | 2.16 (1.15, 4.06) |
| Quartile 4 (worst time) ≥6.2 s | 892 | 38 (4.3) | 0.88 (0.54, 1.44) | 0.87 (0.53, 1.42) | |
| Quartile 3 ≥5.2 to < 6.2 s | 1035 | 46 (4.4) | 1.02 (0.66, 1.59) | 1.05 (0.67, 1.65) | |
| Quartile 2 ≥4.5 to < 5.2 s | 1072 | 46 (4.3) | 1.08 (0.70, 1.68) | 1.10 (0.71, 1.72) | |
| Quartile 1 (best) <4.5 s | 1116 | 42 (3.8) | 1.00 (referent) | 1.00 (referent) | |
| Walking speed | Quartile 1 (slowest) <1.06 m/s | 887 | 50 (5.6) | 1.47 (0.92, 2.34) | 1.43 (0.89, 2.31) |
| Quartile 2 ≥1.06 to < 1.21 m/s | 1091 | 52 (4.8) | 1.37 (0.88, 2.12) | 1.34 (0.86, 2.09) | |
| Quartile 3 ≥1.21 to < 1.36 m/s | 1138 | 50 (4.4) | 1.29 (0.84, 1.99) | 1.22 (0.79, 1.90) | |
| Quartile 4 (fastest) < 1.36 m/s | 1216 | 40 (3.3) | 1.00 (referent) | 1.00 (referent) | |
| Grip strength | Unable | 69 | 3 (4.4) | 1.53 (0.44, 5.27) | 1.54 (0.44, 5.44) |
| Quartile 1 (worst)<36.0 kg | 768 | 54 (7.0) | 1.90 (1.15, 3.16) | 2.01 (1.20, 3.36) | |
| Quartile 2 ≥36.0 to <42.0 kg | 1090 | 60 (5.5) | 1.60 (1.01, 2.54) | 1.61 (1.01, 2.57) | |
| Quartile 3 ≥42.0 to <48.0 kg | 1153 | 40 (3.5) | 1.13 (0.70, 1.82) | 1.15 (0.71, 1.87) | |
| Quartile 4 (best) (≥48 kg) | 1252 | 35 (2.8) | 1.00 (referent)‡ | 1.00 (referent)‡ | |
| Baseline Summary Physical Performance Score (number of tests with poor performance) | 0 (best) | 1796 | 58 (3.3) | 1.00 (referent)‡ | 1.00 (referent)‡ |
| 1–2 | 1744 | 76 (4.4) | 1.21 (0.84, 1.74) | 1.25 (0.86, 1.81) | |
| 3 + (worst) | 636 | 52 (8.2) | 2.04 (1.30, 3.22) | 2.00 (1.26, 3.19) |
Change in SQ≥1
Model 1 adjusted for total lumbar spine bone mineral density, clinical center, smoking status, race, age, height, weight, history of falls, one or more co-morbid conditions and activity level. Model 2 includes additional adjustment for prevalent fracture (SQ≥1 at baseline)
P for trend across quartiles/groups <0.05
Men with concurrent poor performance on several tests (Summary Physical Performance Score ≥3) were about twice as likely to have a new or worsening radiographic vertebral fracture in the primary analyses and this association was unchanged by adjustment for prevalent fractures status (Table 2). Similarly, in analyses restricted to those without prevalent fracture at baseline, the men with the worst Summary Physical Performance Score (≥3) were also about twice as likely to have a new radiographic vertebral fracture compared to those with the best performance (Supplemental Table 2).
Prospective analyses: Physical performance and incident clinical vertebral factures
During 9.8 years (3.0 SD) of follow-up, 149 men (2.6%) experience a clinical vertebral fracture, of whom 132 (91.7%) reported going to the doctor for back pain.
In multivariate models (Table 3), men who were unable to complete the repeated chair stands test had more than 3 times the risk of risk of clinical vertebral fractures compared to men with the best performance on this test, and this association was only slightly attenuated after adjustment for self-reported prevalent vertebral fracture. In contrast, the time to complete the chair stands test was not strongly related to risk of clinical vertebral fractures. None of the other individual physical performance tests (leg power, narrow walk, walking speed and grip strength) were related to risk of clinical vertebral fracture. There was a suggestion that men with poor performance on several physical performance tests had a somewhat higher risk of clinical vertebral fracture compared to men with better performance (p for trend across categories of baseline summary physical performance score = 0.078), but this association was further attenuated by adjustment for prevalent self-reported vertebral fracture (p for trend across categories of baseline summary physical performance score = 0.114).
