Abstract
Three component coupling of a 2-methylenetetrahydropyran, an activated aldehyde or ketone and a secondary nucleophile provides an efficient preparation of tetrahydropyranyl ketides, a unit common to many complex natural products.
2-Methylenetetrahydropyrans are useful intermediates for the synthesis of complex molecules.1 These substrates have been utilized for the preparation of C-glycosides,2 fused polyethers,3 spiroketals,4 and related compounds. Nonetheless, the chemistry of these systems is less developed than that of the corresponding 2,3 dihydropyrans. Efforts to functionalize the exocyclic double bond have largely focused on the introduction of heteroatoms, while protocols for carbon carbon–bond formation, particularly at the terminus, are more limited. Attempts to further elaborate the enol ether include B-alkyl Suzuki coupling,5 radical processes,6 and cycloaddition reactions7 (Scheme 1). Less common are methods that take advantage of the nucleophilic character of the exocyclic enol ether for direct formation of a carbon–carbon bond (2). Such examples have been limited to dimerization processes (3)8 and reaction with epi-sulfonium ions (4).9 As functionalized tetrahydropyrans are integral components of many biologically significant compounds, the development of more general nucleophilic processes could find broad application in complex molecule synthesis.
Scheme 1.

C–C bond forming reactions of 2-methylenetetrahydropyrans.
Our interest in the synthesis of tetrahydropyran containing polyketide natural products such as spirastrellolide A10 led us to consider methods for the synthesis of tetrahydropyranyl ketides like 5. We anticipated that the nucleophilic addition of an exocyclic enol ether 1 to an aldehyde or ketone would give the desired 2-tetrahydropyranylethanol derivative 5 upon reaction of the intermediate oxonium species with a secondary nucleophile (Scheme 2). Such a process could be used to form the C8–C9 bond of spirastrellolide A. Though this mode of reactivity has previously been reported for 2,3-dihydropyrans,11 similar reactions for the corresponding exocyclic enol ethers have remained largely unexplored. This omission is likely due to the propensity of these systems to undergo rapid double bond isomerization (7) upon heating or in the presence of trace amounts of protic acid. Nonetheless, the requisite exocyclic enol ethers are readily available via dehydrohalogenation of a suitably substituted tetrahydropyran12 or by lactone methylenylation.13
Scheme 2.
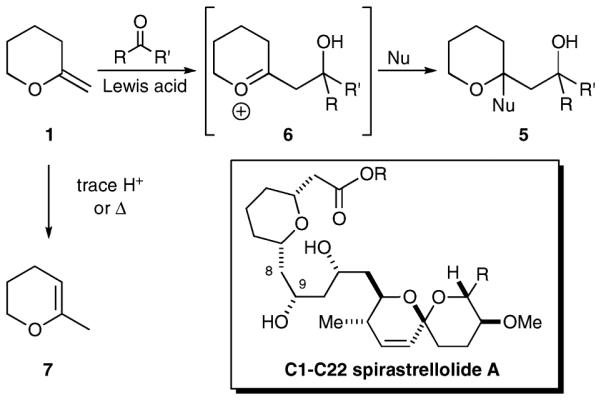
Proposed synthesis of tetrahydropyranyl ketides 5.
In order to evaluate the feasibility of using exocyclic enol ethers in nucleophilic addition processes, we examined the reaction of enol ether 114 with ethyl glyoxylate 815 using Et3SiH as the secondary nucleophile (Table 1). Though a variety of Lewis acids were screened in this application, TiCl4 gave the best results providing the desired 2-hydroxy ester 9 in the highest yields and with the fewest side reactions (entries 1–6). Variation in the reaction stoichiometry and time to addition of reducing agent were evaluated in order to optimize yields further. As shown, the relative percentage of reactive components 1 and 8 has little impact on the yield of the reaction (entries 6 and 7, 10 and 11). More important to the success of this transformation is the ready availability of the nucleophile upon generation of the intermediate oxonium species (entries 6, 8–10). Best results are obtained when Et3SiH is present in the reaction mixture prior to addition of the Lewis acid. In these cases, reduction in the amount of TiCl4 utilized results in a corresponding decrease in the product yield (entries 10, 12–14). Based on these findings, the protocol that unfolds is one in which (1) activation of the aldehyde with equimolar amounts of Lewis acid to facilitate rapid addition of the exocyclic enol ether prior to double bond isomerization, and (2) immediate reaction of the oxonium intermediate thus formed with an appropriate nucleophile to minimize side reactions.
Table 1.
