Abstract
Recombinant antibody phage library technology provides multiple advantages, including that human antibodies can be generated against proteins that are highly conserved between species. We used this technology to isolate and characterize an anti-EphA2 single-chain antibody. We show that the antibody binds the antigen with 1:1 stoichiometry and has high specificity for EphA2. The crystal structure of the complex reveals that the antibody targets the same receptor surface cavity as the ephrin ligand. Specifically, a lengthy CDR-H3 loop protrudes deep into the ligand-binding cavity, with several hydrophobic residues at its tip forming an anchor-like structure buried within the hydrophobic Eph pocket, in a way similar to the ephrin receptor-binding loop in the Eph/ephrin structures. Consequently, the antibody blocks ephrin binding to EphA2. Furthermore, it induces apoptosis and reduces cell proliferation in lymphoma cells lines. Since Ephs are important mediators of tumorigenesis, such antibodies could have applications both in research and therapy.
Keywords: Crystal structure, eph receptor, single-chain antibody
Introduction
Monoclonal antibodies (mAbs) are important tools in research, diagnostics and therapy (Siddiqui, 2010). MAbs with suitable specificities can be highly valuable tools for the modulation of cell signaling and have great potential as cancer therapeutics (Sliwkowski & Mellman, 2013). Typically, mAbs are prepared using hydridoma cell lines (Kohler & Milstein, 1975; Yagami et al., 2013). Generation of such mAbs with desired specificity, however, is difficult when the target antigen or epitope is conserved between human and mouse. Another issue complicating the development of therapeutic mAbs is the fact that the successful outcome of the humanization process, generally implemented to reduce the immunogenicity of the rodent protein, cannot be guaranteed (Stern & Herrmann, 2005). In addition, commercially available mAbs for research are well known to often have serious quality issues, where one batch of the antibody functions quite differently from another batch from the same manufacturer (Couchman, 2009; Saper, 2005).
Recombinant antibody phage library technology is an alternative and powerful method to generate antibodies without immunization (Hoogenboom et al., 1998). A single selection experiment with a high quality naïve or synthetic antibody library can produce a large number of unique antibodies (Brockmann, 2011). The library technology provides several advantages in comparison to the conventional hydridoma technology. First, because the antigen and the selection conditions can be freely chosen, antibodies that recognize a conserved antigen or target a specific protein isoform or epitope, can be systematically generated. Second, very small amount of antigen is needed and human antibodies can be directly obtained from human immunoglobulin genebased libraries. Moreover, antibody genes are immediately obtained, the antibodies can be easily further modified and their production quality can be maintained. Hence, high quality antibody phage libraries provide an efficient way to generate antibodies that might be difficult to obtain with traditional methods.
We describe here the production, characterization and structural analysis of an EphA2-specific human single-chain antibody (scFv). We have studied the Eph family of tyrosine kinase receptors extensively in the past and are in constant need of good-quality antibodies for biochemical, structural and functional studies. The generated scFv shows high specificity for EphA2, blocks ligand binding, and its structure bound to the ligand-binding domain of the receptor shows interesting features that are discussed below.
Methods
Phage display selections for the production of anti-EphA2 scFv
The ligand-binding domain of EphA2 was expressed in 293-HEK cells as Fc-fusion and purified by Protein A-Sepharose affinity and Superdex-200 size exclusion-chromatography as described earlier (Himanen et al., 2009). The EphA2 specific binders were selected by phage display from synthetic single-chain antibody fragment (scFv) phage libraries ScFvM (Huovinen et al., 2013) and ScFvP (Brockmann, 2011). The methods used for M13 phage display have been described by Huovinen et al. (2013). Purified recombinant EphA2 was first immobilized on Dynabeads® M-270 Epoxy (Life Technologies Inc., Carlsbad, CA) using 20 μg of antigen per mg of beads, according to the instructions of the Dynabeads® Antibody Coupling Kit. Phosphate buffered saline (PBS), supplemented with 0.05% n-dodecyl β-D-maltoside (DDM) and 0.01% cholesterol hemisuccinate (CHS), was used for the last washing step and to resuspend the beads. At the first selection round 1012 colony-forming units (cfu) of each of the two scFv phage libraries were used as mixed and at the second round the phage input was 1011 cfu from the first round output. The mass of antigen-coupled beads used for the first and second round selections was 0.5 mg or 0.05 mg, respectively. The phages were incubated with the beads in TBS (50mM Tris, 150mM NaCl, 1% BSA, pH 7.5) containing 0.05% DDM and 1% bovine serum albumin (BSA) for 30–60 min at room temperature with rotation. The unbound phages were removed by washing twice with the same buffer, followed by one wash with TBS+0.02% NaN3+0.05% Tween-20. Elution of the bound phages was performed with trypsin.
Enrichment of specific phages was monitored by a phage immunoassay as described by Huovinen et al. (2013). The plates (Maxisorp, Nunc, Denmark) were coated with antigen by incubating a saturating amount of EphA2 in 0.1M NaH2PO4 at +35 °C, o/n. The wells were washed twice and blocked with 50mM NaH2PO4, 0.1% Germall II, 6% Sorbitol and 1% milk by incubating at room temperature for 2 h.
ELISA screening
After two rounds of phage display selection, the scFvs genes were cut off from the phagemid vector by SfiI digestion and cloned as a pool into the vector pEB06H for single-clone immunoactivity screening (Huovinen et al., 2013). In this vector, the scFv is fused to the gene encoding for bacterial alkaline phosphatase (AP). Electro-competent E. coli XL1-Blue cells (Stratagene) were transformed, and scFv-AP fusion protein was expressed in a 96-well format. The activity of antibody fragments was determined on EphA2 coated Maxisorp plates by ELISA as described by Paino et al. (2013). As a cross reactivity control the assay was also performed on plates coated with EphB2ECD-Fc fusion protein.