Table 3.
Risk of incident clinical vertebral fracture by category of baseline physical performance in MrOS men
| Test of physical performance | N in group |
N (%) fractured |
Model 1* HR (95% CI) |
Model 2* HR (95% CI) |
|
|---|---|---|---|---|---|
| Repeated chair stands | Unable | 140 | 10 (7.1) | 3.66 (1.68, 7.94) | 3.38 (1.54, 7.40) |
| Quartile 4 (worst) ≥12.6 s | 1419 | 53 (3.7) | 1.58 (0.96, 2.61) | 1.57 (0.95, 2.58) | |
| Quartile 3 ≥10.5 to <12.6 s | 1421 | 26 (1.8) | 0.92 (0.53, 1.59) | 0.93 (0.54, 1.61) | |
| Quartile 2 ≥9.0 to <10.5 s | 1423 | 33 (2.3) | 1.07 (0.64, 1.79) | 1.07 (0.64, 1.79) | |
| Quartile 1 (best) < 8.9 s | 1410 | 27 (1.9) | 1.00 (referent)† | 1.00 (referent)‡ | |
| Leg power | Quartile 1 (worst) <164.7 watts | 1312 | 38 (2.9) | 1.10 (0.58, 2.08) | 1.10 (0.58, 2.08) |
| Quartile 2 ≥164.7 to < 207.4 watts | 1347 | 48 (3.6) | 1.51 (0.86, 2.66) | 1.53 (0.87, 2.69) | |
| Quartile 3 ≥207.4 to <247.8 watts | 1312 | 41 (2.4) | 1.16 (0.65, 2.04) | 1.17 (0.66, 2.07) | |
| Quartile 4 (best) ≥247.8 watts | 1350 | 21 (1.6) | 1.00 (referent) | 1.00 (referent) | |
| Narrow walk | Unable | 439 | 12 (2.7) | 1.08 (0.52, 2.26) | 1.05 (0.50, 2.20) |
| Quartile 4 (worst time) ≥6.2 s | 1350 | 45 (3.3) | 1.19 (0.71, 2.02) | 1.16 (0.69, 1.96) | |
| Quartile 3 ≥5.2 to < 6.2 s | 1340 | 38 (2.8) | 1.19 (0.72, 1.97) | 1.15 (0.69, 1.92) | |
| Quartile 2 ≥4.5 to < 5.2 s | 1342 | 27 (2.0) | 0.93 (0.54, 1.59) | 0.92 (0.54, 1.59) | |
| Quartile 1 (best time) <4.5 s | 1342 | 27 (2.0) | 1.00 (referent) | 1.00 (referent) | |
| Walking speed | Quartile 1 (slowest) <1.06 m/s | 1446 | 47 (3.3) | 1.06 (0.67, 1.70) | 1.02 (0.64, 1.63) |
| Quartile 2 ≥1.06 to < 1.21 m/s | 1452 | 36 (2.5) | 0.85 (0.53, 1.35) | 0.86 (0.54, 1.36) | |
| Quartile 3 ≥1.21 to < 1.36 m/s | 1455 | 25 (1.7) | 0.61 (0.37, 1.02) | 0.60 (0.36, 1.00) | |
| Quartile 4 (fastest) < 1.36 m/s | 1460 | 41 (2.8) | 1.00 (referent) | 1.00 (referent) | |
| Grip strength | Quartile 1 (worst strength) <36.0 kg | 1321 | 43 (3.3) | 1.42 (0.79, 2.56) | 1.43 (0.79, 2.56) |
| Quartile 2 ≥36.0 to <42.0 kg | 1505 | 44 (2.9) | 1.37 (0.80, 2.35) | 1.38 (0.81, 2.37) | |
| Quartile 3 ≥42.0 to <48.0 kg | 1472 | 39 (2.7) | 1.48 (0.87, 2.51) | 1.52 (0.89, 2.58) | |
| Quartile 4 (best strength) ≥48 kg | 1515 | 23 (1.5) | 1.00 (referent) | 1.00 (referent) | |
| Baseline Summary Physical Performance Score (number of tests with poor performance) | 0 (best) | 2157 | 40 (1.9) | 1.00 (referent) | 1.00 (referent) |
| 1–2 | 2294 | 62 (2.7) | 1.24 (0.82, 1.88) | 1.23 (0.81, 1.86) | |
| 3 + (worst) | 1149 | 33 (3.8) | 1.58 (0.95, 2.62) | 1.51 (0.91, 2.51) |
Model 1 Adjusted for total lumbar spine bone mineral density, clinical center, smoking status, race, age, height, weight, history of falls, one or more co-morbid conditions and activity level. Model 2 additionally adjusted for prevalent vertebral fracture (assessed by self-report.)