Reaction of enol ether 1 with ethyl glyoxylate 8 and Et3SiH

| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Ratio 1 : 8 | Lewis acid | Equiv. LA | Timea | Yield (%) |
| 1 | 1 : 1.2 | BF3·OEt2 | 1 | 1 h | — |
| 2 | 1 : 1.2 | SnCl2 | 1 | 1 h | — |
| 3 | 1 : 1.2 | ZnCl2 | 1 | 1 h | — |
| 4 | 1 : 1.2 | EtAlCl2 | 1 | 1 h | — |
| 5 | 1 : 1.2 | Et2AlCl | 1 | 1 h | 1 |
| 6 | 1 : 1.2 | TiC14 | 1 | 1 h | 48 |
| 7 | 1.5 : 1 | TiCl4 | 1 | 1 h | 56 |
| 8 | 1 : 1.2 | TiCl4 | 1 | 20 min | 45 |
| 9 | 1 : 1.2 | TiCl4 | 1 | 10 min | 59 |
| 10 | 1 : 1.2 | TiCl4 | 1 | — b | 86 |
| 11 | 1.2: 1 | TiCl4 | 1 | — b | 84 |
| 12 | 1 : 1.2 | TiCl4 | 0.5 | — b | 45 |
| 13 | 1 : 1.2 | TiCl4 | 0.2 | — b | 23 |
| 14 | 1 : 1.2 | TiCl4 | 0.1 | — b | 10 |
Time reaction mixture stirred prior to addition of Et3SiH.
Et3SiH added before the Lewis acid.
The scope of this transformation was next evaluated by varying both carbonyl and nucleophile components. As shown in Table 2, both 2-methylenetetrahydropyrans (1 and 15) and the corresponding chroman derivative (14) can be utilized as the enol component in these transformations. Addition of these substrates to activated aldehydes 8 and 17 occurs readily at −78 1C. Both hydride and allyl functions can be subsequently incorporated at the C2 position upon treatment of the intermediate oxonium ion with triethylsilane or allyltrimethylsilane, respectively. Similar reactivity is observed with 2,3 butanedione 16 (entries 3, 9, 13). The resulting aldol equivalents are produced in excellent yields. This reaction is significant in that ketones are typically poor electrophiles in intermolecular aldol reactions because the reaction equilibrium lies far to the left. Further, use of a ketone electrophile provides access to compounds that contain a quaternary center b to the tetrahydropyran ring. When allyltrimethylsilane is used as the secondary nucleophile (entries 4, 10), two oxygenated quaternary centers are generated in a single process.
Table 2.
Three component coupling reaction of 2-methylenetetrahydropyrans

|
In cases where a pre existing stereocenter is present at C6 of the tetrahydropyran, the three component coupling proceeds to give the corresponding 2,6-cis tetrahydropyrans as the only isolated products (entries 11–13). The stereochemistry of these compounds 22 and 23 was determined by nOe. The observed stereoselectivity can be rationalized as shown in Fig. 1. The observed stereoselectivity is consistent with addition of the secondary nucleophile to an oxonium ion intermediate that exists in a half chair conformation in which the C6 substituent is pseudo equatorial (24B).16 Subsequent axial attack of the nucleophile is then anticipated on both steric and stereoelectronic grounds.17 Similar selectivity is observed in the addition of nucleophiles to related tetrahydropyranyl oxonium species.18
Fig. 1.

Stereochemistry of nucleophile addition.
In conclusion, we have demonstrated a facile synthesis of tetrahydropyranyl ketide derivatives using a three component coupling protocol. These studies demonstrate that 2-methylene tetrahydropyrans are suitable nucleophiles for addition to activated aldehydes and ketones. Competing double bond iso-merization is not a factor. The resulting 2-(tetrahydropyran 2-yl)-alcohols will be useful intermediates for complex molecule synthesis. Current efforts are aimed at the synthesis of spirastrellolide A and related polyketide natural products. These studies will be reported in due course.
Supplementary Material
Acknowledgments
Financial support from the National Institutes of Health (R01 GM064357) is gratefully acknowledged. We thank Mr. Alex Augatis for assistance with nOe studies.
