Abstract
Objective
To use fMRI to investigate whether hippocampal and entorhinal activation during learning is altered in the earliest phase of mild cognitive impairment (MCI).
Methods
Three groups of older individuals were studied: 10 cognitively intact controls, 9 individuals at the mild end of the spectrum of MCI, and 10 patients with probable Alzheimer disease (AD). Subjects performed a face-name associative encoding task during fMRI scanning, and were tested for recognition of stimuli afterward. Data were analyzed using a functional-anatomic method in which medial temporal lobe (MTL) regions of interest were identified from each individual’s structural MRI, and fMRI activation was quantified within each region.
Results
Significantly greater hippocampal activation was present in the MCI group compared to controls; there were no differences between these two groups in hippocampal or entorhinal volumes. In contrast, the AD group showed hippocampal and entorhinal hypoactivation and atrophy in comparison to controls. The subjects with MCI performed similarly to controls on the fMRI recognition memory task; patients with AD exhibited poorer performance. Across all 29 subjects, greater mean entorhinal activation was found in the subgroup of 13 carriers of the APOE ε4 allele than in the 16 noncarriers.
Conclusions
The authors hypothesize that there is a phase of increased medial temporal lobe activation early in the course of prodromal Alzheimer disease followed by a subsequent decrease as the disease progresses.
Since the earliest pathologic changes in Alzheimer disease (AD) are thought to occur in medial temporal lobe (MTL) brain regions, particularly the hippocampal formation (HF) and entorhinal cortex (EC), there is growing interest in using fMRI to assess the integrity of MTL function in very early AD. Studies conducted in patients with a clinical diagnosis of AD consistently show that MTL activation is decreased in comparison to older controls.1-5 Relatively few fMRI studies have investigated subjects whose cognitive function falls between that of normal aging and mild AD, a condition often referred to as mild cognitive impairment (MCI), and results thus far have been inconsistent.1,5,6
It is increasingly clear that groups of individuals with MCI are heterogenous,7 and this clinical heterogeneity may, in part, explain differences between previous fMRI studies of MCI. If MCI encompasses the entire transitional continuum between normal aging and dementia, as has been proposed,7 then individuals at the boundary between normal aging and MCI should have less underlying pathology and less memory impairment than individuals at the boundary between MCI and AD. Our previous fMRI study demonstrated that this range of clinical impairment is reflected in MTL activation, with increased activation in MCI subjects who were more impaired compared with those who were less impaired.6 It is not yet clear, however, whether MTL activation is increased or decreased in MCI subjects with very mild symptoms compared to normal older individuals. It is also unknown whether changes in MTL function in very mildly impaired individuals precede significant atrophy of MTL regions.
To address these questions, we performed an fMRI study using a face-name associative memory task in three groups of older persons: 1) cognitively intact individuals, 2) subjects with MCI, and 3) patients with probable AD. For the MCI group, we specifically selected individuals with mild functional difficulty in daily life, suggesting that they were on the mild end of the MCI spectrum. Based on our previous findings, we hypothesized that patients with AD would demonstrate decreased MTL activation, but the MCI subjects would exhibit increased MTL activation in comparison to older controls. Moreover, we hypothesized that while patients with AD would demonstrate decreased volume in MTL regions, the increased activation in early MCI subjects would be present in the absence of significant MTL atrophy.
Methods
The study involved 29 older individuals. Of these, 18 were participants in a longitudinal study of aging and the evolution of AD, and were recruited through the print media (rather than from a clinical or other medical referral source).8 Another 11 subjects were recruited from tertiary memory disorders clinics. All subjects provided informed consent in accordance with the Human Research Committee guidelines of the Massachusetts General Hospital (Boston, MA). None of the subjects in the present study were participants in our previous fMRI studies.4,6
All subjects underwent a comparable clinical, neuropsychological, and laboratory evaluation, and were required to be free of significant underlying medical, neurologic, or psychiatric conditions. Based on the results of this evaluation, subjects were assigned to one of three clinical groups.
Ten subjects met criteria for normal cognitive function in that they had an overall Clinical Dementia Rating (CDR) of 0. All 10 of these normal older controls were participants in the longitudinal study mentioned above.
Nine individuals met the following criteria for MCI9 in that they 1) had a memory complaint corroborated by an informant, 2) had normal general cognitive function, 3) had normal activities of daily living, 4) had an overall CDR rating of 0.5, and 5) were not demented. Given the present study’s focus on the very mild end of the MCI spectrum, we selected subjects whose CDR Sum of Boxes score ranged from 0.5 to 1.5, with at least a 0.5 in the memory subcategory, and we did not require that subjects perform below specific cutoffs on psychometric testing. All but one were participants in the longitudinal study.
Ten subjects met clinical research criteria for probable AD.10 All of the patients with AD were recruited from tertiary memory disorders clinics, and had either been off cholinesterase inhibitors for at least 30 days prior to scanning or had never taken these medications.