P <0.005 across quartiles/groups
DISCUSSION
In general, we found modest cross-sectional associations between poor performance in a number of domains and an increased likelihood of prevalent radiographic vertebral fracture. Men with concurrent poor performance on several measures (3 or more) were more likely to have a prevalent radiographic vertebral fracture than those who did not have poor performance on any measure. In prospective analyses, with the exception of walking speed, poorer performance was associated in a graded manner with an increased risk of incident radiographic vertebral fracture. Men with current poor performance on several tests had a higher risk of radiographic vertebral fracture over time compared to men who did not have poor performance on any test. In contrast, for incident clinical vertebral fractures, only inability to complete the repeat chair stands test, but not the time to complete this test, nor any other measure of physical performance was associated with increased risk of clinical vertebral fracture.
There are several reasons why poor physical performance may be associated with radiographic vertebral fractures. First, at least some vertebral fractures are likely the result of a fall. Oe report has found that the vast majority of vertebral fracture-related emergency department visits were due to a fall;16 another report found that in MrOS, about half of clinical vertebral fractures were precipitated by a fall.17 This suggests that poor physical function may influence the risk of these fractures through an increased risk of falling.18 However, most radiographic vertebral fractures never come to clinical attention and their etiology is not well described, especially in men.19 Second, the mass, quality and function of both muscle and bone show a parallel decline with age in men,14,20,21 suggesting that a common factor may contribute to declines in each system. Third, the “mechanostat” model theorizes that bone responds to local mechanical forces,22 adapting to usual, every day strain which is likely reflected in body size and measures of physical performance. Specifically, two small studies23,24 suggest that back extensor muscle weakness may be associated with vertebral fracture and reduced BMD, although a larger study in women did not find that physical performance was a strong risk factor for vertebral fractures once other clinical characteristics were known.25 Fourth, participants with prevalent vertebral fracture tended to be less healthy than those without fracture, suggesting vertebral fractures may be the result of poor health status. However, our results were generally little changed by adjustment for our measured confounding factors, including comorbid medical conditions. Finally, muscle may have dominant role in the synchronization of the musculoskeletal system; if this is the case, then one would expect that declines in muscle (which may be reflected in declines in strength or physical performance) would precede decrements in bone (such as vertebral fractures),26 thereby increasing the risk of fracture in those with poor performance.
There was an association between physical performance and incident radiographic, but not incident clinical vertebral fractures. There are several potential reasons for this discrepancy. First, there were differences in methodology between identification of clinical versus radiographic fractures: clinical fractures were identified using community acquired images; different readers assessed SQ grades for the clinical and radiographic fractures; and clinical fractures must have come to the attention of the participant and then reported to their physician. In contrast, radiographic fractures may not necessarily have come to clinical attention. In women, only one-quarter to one-third of radiographic fractures come to clinical attention.19,27,28 We posit that the lack of association between physical performance and clinical fractures is due to bias towards the null due to substantial misclassification of the outcome. On the one hand, this explanation suggests that radiographic fractures should be used as the gold standard in research studies. However, other evidence in women suggests that clinical vertebral fractures are more strongly associated with clinical outcomes than radiographic fractures.19,29,30 These apparently discrepant findings demonstrate the need for additional research in this area, particularly for men.
The associations between physical performance and radiographic vertebral fracture were somewhat stronger for incident compared to prevalent fractures. This observation may be due to the possibility that many prevalent fractures in older men may be of diverse origins (such as traumatic events at much younger ages as well as osteoporotic changes) whereas incident radiographic vertebral fractures are more often osteoporotic in origin.