Footnotes
Electronic supplementary information (ESI) available: Experimental procedures and characterization data for all new compounds. See DOI: 10.1039/b916190b
Notes and references
- 1.(a) Taillefumier C, Chapleur Y. Chem. Rev. 2004;104 doi: 10.1021/cr030640v. For general reviews on the synthesis and use of exo glycals, see: [DOI] [PubMed] [Google Scholar]; (b) Lin CH, Lin HC, Yang WB. Curr. Top. Med. Chem. 2005;5 doi: 10.2174/156802605774642980. [DOI] [PubMed] [Google Scholar]
- 2.(a) RajanBabu TV, Reddy GS. J. Org. Chem. 1986;51 [Google Scholar]; (b) Johnson CR, Johns BA. Synlett. 1997:1406. [Google Scholar]; (c) Herpin TF, Motherwell WB, Tozer MJ. Tetrahedron: Asymmetry. 1994;5 [Google Scholar]; (d) Campbell AD, Paterson DE, Taylor RJK, Raynham TM. Chem. Commun. 1999:1599. [Google Scholar]; (e) Link JT, Sorensen BK. Tetrahedron Lett. 2000;41 [Google Scholar]; (f) Walker JR, Alshafie G, Nieves N, Ahrens J, Clagett Dame M, Abou Issa H, Curley RW., Jr. Bioorg. Med. Chem. 2006;14 doi: 10.1016/j.bmc.2005.12.044. see also ref. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Fuwa H, Kainuma N, Tachibana K, Sasaki M. Tetrahedron Lett. 2004;45 [Google Scholar]; (b) Nicolaou KC, Cole KP, Frederick MO, Aversa RJ, Denton RM. Angew. Chem., Int. Ed. 2007;46 doi: 10.1002/anie.200703742. [DOI] [PubMed] [Google Scholar]; (c) Sasaki M, Fuwa H. Nat. Prod. Rep. 2008;25 doi: 10.1039/b705664h. and references therein. [DOI] [PubMed] [Google Scholar]
- 4.(a) Ireland RE, Thaisrivongs S, Dussault PH. J. Am. Chem. Soc. 1988;110 [Google Scholar]; (b) Haudrechy A, Sinay P. Carbohydr. Res. 1991;256 [Google Scholar]; (c) Hayes P, Maignan C. Synthesis. 1999:783. [Google Scholar]; (d) Cuzzupe AN, Hutton CA, Lilly MJ, Mann RK, McRae KJ, Zammit S, Rizzacasa MA. J. Org. Chem. 2001;66 doi: 10.1021/jo001646c. [DOI] [PubMed] [Google Scholar]; (e) El Sous M, Ganame D, Treggloan PA, Rizzacasa MA. Org. Lett. 2004;6 doi: 10.1021/ol048811l. [DOI] [PubMed] [Google Scholar]; (f) Lindsey CC, Wu KL, Pettus TRR. Org. Lett. 2006;8 doi: 10.1021/ol0606886. [DOI] [PubMed] [Google Scholar]; (g) Zhou G, Zheng D, Da S, Xie Z, Li Y. Tetrahedron Lett. 2006;47 [Google Scholar]
- 5.(a) Johns BA, Pan YT, Elbein AD, Johnson CR. J. Am. Chem. Soc. 1997;119 [Google Scholar]; (b) Sasaki M, Fuwa H, Inoue M, Tachibana K. Tetrahedron Lett. 1998;39 [Google Scholar]; (c) Sasaki M, Fuwa H. Synlett. 2004:1851. and references therein. [Google Scholar]; (d) Ciblat S, Kim J, Stewart CA, Wang J, Forgione P, Clyne D, Paquette LA. Org. Lett. 2007;9 doi: 10.1021/ol063083i. [DOI] [PubMed] [Google Scholar]
- 6.(a) Motherwell WB, Ross BC, Tozer MJ. Synlett. 1989:68. [Google Scholar]; (b) Myers AG, Gin DY, Widdowson KL. J. Am. Chem. Soc. 1991;113 [Google Scholar]; (c) Cipolla L, Liguori L, Nicotra F, Torri G, Vismara E. Chem. Commun. 1996:1253. [Google Scholar]; (d) Dang HS, Elsegood MRJ, Kim KM, Roberts BP. J. Chem. Soc., Perkin Trans. 1. 1999:2061. [Google Scholar]; (e) Vauzeilles B, Sinay P. Tetrahedron Lett. 2001;42 [Google Scholar]