The CDR ratings were derived from a semi-structured interview. This interview generates both an overall CDR rating and a measure known as the CDR Sum of Boxes. The interview was based on the Initial Subject Protocol that was used in the development of the CDR scale.11,12 It includes a set of questions regarding functional status, asked of the subject and a collateral source (e.g., family member or friend), and a standardized neurologic, psychiatric, and mental status evaluation of the subject. In the current study, each interview was administered by a skilled clinician (e.g., psychiatrist, neurologist, neuropsychologist, or physician’s assistant) and took approximately 1 to 2 hours to complete. The mean inter-rater reliability of the CDR ratings was high (r = 0.99, p < 0.0001), as was the inter-rater reliability of the 6 CDR subcategories (r = 0.90) that were used to generate the overall CDR rating.8 The CDR Sum of Boxes represents the sum of the ratings in each of the six CDR subcategories.
MRI procedures
Data acquisition
Subjects were scanned using a Siemens Trio 3.0 Tesla scanner (Siemens Medical Systems, Iselin, NJ) with a three-axis gradient head coil. First, high resolution structural data were acquired (Siemens MP-RAGE sequence: repetition time (TR)/inversion time (TI)/echo time (TE) 2,730/1,000/3 msec, field of view (FOV) = 256, FA = 7°, 128 sagittal slices, thickness = 1.33 mm, matrix 192 × 256. Due to subject discomfort, one subject’s high-resolution structural data were acquired at a separate session on a 1.5 Tesla General Electric scanner using an SPGR sequence: TR/TE = 35/5 msec, FOV = 240, FA = 45°, 124 coronal slices, thickness = 1.5 mm, matrix 256 × 256. Next, blood oxygen level-dependent (BOLD) functional data were acquired (Siemens gradient echo T2* sequence: TR/TE = 2,500/30 msec, FA = 90°, 30 slices, 5-mm thick with 1-mm gap, voxel dimensions = 3.125 mm2). Functional data were acquired in an oblique coronal orientation beginning at the occipital pole, perpendicular to the anterior-posterior commissure line, to maximize in-plane resolution in the hippocampus. Scanning time for each functional run was 4 minutes and 15 seconds, consisting of 102 time points per run (the first 4 time points were discarded for T1 stabilization).
fMRI activation task
The associative encoding task involved the learning of faces (previously unfamiliar to the subjects) paired with fictional first names.4,13 The face stimuli were color photographs taken with a digital camera (Fuji 800) with resolution of 924 × 1,096 mp. The photographs were of individuals who varied in age (range 18 to 90 years) and ethnicity, with equal numbers of male and female faces. Popular first names were obtained from the public lists on the Internet of the most commonly used names for each decade from 1910 through 1990. First names were assigned to each face by the investigators. The face stimuli were centered on a black background with the first name printed in white below the face, forming a face-name pair. Visual stimuli were presented using a Macintosh Computer (Apple) connected to a Sharp 2000 color LCD projector. Images were projected through a collimating lens (Buhl Optical) onto a screen attached to the head coil during functional data acquisition.
Subjects were given explicit instructions to try to remember which name was associated with which face for later testing, and were asked to indicate with a button press whether each name “fit” each face. These stimuli were used in a block design paradigm with three conditions: 1) Novel: Each novel face-name pair was presented once for 5 seconds. Subjects viewed seven novel face-name pairs during each Novel block, seeing a total of 84 novel face-name pairs over the course of six functional runs. 2) Repeated: Two repeated face-name pairs (one male and one female) were presented for 5 seconds each, with the male and female face-name pairs alternating throughout each Repeated block: The two repeated face-name pairs were first shown to the subject in a practice run immediately prior to the functional runs, so that these stimuli were already somewhat familiar to the subjects prior to beginning the functional runs. Each repeated face-name pair was presented a total of 49 times over the course of the session. 3) Fixation: Subjects were instructed to look at a white fixation cross (+) presented in the center of the visual field on a black background in order to focus the subject’s attention in the visual domain.
Approximately 5 minutes after the scanning session was completed, all subjects underwent a brief forced-choice recognition test for a subset of the face-name pairs presented in the magnet. Fourteen of the faces shown during that scanning session were presented on a computer monitor outside the scanning room. Each face was shown with two names printed underneath: the correct name that was paired with the face during scanning and one incorrect name that was previously paired with a different face during scanning. The position of the correct name was counterbalanced across the post-scan test stimuli, and subjects were instructed to indicate the correct name by pointing to it on the computer monitor.