Our results are consistent with previous reports that have demonstrated a cross-sectional association between vertebral fracture and self-reported function. A small case-control study showed that men with vertebral fracture were more likely to report poor physical function than men without vertebral fracture.31 Also, in older men and women, those with radiographic vertebral fractures were more likely to report disability and use of walking aids than those without fracture.32
Our results suggest that physical performance may have clinical utility in identifying individuals at risk of fractures. In particular, in men, inability to rise from a chair without the use of the arms is a strong risk factor for both vertebral fracture as shown here (2–3 fold increased risk) and hip fracture (8 fold increased risk), as previously reported.7 Chair rise inability is simple to measure in a clinical setting, and may ultimately prove valuable in indentifying whom to treat and the course of treatment to undertake; however, this must be further developed in additional analyses. These results suggest that clinical interventions to improve physical performance could also reduce the risk of future vertebral fractures, especially in patients with existing vertebral fractures who are at high risk.
This study has a number of strengths. MrOS is a well characterized cohort of men with extensive data on physical performance and excellent assessment of vertebral fracture status. However, a few limitations must be noted. First, the MrOS cohort is comprised of ambulatory, community dwelling, mostly white men, so our ability to extrapolate our results to other populations, such as women, non-whites, and the institutionalized is limited. Second, the MrOS cohort is healthy and well-functioning at baseline, and associations between physical performance and vertebral fracture may differ in the infirm. Third, although our assessment of radiographic vertebral fractures is consistent with many other studies, we do not know the cause of each fracture and thus cannot determine if risk factors differ for fractures that occur subsequent to a fall compared to fractures that occur under other circumstances. Fourth, we do not have measures of trunk muscle strength or function. Finally, our composite measure of function, Summary Physical Performance Score, is unique to MrOS. While this measure has been linked to hip fractures in MrOS, it has not been formally validated, so extrapolation of these results to other populations may be difficult.
We conclude that, in general, poor physical performance is associated with greater likelihood of prevalent radiographic vertebral fracture and an increased risk of incident radiographic vertebral fracture. There was little association between poor physical performance and risk of clinical vertebral fractures. Randomized trials that evaluate interventions to improve physical performance in older adults should consider adding radiographic vertebral fractures as secondary outcomes.
Supplementary Material
Acknowledgement
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 TR000128. PMC developed the concept for the paper and drafted the manuscript; TLB completed the statistical analyses. JTS completed the radiographic assessment of the prevalent and incident radiographic vertebral fractures. All authors critically reviewed the paper and assisted with interpretation of the analyses.
References
- 1.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. Jama. 2011 Jan 5;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cawthon PM, Fox KM, Gandra SR, et al. Clustering of strength, physical function, muscle, and adiposity characteristics and risk of disability in older adults. J Am Geriatr Soc. 2011 May;59(5):781–787. doi: 10.1111/j.1532-5415.2011.03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cawthon PM, Fox KM, Gandra SR, et al. Do Muscle Mass, Muscle Density, Strength, and Physical Function Similarly Influence Risk of Hospitalization in Older Adults? Journal of the American Geriatrics Society. 2009;57(8):1411–1419. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. [3/2/1995];N.Engl.J Med. 1995 332:556–561. doi: 10.1056/NEJM199503023320902. [see comments]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 6.Nyberg L, Gustafson Y, Berggren D, Brannstrom B, Bucht G. Falls leading to femoral neck fractures in lucid older people. J Am Geriatr Soc. 1996;44:156–160. doi: 10.1111/j.1532-5415.1996.tb02432.x. 1996. [DOI] [PubMed] [Google Scholar]