- 7.(a) Ireland RE, Haebich D. Chem. Ber. 1981;114 Hetero Diels Alder: [Google Scholar]; (b) Hayes P, Maignan C. Synlett. 1994:409. [Google Scholar]; (c) Tietze LF, Schneider G, Wolfling J, Nobel T, Wulff C, Christian I, Rubeling A. Angew. Chem., Int. Ed. 1998;37 doi: 10.1002/(SICI)1521-3773(19981002)37:18<2469::AID-ANIE2469>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]; (d) Gallos JK, Sarli VC, Stathakis CI, Koftis TV, Nachmia VR, Coutouli Argyropoulou E. Tetrahedron. 2002;58 [Google Scholar]; (e) Zhou G, Zheng D, Da S, Xie Z, Li Y. Tetrahedron Lett. 2006;47 Intramolecular Diels Alder. [Google Scholar]; (f) Wender PA, Keenan RM, Lee HY. J. Am. Chem. Soc. 1987;109 1,3 Dipolar cycloaddition. [Google Scholar]; (g) Gallos JK, Koftis T. J. Chem. Soc., Perkin Trans. 1. 2001:415. [Google Scholar]; (h) Colinas PA, Jager V, Lieberknecht A, Bravo RD. Tetrahedron Lett. 2003;44 [Google Scholar]; (i) Li X, Takahashi H, Hideyo O, Ikegami S. Tetrahedron Lett. 2004;45 [Google Scholar]; (j) Enderlin G, Taillefumier C, Didierjean C, Chapleur Y. Tetrahedron: Asymmetry. 2005;16 [Google Scholar]; (k) Zhang PZ, Li XL, Chen H, Li YN, Wang R. Tetrahedron Lett. 2007;48 [Google Scholar]
- 8.(a) Lay L, Nicotra F, Panza L, Russo G, Caneva E. J. Org. Chem. 1992;57 [Google Scholar]; (b) Fráter G, Schroder F. J. Org. Chem. 2007;72 doi: 10.1021/jo061668k. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Smoliakova IP, Koikov LN. Org. Lett. 2002;4 doi: 10.1021/ol026718w. [DOI] [PubMed] [Google Scholar]
- 10.(a) Williams DE, Roberge M, Van Soest R, Andersen RJ. J. Am. Chem. Soc. 2003;125 doi: 10.1021/ja0348602. [DOI] [PubMed] [Google Scholar]; (b) Williams DE, Lapawa M, Feng X, Tarling T, Roberge M, Andersen RJ. Org. Lett. 2004;6 doi: 10.1021/ol0490983. [DOI] [PubMed] [Google Scholar]; (c) Warabi K, Williams DE, Patrick BO, Roberge M, Andersen RJ. J. Am. Chem. Soc. 2007;129 doi: 10.1021/ja068271i. [DOI] [PubMed] [Google Scholar]
- 11.(a) Mukaiyama T, Wariishi K, Sato Y. Chem. Lett. 1988:1101. [Google Scholar]; (b) Sugimura H, Osumi K. Tetrahedron Lett. 1989;30 [Google Scholar]; (c) Ghosh AK, Kawahama R. Tetrahedron Lett. 1999;40 doi: 10.1016/S0040-4039(98)02633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ghosh AK, Kawahama R. Tetrahedron Lett. 1999;40 doi: 10.1016/S0040-4039(98)02633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ghosh AK, Kawahama R, Wink D. Tetrahedron Lett. 1999;40 doi: 10.1016/S0040-4039(00)01602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ghosh AK, Xu CX, Sarang SS, Wink D. Org. Lett. 2005;7 doi: 10.1021/ol048302j. [DOI] [PubMed] [Google Scholar]
- 12.(a) Prugh JD, Stanley CS, Deana AA, Ramjit HG. Tetrahedron Lett. 1985;26 For example, see: [Google Scholar]; (b) Prandi J, Beau JM. Tetrahedron Lett. 1989;30 [Google Scholar]; (c) Taillefumier C, Chapleur Y. Can. J. Chem. 2000;78 [Google Scholar]
- 13.(a) Petasis NA, Bzowej EI. J. Am. Chem. Soc. 1990;112 [Google Scholar]; (b) Petasis NA, Lu SP. Tetrahedron Lett. 1995;36 [Google Scholar]
- 14.Neokosmidi A, Ragoussis V, Evangelatos G. J. Agric. Food Chem. 2004;52 doi: 10.1021/jf0497758. see also ref. 4d and 7a. [DOI] [PubMed] [Google Scholar]
- 15.Evans DA, MacMillan DWC, Campos KR. J. Am. Chem. Soc. 1997;119 Ethyl glyoxylate was freshly distilled before use according to the procedure of Evans: [Google Scholar]
- 16.Woods RJ, Andrews CW, Bowen JP. J. Am. Chem. Soc. 1992;114 [Google Scholar]
- 17.(a) Deslongchamps P. Stereoelectronic Effects in Organic Chemistry. Pergamon; New York: 1983. p. 211. [Google Scholar]; (b) Stevens RV, Lee AWM. J. Am. Chem. Soc. 1979;101 [Google Scholar]; (c) Stevens RV. Acc. Chem. Res. 1984;17 [Google Scholar]
- 18.(a) Brown DS, Ley SV, Bruno M. Heterocycles. 1989;28 [Google Scholar]; (b) Lucero CG, Woerpel KA. J. Org. Chem. 2006;71 doi: 10.1021/jo0522963. [DOI] [PubMed] [Google Scholar]; (c) Crawford C, Nelson A, Patel I. Org. Lett. 2006;8 doi: 10.1021/ol0614323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