Data analysis
Functional-anatomic MRI data analysis
Freesurfer/FS-FAST analysis software was used to analyze the fMRI data (http://surfer.nmr.mgh.harvard.edu). Each of the six functional MRI runs was motion-corrected to the first run using the Analysis of Functional NeuroImages software14 and then spatially smoothed using a three-dimensional Gaussian filter (FWHM = 5 mm). The stimulus effects at each voxel were estimated by fitting the amplitudes of two boxcar functions (one for Novel and one for Repeated conditions) convolved with a gamma function to the BOLD signal across all runs.15 The boxcar was delayed by 5 seconds with respect to block onset to account for the hemodynamic delay. A baseline offset and linear trend were also fit for each run. The residual error was used to estimate the variance of the noise.15
Each subject’s fMRI dataset was then coregistered to that subject’s structural MRI dataset so that each individual’s fMRI data could be localized with reference to their own neuroanatomic space.16 Activation maps were generated for the contrast of Novel vs Repeated (NvR) conditions, which held the visual complexity of the stimuli constant, and thus provided information on the encoding of novel face-name pairs compared with the viewing of familiar face-name pairs.
The structural MRI data were also used to generate two anatomic MTL regions of interest (ROI): the hippocampal formation (hippocampus proper, dentate gyrus, and subiculum) and entorhinal cortex (including subjacent white matter). ROIs were drawn manually (by B.C.D. and D.H.S.) on multiple slices of the structural MRI in both the right and left hemisphere, following previously published protocols.17,18 Reliability data for these procedures have been previously reported.18 Raw ROI volumetric data were analyzed, as were ROI data divided by mid-sagittal intracranial area to account for the potential contribution of differences in head size.19
The extent of fMRI activation in the NvR contrast was examined within each ROI, using a modified version of a previously reported method.6,20 Extent of activation was defined as the number of voxels activated over the significance threshold (p < 0.01) within the structural ROI (i.e., number of significantly activated voxels for a given contrast). For correlational analyses, we examined both absolute extent of activation (the absolute number of activated voxels) and relative extent of activation (the number of activated voxels divided by the total number of voxels in the ROI); for group comparisons, we examined relative extent of activation.
APOE genotyping
The APOE polymorphisms were genotyped by restriction fragment length analysis following PCR from 10 nanograms of genomic DNA, as described previously.21
Statistical analyses
Group comparisons were performed using analysis of variance (ANOVA), with a priori-specified planned contrasts, to evaluate differences between subject groups with regard to specific characteristics of interest. Pearson correlations and partial correlations (adjusting for covariates) were performed to examine relationships among the primary variables of interest. In addition, the effects of potential confounding factors, such as age and education, were analyzed as covariates. These statistical analyses were performed using SPSS 11.0 (Chicago, IL).
Results
Subject characteristics
The elderly controls (n = 10) demonstrated normal cognition, with overall CDR ratings of 0, CDR Sum-of-Boxes scores of 0, and a mean Mini-Mental State Examination22 (MMSE) score of 29.7 (range = 29 to 30). The MCI group (n = 9) had a mean CDR Sum-of-Boxes score of 0.94 (0.5 [n = 5] and 1.5 [n = 4]), and a mean MMSE score of 29.6 (range = 27 to 30). The subjects in the AD group (n = 10) were mildly to moderately impaired with a mean MMSE score of 21.1 (range = 15 to 24). Of the 29 subjects, 13 were heterozygous carriers of the APOE ε4 allele: two control, four MCI, and seven AD subjects. The demographic, clinical, psychometric, and genetic data for the three subject groups are presented in the table. The mean educational level of the MCI subjects was high at 18.4 years, which was greater than that of the other two groups (p < 0.05). Performance on the California Verbal Learning Test23 did not differ between the MCI and control groups with or without adjustment for educational level.
Table 1.
Participant characteristics
| Controls | MCI | AD | |
|---|---|---|---|
| n | 10 | 9 | 10 |
| Age, y | 71.5 ± 2.9 | 73.9 ± 7.3 | 77.6 ± 8.0 |
| Education, y | 14.9 ± 3.0 | 18.4 ± 2.4* | 13.0 ± 3.1 |
| M/F | 4/6 | 5/4 | 3/7 |
| APOE ε4 carriers, n (%) | 2 (22)† | 4 (44) | 7 (70) |
| CDR sum-of-boxes | 0 ± 0 | 0.94 ± 0.53† | ‡ |
| MMSE | 29.7 ± 0.5 | 29.6 ± 0.5† | 21.1 ± 3.1§ |
| CVLT total learning score | 46.9 ± 8.4 | 50.1 ± 12.4† | ‡ |
| CVLT delayed free recall | 10.2 ± 3.5 | 10.8 ± 4.3† | ‡ |
| Post-scan memory¶ | 87.1 ± 12.5% | 84.9 ± 6.6% | 65.7 ± 11.6%§ |
Values represent mean ± SD.
Data not available for one subject in this group.
Data not collected for this group.
p < 0.05.
p < 0.005.
Post-scan recognition memory test performance, % correct.
MCI = mild cognitive impairment; AD = Alzheimer disease; CDR = Clinical Dementia Rating; MMSE = Mini-Mental State Examination; CVLT = California Verbal Learning Test.