- 7.Cawthon PM, Fullman RL, Marshall L, et al. Physical performance and risk of hip fractures in older men. J Bone Miner Res. 2008 Jul;23(7):1037–1044. doi: 10.1359/JBMR.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schousboe JT, Rosen HR, Vokes TJ, et al. Prediction Models of Prevalent Radiographic Vertebral Fractures Among Older Men. J Clin Densitom. 2013 Nov 27; doi: 10.1016/j.jocd.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005 Oct;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell T, Cawthon PM, Marshall LM, Brand R. Consistency of Leg Extension Power Assessments in Older Men: The Osteoporotic Fractures in Men (MrOS) Study. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2009 Nov;88(11):934–940. doi: 10.1097/PHM.0b013e3181bbddfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harkonen R, Harju R, Alaranta H. Accuracy of the Jamar dynamometer. J Hand Ther. 1993 Oct-Dec;6(4):259–262. doi: 10.1016/s0894-1130(12)80326-7. [DOI] [PubMed] [Google Scholar]
- 12.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 13.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. [2/1993];J Clin.Epidemiol. 1993 46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 14.Cawthon PM, Ewing SK, McCulloch CE, et al. Loss of hip BMD in older men: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2009 Oct;24(10):1728–1735. doi: 10.1359/JBMR.090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994 Aug;10(4):405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 16.Oudshoorn C, Hartholt KA, Zillikens MC, et al. Emergency department visits due to vertebral fractures in the Netherlands, 1986–2008: steep increase in the oldest old, strong association with falls. Injury. 2012 Apr;43(4):458–461. doi: 10.1016/j.injury.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Freitas SS, Barrett-Connor E, Ensrud KE, et al. Rate and circumstances of clinical vertebral fractures in older men. Osteoporos Int. 2008 May;19(5):615–623. doi: 10.1007/s00198-007-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan BK, Marshall LM, Winters KM, Faulkner KA, Schwartz AV, Orwoll ES. Incident Fall Risk and Physical Activity and Physical Performance among Older Men: The Osteoporotic Fractures in Men Study. Am J Epidemiol. 2007 Mar 15;165(6):696–703. doi: 10.1093/aje/kwk050. [DOI] [PubMed] [Google Scholar]
- 19.Ensrud KE, Schousboe JT. Clinical practice. Vertebral fractures. N Engl J Med. 2011 Apr 28;364(17):1634–1642. doi: 10.1056/NEJMcp1009697. [DOI] [PubMed] [Google Scholar]
- 20.Cawthon PM, Ewing SK, Mackey DC, et al. Change in hip bone mineral density and risk of subsequent fractures in older men. J Bone Miner Res. 2012 Oct;27(10):2179–2188. doi: 10.1002/jbmr.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. The journals of gerontology. 2006 Oct;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 22.Frost HM. A 2003 update of bone physiology and Wolff's Law for clinicians. The Angle orthodontist. 2004 Feb;74(1):3–15. doi: 10.1043/0003-3219(2004)074<0003:AUOBPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Sinaki M, Itoi E, Wahner HW, et al. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone. 2002 Jun;30(6):836–841. doi: 10.1016/s8756-3282(02)00739-1. [DOI] [PubMed] [Google Scholar]
- 24.Sinaki M, Wollan PC, Scott RW, Gelczer RK. Can strong back extensors prevent vertebral fractures in women with osteoporosis? Mayo Clin Proc. 1996 Oct;71(10):951–956. doi: 10.1016/S0025-6196(11)63768-3. [DOI] [PubMed] [Google Scholar]
- 25.Nevitt MC, Cummings SR, Stone KL, et al. Risk factors for a first-incident radiographic vertebral fracture in women > or = 65 years of age: the study of osteoporotic fractures. J Bone Miner Res. 2005 Jan;20(1):131–140. doi: 10.1359/JBMR.041003. [DOI] [PubMed] [Google Scholar]
- 26.Digirolamo DJ, Kiel DP, Esser KA. Bone and skeletal muscle: neighbors with close ties. J Bone Miner Res. 2013 Apr 29; doi: 10.1002/jbmr.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper C, Atkinson EJ, O'Fallon WM, Melton LJ. Incidence of clinically diagnosed vertebral fractures: A population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7:221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 28.Fink HA, Milavetz DL, Palermo L, et al. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res. 2005 Jul;20(7):1216–1222. doi: 10.1359/JBMR.050314. [DOI] [PubMed] [Google Scholar]
- 29.Fink HA, Ensrud KE, Nelson DB, et al. Disability after clinical fracture in postmenopausal women with low bone density: the fracture intervention trial (FIT) Osteoporos Int. 2003;14(1):69–76. doi: 10.1007/s00198-002-1314-y. [DOI] [PubMed] [Google Scholar]
- 30.Nevitt MC, Ettinger B, Black DM, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Annals of internal medicine. 1998;128(10):793–800. doi: 10.7326/0003-4819-128-10-199805150-00001. [DOI] [PubMed] [Google Scholar]
- 31.Scane AC, Francis RM, Sutcliffe AM, Francis MJ, Rawlings DJ, Chapple CL. Case-control study of the pathogenesis and sequelae of symptomatic vertebral fractures in men. Osteoporos Int. 1999;9(1):91–97. doi: 10.1007/s001980050120. [DOI] [PubMed] [Google Scholar]
- 32.Burger H, Van Daele PL, Grashuis K, et al. Vertebral deformities and functional impairment in men and women. J Bone Miner Res. 1997 Jan;12(1):152–157. doi: 10.1359/jbmr.1997.12.1.152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.