Performance on fMRI associative memory task
The MCI subjects did not differ from the older controls in their performance on the post-scan recognition memory task: MCI subjects correctly recognized 85% (SD = 6.6%) of the names associated with the faces, while controls recognized 87% (SD = 12.5%; group mean comparison, p = 0.66). The AD patients correctly recognized 66% (SD = 11.6%) of the names, a difference from that of controls and MCIs (p < 0.005). The addition of education as a covariate in this analysis did not affect the results. There was no difference in task performance between APOE ε4 carriers and non-carriers (p = 0.32). Performance on this test was not correlated with the subjects’ age (p = 0.23) or education (p = 0.12).
fMRI data: Group differences in HF and EC activation
The extent of MTL activation in the NvR contrast was examined using two-way repeated measures ANOVA with hemisphere (right or left) as the within-group factor and subject group and APOE genotype (ε4 carrier or noncarrier) as between-group factors. Where not explicitly stated, these analyses did not identify genotype or hemisphere effects, nor interactions between factors. For the HF, a main effect was found for subject group (F[2, 22] = 9.0, p < 0.001). Planned contrasts between groups showed that MCI subjects had a greater extent of hippocampal activation than controls (p < 0.03), and that patients with AD had a lesser extent of hippocampal activation than controls and MCI subjects (p < 0.005). The same analysis for the EC demonstrated main effects for group (F[2, 22] = 4.5, p < 0.02) and genotype (F[2, 22] = 7.4, p < 0.02). In this analysis, MCI subjects did not differ from controls in extent of entorhinal activation (p = 0.44), but patients with AD showed a lesser extent of entorhinal activation than controls and MCI subjects (p < 0.02). Interestingly, carriers of the APOE ε4 allele activated a greater extent of EC than noncarriers (p < 0.02). The results of similar analyses with age and education entered as covariates did not differ. Figure 1 provides examples of the anatomic ROIs and functional activation maps. Figure 2 shows MTL extent of activation data, and figure 3 illustrates genotype effects on activation.
Figure 1.
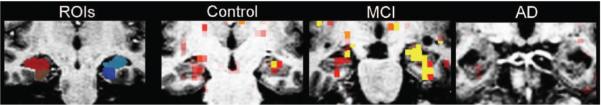
Representative coronal views of MRI data from single subjects. Regions of interest (ROIs): examples of anatomic ROIs for hippocampal formation (red and light blue) and entorhinal cortex (brown and dark blue), as manually delineated on high resolution T1-weighted structural MRI scans. Control, mild cognitive impairment (MCI), Alzheimer disease (AD): examples of fMRI activation maps from the individual subject in each group with the median extent of HF activation (novel vs repeated contrast, p < 0.01) overlaid on that subject’s T1-weighted structural scan.
Figure 2.
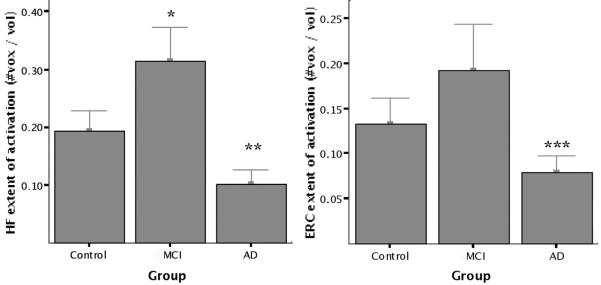
Group mean fMRI activation data. Extent of fMRI activation is defined as total (left + right) number of voxels within each region of interest (ROI) showing task-related fMRI activation in novel vs repeated contrast (p < 0.01) divided by total number of voxels in ROI. Greater hippocampal (HF) activation was present in the mild cognitive impairment (MCI) group than controls (*p < 0.03), and lesser hippocampal (**p < 0.005) and entorhinal (ERC; ***p < 0.02) activation was present in the Alzheimer disease (AD) group.
Figure 3.
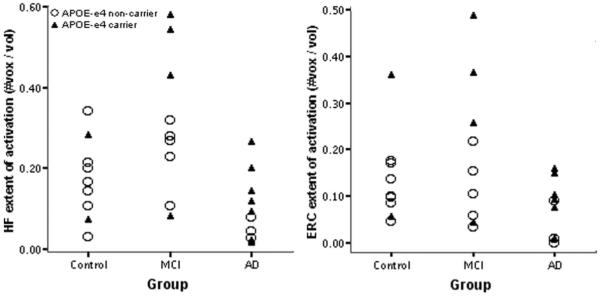
Hippocampal (HF) and entorhinal (ERC) activation by clinical group and APOE genotype. Data points indicate total (left + right) number of voxels within each region of interest (ROI) showing task-related fMRI activation in the novel vs repeated contrast (p < 0.01) divided by total number of voxels in ROI. Among all 29 subjects, greater entorhinal activation was present in APOE E4 carriers than in noncarriers (p < 0.02). MCI = mild cognitive impairment; Alzheimer disease = AD.
Structural MRI data: group differences in HF and EC volume
Next, the volumes of MTL ROIs were examined. For the HF, ANOVA analyses as performed above demonstrated a main effect for group (F[2, 22] = 13.5, p < 0.0005). Similarly, this analysis for the EC demonstrated a main effect for group (F[2, 22] = 11.1, p < 0.0005). Planned contrasts showed that the MCI subjects and controls did not differ in hippocampal (p = 0.87) or entorhinal volumes (p = 0.56), but that patients with AD had smaller hippocampal (p < 0.0005) and entorhinal volumes (p < 0.0005). Similar results were found when volumetric data adjusted for intracranial area were analyzed. There were no genotype or hemisphere effects, nor interactions. Figures 4 and 5 show MTL volumetric data.
Figure 4.
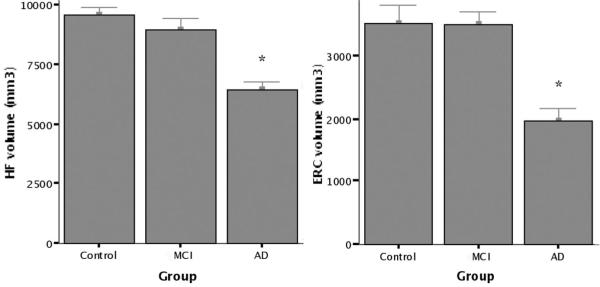
Group mean volumetric MRI data. Standard manual volumetric protocols were used to measure regions of interest (ROIs) for the hippocampal formation (HF) and entorhinal cortex (ERC) from each individual subject’s structural MRI. Total volume (left + right) is displayed for each ROI. There was no significant difference in volumes between controls and mild cognitive impairment (MCI), but patients with Alzheimer disease (AD) demonstrated evidence of atrophy (*p < 0.0005).
Figure 5.
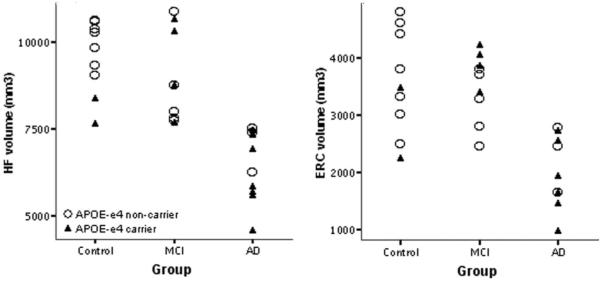
Hippocampal (HF) and entorhinal (ERC) volume by clinical group and APOE genotype. Data points indicate total (left + right) volume of each region of interest (ROI). As shown in figure 3, the mean volumes of both ROIs are smaller in the Alzheimer disease (AD) group; no APOE effects or group by genotype interactions were observed. MCI = mild cognitive impairment.
fMRI and volumetric data: relationship to memory task performance
We analyzed the relationships of the functional and structural MRI variables to memory task performance. No significant correlations between these variables were found within individual subject groups (e.g., controls or MCI), likely due to small sample size. Across the entire group of 29 subjects, there were correlations between performance and extent of MTL activation (absolute number of voxels activated in the NvR contrast) in the right EC (r = 0.49, p < 0.01) and left HF (r = 0.42, p < 0.03), along with a similar trend for the right HF (r = 0.33, p = 0.08). As for structural MRI measures, correlations were found between memory performance and ROI volumes for the right HF (r = 0.47, p < 0.02) and right EC (r = 0.41, p < 0.03); a trend was present for the left HF (r = 0.32, p = 0.09). A stepwise multiple linear regression model showed that, of these variables, memory task performance was best predicted by a combination of right EC and HF activation and right HF volume (R2 = 0.55, F = 10.0, p < 0.0005).
Discussion
Our findings show that subjects with MCI with relatively mild functional difficulties in daily life demonstrate greater hippocampal activation during memory task performance than older control subjects. Conversely, patients with AD show significantly less hippocampal activation than controls or MCI subjects. The subjects with MCI performed similarly to controls on the fMRI recognition memory task; patients with AD exhibited poorer performance. These findings support the hypothesis that the degree of memory-related MTL activation in MCI is related to subjects’ overall level of clinical impairment along this transitional continuum between normal aging and dementia. Moreover, compared to older controls, individuals in the early stages of MCI demonstrate increased MTL activation when a memory task is administered that they can still perform.
We hypothesize that the variation in findings among previous reports likely relates in large part to differences in the subjects’ clinical characteristics. For example, an MCI cohort in a recent fMRI study probably represented the relatively more impaired end of the MCI continuum, since they had sought clinical evaluation for a memory complaint and demonstrated impairment on psychometric memory testing similar to that of patients with AD—MTL activation in this group was similar to that of patients with AD.5 In contrast, the subjects in another previous fMRI study were recruited from a community-based longitudinal study rather than a clinic, which may have resulted in greater heterogeneity in the subject population; heterogeneity was also present in MTL activation.1 In our previous fMRI study we purposely selected individuals who represented a broad spectrum of persons with MCI (as their CDR Sum of Boxes scores ranged from 0.5 to 3.0), and demonstrated that MTL activation was systematically related to their overall degree of clinical impairment.6 In the present study, we specifically focused on individuals who represented the mild end of the MCI spectrum. MTL activation in these subjects was significantly greater than that of older controls.
The variability of fMRI findings in previous studies of MCI may also relate to subjects’ performance on the specific memory task employed in the fMRI paradigm, since greater MTL activation during encoding is associated with better recognition memory performance in control subjects.24-26 For example, in a previous fMRI study of MCI, both MTL activation and fMRI memory task performance in the MCI subjects were more similar to patients with AD than to controls.5 We previously demonstrated that, within a different group of MCI subjects, greater MTL activation was associated with better recognition memory performance on the fMRI task.6 In the present study, the MCI subjects performed similarly to controls on the fMRI recognition memory task, yet they recruited a greater extent of the hippocampus during the encoding of novel face-name pairs. Conversely, patients with AD exhibited poor memory performance on the fMRI task and showed less activation than controls or MCI subjects.
Differences between these previous studies in the localization of fMRI activation within the MTL are likely related to the demands of the memory task being performed by the subjects. That is, performance of the associative memory encoding task used in the present study was related to activation in anterior hippocampal and entorhinal regions, whereas performance on the scene encoding task employed in our previous study was related to activation in posterior parahippocampal regions.
Although these fMRI studies have been cross-sectional, they and other neuroimaging and postmortem investigations suggest that alterations in MTL function follow a nonlinear trajectory over the course of prodromal AD.27-29 The present fMRI data, along with those from our previous investigation of MCI subjects and from a study of individuals at elevated genetic risk for AD, support the hypothesis that there is a phase of increased MTL activation very early in the course of AD.6,30 Once the burden of pathology and neuronal loss accrues past a certain level and memory impairment becomes more pronounced later in the course of prodromal AD, and certainly by the time AD is diagnosed clinically, memory-related MTL activation is decreased.1-5
There are a number of potential reasons for hyper-activation within MTL regions during memory task performance in MCI subjects. First, this may reflect the need for memory circuits to recruit additional neural resources in order to compensate for the accumulation of AD neuropathology, a mechanism that has been suggested by physiologic studies of animal models.31 It is also possible that individuals with MCI encode information using a different cognitive processing strategy, which may in turn drive increased MTL activation.32 In this context, the MCI subjects in the present study were highly educated, and thus it is possible that such neural or cognitive changes may reflect cognitive reserve.33,34 Alternatively, given that the MTL is highly interconnected with neocortical brain regions, changes in its activation may reflect differences in the recruitment of networks of brain regions outside the MTL, which have been observed in patients with AD.35-38 Finally, there is increasing evidence that mechanisms involved in neuroplasticity become aberrant in AD and contribute to neurodegeneration.39 From this perspective, hyperactivation of MTL regions could be a marker of the pathophysiologic process itself.6 Thus, these neuroimaging data may represent the macroscopic signature of a complex cycle of degenerative and compensatory processes at work in the AD brain prior to the development of frank dementia.
It should be noted that the mean hippocampal and entorhinal volumes in the MCI group in the present study did not differ from those of the controls, while the patients with AD exhibited smaller MTL volumes than both of the other groups. Even after correcting for volume, hippocampal activation was greater in MCI and diminished in AD compared to controls. This suggests that functional alterations within MTL regions during the evolution of AD pathology may precede the development of significant atrophy.
Another finding in the present study was that a greater extent of memory-related activation within the MTL was seen among individuals carrying an APOE ε4 allele. This result suggests a genotypic contribution to the physiologic phenotype of MTL activation, and is similar to findings reported in cognitively intact middle-aged individuals with a family history of AD.30 It is, however, unclear whether increased memory-related MTL activation in association with an APOE ε4 allele only begins to appear as individuals age, or whether it is a lifelong genotype-phenotype characteristic. In either case, increased MTL activation in APOE ε4 carriers may represent attempted compensation for the diminished synaptic plasticity associated with this genotype, which has been observed in animal models40 and postmortem human tissue.41 We did not observe a relationship between memory task performance and APOE genotype; such a relationship has primarily been reported in studies with larger sample sizes.42
Although our focus on MTL function in individuals representing the mild end of the MCI spectrum had a number of advantages, it also led to several limitations. First, an objective memory impairment on neuropsychological testing was not required. As a result, the MCI subjects in this study may not be comparable to those in many other studies, and in fact represent a circumscribed subgroup of MCI within the larger longitudinal study. Second, longitudinal follow-up will be needed to determine whether the very mild MCI subjects in the present study are in the earliest phase of AD. Previous work by our group suggests that many such subjects demonstrate cognitive decline within 3 years and a proportion convert to clinical AD.8 The increased proportion of APOE ε4 carriers in the very mild MCI group further suggests that the subjects are more likely to develop clinical AD in the future. Third, differences in MTL activation may be associated with between-group differences in other factors, such as APOE genotype or educational level, in addition to severity of clinical impairment. Other studies have suggested that cognitive reserve or intelligence appear to modulate both brain activation during memory task performance and clinicians’ ability to detect cognitive impairment.33,34 The MCI group in this study was relatively highly educated, which could be reflected in MTL activation, although statistical adjustment did not indicate that this factor confounded the results. As is illustrated in figure 3, even within this small sample of MCI subjects there is a range of MTL activation. Thus, the examination of brain activation data with respect to potential confounding factors, such as APOE genotype or level of education, may reveal distinct subgroups within MCI, but the necessary multifactorial analyses require relatively large sample sizes. Our ongoing work with larger samples of subjects representing the broader spectrum of MCI should enable some of these issues to be addressed.
It is important to recognize that the interpretation of fMRI data may also be confounded by alterations in resting metabolism or perfusion,43,44 which are present in patients with AD, APOE ε4 carriers,45 and cognitively impaired individuals prior to dementia.45,46 Since the primary imaging measure in the present study was a comparison between activation during novel and familiar face-name pair encoding without a baseline condition, this potential confound seems less likely. Also, note that the blood-oxygen level dependent (BOLD) signal measured in fMRI reflects a coupled neural and vascular response, and therefore findings may be confounded by non-neural factors, including changes in neurovascular coupling47-49 or vascular physiology.50,51
The data in this report and our previous study provide convergent evidence supporting the hypothesis that there is a phase of increased medial temporal activation early in the course of prodromal AD, prior to clinical dementia. This increase can be seen in the absence of significant MTL atrophy, suggesting that physiologic alterations may precede significant structural abnormalities in very early AD.52 Thus, it may be possible to detect such alterations early in the prodromal phase of the disease, at a time when disease-modifying or neuroprotective strategies may have great clinical impact.53 Further fMRI studies investigating the relationship between MTL activation, memory performance, and clinical status across the continuum of MCI should clarify whether fMRI measures can be translated into markers for early disease detection or a tool for monitoring the effects of disease-modifying therapies.
Acknowledgment
The authors thank the staff of the MGH Gerontology Research Unit for assistance with subject recruitment, evaluation, and data management, Mary Foley and Larry White for assistance with MRI data collection, and Dr. Steven Salloway for assistance with AD patient recruitment. They also thank Dr. Marc Vangel, who is supported by the MGH Mallinckrodt General Clinical Research Center, for consultation in biostatistics. They express appreciation to their subjects for their participation.
Supported by the National Institute on Aging (PO1-AG04953; K23-AG22509), National Institute of Neurologic Disorders and Stroke (K23-NS02189), Beeson Scholars in Aging Program (American Federation for Aging Research), Clinical Investigator Training Program (Harvard/MIT Health Sciences and Technology–Beth Israel Deaconess Medical Center, in collaboration with Pfizer Inc.), Mental Illness and Neuroscience Discovery (MIND) Institute, Eli Lilly Pharmaceuticals, MA ADRC (P50-AG05134), NCRR (P41-RR14075), and NCRR BIRN Morphometry Project (BIRN004).
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Ann Neurol. 1999;45:466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Rombouts SA, Barkhof F, Veltman DJ, et al. Functional MR imaging in Alzheimer’s disease during memory encoding. AJNR Am J Neuroradiol. 2000;21:1869–1875. [PMC free article] [PubMed] [Google Scholar]
- 3.Kato T, Knopman D, Liu H. Dissociation of regional activation in mild AD during visual encoding: a functional MRI study. Neurology. 2001;57:812–816. doi: 10.1212/wnl.57.5.812. [DOI] [PubMed] [Google Scholar]
- 4.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machulda MM, Ward HA, Borowski B, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61:500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickerson BC, Salat DH, Bates JF, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 8.Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert M. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000;57:675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 10.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 11.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology. 1997;48:1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- 13.Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS. Encoding novel face-name associations: a functional MRI study. Hum Brain Mapp. 2001;14:129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Burock MA, Dale AM. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum Brain Mapp. 2000;11:249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 17.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 18.Goncharova II, Dickerson BC, Stoub TR, DeToledo-Morrell L. MRI of human entorhinal cortex: a reliable protocol for volumetric measurement. Neurobiol Aging. 2001;22:737–745. doi: 10.1016/s0197-4580(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 19.den Heijer T, Vermeer SE, Clarke R, et al. Homocysteine and brain atrophy on MRI of non-demented elderly. Brain. 2003;126:170–175. doi: 10.1093/brain/awg006. [DOI] [PubMed] [Google Scholar]
- 20.Sperling R, Greve D, Dale A, et al. Functional MRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci USA. 2002;99:455–460. doi: 10.1073/pnas.012467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test, research edition, manual. The Psychological Corporation, Harcourt Brace Jovanovich; San Antonio: 1987. [Google Scholar]
- 24.Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- 25.Wagner AD, Schacter DL, Rotte M, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 26.Sperling R, Chua E, Cocchiarella A, et al. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- 28.Head E, Lott IT, Hof PR, et al. Parallel compensatory and pathological events associated with tau pathology in middle aged individuals with Down syndrome. J Neuropathol Exp Neurol. 2003;62:917–926. doi: 10.1093/jnen/62.9.917. [DOI] [PubMed] [Google Scholar]
- 29.Haier RJ, Alkire MT, White NS, et al. Temporal cortex hypermetabolism in Down syndrome prior to the onset of dementia. Neurology. 2003;61:1673–1679. doi: 10.1212/01.wnl.0000098935.36984.25. [DOI] [PubMed] [Google Scholar]
- 30.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stern EA, Bacskai BJ, Hickey GA, Attenello FJ, Lombardo JA, Hyman BT. Cortical synaptic integration in vivo is disrupted by amyloid-beta plaques. J Neurosci. 2004;24:4535–4540. doi: 10.1523/JNEUROSCI.0462-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandzia JL, Black SE, McAndrews MP, Grady C, Graham S. fMRI differences in encoding and retrieval of pictures due to encoding strategy in the elderly. Hum Brain Mapp. 2004;21:1–14. doi: 10.1002/hbm.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarmeas N, Zarahn E, Anderson KE, et al. Cognitive reserve-mediated modulation of positron emission tomographic activations during memory tasks in Alzheimer disease. Arch Neurol. 2004;61:73–78. doi: 10.1001/archneur.61.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rentz DM, Huh TJ, Faust RR, et al. Use of IQ-adjusted norms to predict progressive cognitive decline in highly intelligent older individuals. Neuropsychology. 2004;18:38–49. doi: 10.1037/0894-4105.18.1.38. [DOI] [PubMed] [Google Scholar]
- 35.Becker JT, Mintun MA, Aleva K, Wiseman MB, Nichols T, DeKosky ST. Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer’s disease. Neurology. 1996;46:692–700. doi: 10.1212/wnl.46.3.692. [DOI] [PubMed] [Google Scholar]
- 36.Woodard JL, Grafton ST, Votaw JR, Green RC, Dobraski ME, Hoffman JM. Compensatory recruitment of neural resources during overt rehearsal of word lists in Alzheimer’s disease. Neuropsychology. 1998;12:491–504. doi: 10.1037//0894-4105.12.4.491. [DOI] [PubMed] [Google Scholar]
- 37.Backman L, Andersson JL, Nyberg L, Winblad B, Nordberg A, Almkvist O. Brain regions associated with episodic retrieval in normal aging and Alzheimer’s disease. Neurology. 1999;52:1861–1870. doi: 10.1212/wnl.52.9.1861. [DOI] [PubMed] [Google Scholar]
- 38.Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer’s disease. J Neurosci. 2003;23:986–993. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mesulam MM. Neuroplasticity failure in Alzheimer’s disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 40.Masliah E, Mallory M, Ge N, Alford M, Veinbergs I, Roses AD. Neuro-degeneration in the central nervous system of apoE-deficient mice. Exp Neurol. 1995;136:107–122. doi: 10.1006/exnr.1995.1088. [DOI] [PubMed] [Google Scholar]
- 41.Arendt T, Schindler C, Bruckner MK, et al. Plastic neuronal remodeling is impaired in patients with Alzheimer’s disease carrying apolipoprotein epsilon 4 allele. J Neurosci. 1997;17:516–529. doi: 10.1523/JNEUROSCI.17-02-00516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett DA, Wilson RS, Schneider JA, et al. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003;60:246–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- 43.Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 46.Johnson KA, Jones K, Holman BL, et al. Preclinical prediction of Alzheimer’s disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 47.Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci. 2000;12(Suppl 2):24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- 48.Huettel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage. 2001;13:161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- 49.D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 50.Johnson SC, Saykin AJ, Baxter LC, et al. The relationship between fMRI activation and cerebral atrophy: comparison of normal aging and Alzheimer disease. Neuroimage. 2000;11:179–187. doi: 10.1006/nimg.1999.0530. [DOI] [PubMed] [Google Scholar]
- 51.Mueggler T, Sturchler-Pierrat C, Baumann D, Rausch M, Staufenbiel M, Rudin M. Compromised hemodynamic response in amyloid precursor protein transgenic mice. J Neurosci. 2002;22:7218–7224. doi: 10.1523/JNEUROSCI.22-16-07218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 53.DeKosky ST, Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302:830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]