Cloning of FLAG-tagged scFv constructs
The genes originating from the human synthetic scFv antibody library (pEB06H/scFv vector; (Brockmann, 2011)), and encoding EphA2-specific scFv antibodies were expressed as fusions to AP. The gene cassette in this vector has the following orientation: SfiI (restriction enzyme site) – scFv – SfiI/AvaI – AP – Histidine tag – STOP codon – HindIII. By virtue of the construction, the scFv genes can easily be switched between appropriate vectors using SfiI enzyme to maintain the cloning frame and the gene orientation. Due to high background staining in COS-7 cells by several anti-AP antibodies that were tested, scFv genes were subcloned into newly synthesized vector (His-FLAG-pEB06) as a scFv-His-FLAG fusion. NsiI-(SfiI)-HindIII fragment was removed from the pEB06H backbone vector, and a new synthetic gene cassette containing NsiI – SfiI/AvaI – TEVPro (protease cleavage site) – His6 – Gly-Ser-Gly-linker – FLAG – STOP – HindIII was inserted in-frame into pEB06H to create His-FLAG-pEB06 (GeneArt Gene Synthesis, Life Technologies, Carlsbad, CA). The EphA2-encoding scFv genes were subcloned into SfiI/AvaI-digested His-FLAG-pEB06, and the construct was checked by restriction site mapping. ScFv antibodies were expressed in bacteria, purified using HisSpinTrap (GE Healthcare) columns, and used to detect Eph receptor expression on COS-7 cells by confocal microscopy.
Production of monomeric anti-EphA2 sc-Fv for crystallization
The scFv-AP fusion protein, described above, is a dimer as AP is a homodimer. To produce monomeric scFv for crystallization experiments, the D2 scFv was cloned into the pEB04 vector with Sfi-digestion. This construct generates scFv with a C-terminal His-tag. The scFv-6xHis protein was expressed in E. coli (AD494) and purified on a Chelating Sepharose (GE Healthcare, Ridgewood, NJ) column according to manufacturer’s instructions.
Pull-down assay
Pull-down experiment was performed as described earlier (Himanen et al., 2004). In short, 10 μg of the ligand-binding or extracellular domains of Eph receptors were incubated with the D2 scFv at room temperature for 30 min in Hepesbuffered saline, Protein A-Sepharose beads (GE Healthcare) were added and mixed for 30 min. The harvested beads were washed and the bound proteins were separated on a 5–20% PAGE gels (Bio-Rad Laboratories Inc., Hercules, CA). The stoichiometry of the receptor/antibody binding was evaluated by size exclusion chromatography. The proteins were diluted to 0.5 mg/ml, applied on a Superdex-200 10/300 column (GE Healthcare, Ridgewood, NJ), then mixed in a uni-molar ratio and run again.
Cell cultivation, transfection and imaging
COS-7 (African green monkey kidney fibroblast-like; ATCC CRL-1651) cells were cultivated in DMEM (Dulbecco’s modified eagle) medium supplemented with 10 % fetal calf serum, 4mM glutamine and 100 U/ml Pen/Strep antibiotics at +37 °C. Cells cultivated on glass slides were transfected at 90% confluency with pDT101 expression vector encoding full-length human EphA2 protein, or with myc-FLAG-tagged vector encoding human EphA7 (hEphA7) (RC226293, Origene). Transfections were performed with Fugene6 (Promega, Madison, WI) transfection reagent according to manufacturer’s instructions. Transfected and control (non-transfected) cells were incubated at 37 °C for 24 h before fixation (4% formalin in PBS) and permeabilization (when applicable; 0.1% Triton-X100 in PBS). Antibody staining was performed at room temperature. Overexpression of FLAG-tagged hEphA7 was visualized in permeabilized cells using anti-FLAG antibodies. Binding of anti-EphA2 scFv anti-bodies to overexpressed EphA2 and EphA7 receptors was detected by rabbit anti-DYKDDDDK (FLAG) Tag Antibody (#2368, Cell Signaling Technologies Inc., Danvers, MA) in non-permeabilized cells. As controls, commercial rabbit anti-EphA2 and rat anti-EphA7 (Santa Cruz Biotechnology Inc., Dallas TX) were used in similar manner. Primary antibody binding was visualized by secondary mouse/rabbit/rat Alexa Fluor (AF) 488/568-labeled antibodies when applicable. All antibodies were diluted in PBS supplemented with 3% BSA. The wells were incubated at room temperature for 1.5 h in the presence of primary antibodies, after which the cells were washed three times with PBS and AF-conjugated secondary antibodies were added. After 30 min incubation at room temperature, the cells were washed and nuclei stained with DAPI. Fixed, permeabilized and immunostained cells were then mounted on microscope slides in Mowiol 4-88 (Calbiochem-Novabiochem, San Diego, CA), 25% glycerol, 0.1M Tris-HCl, pH 8.5, containing 25 mg/ml Dabco (Sigma-Aldrich, St. Louis, MO) and examined with Zeiss LSM780 confocal microscope using a Plan-Apochromat objective (63× oil). The images were processed with BioImageXD (Kankaanpaa et al., 2012).
Lymphoma cell culture and apoptosis assays
Lymphoma cell lines Raji, Ly-19 and Toledo were maintained in RPMI 1640 with 10% fetal bovine serum, 1% L-glutamine and 1% penicillin/streptomycin. Human lymphoma cell lines treated with single-chain anti-EphA2 antibody were analyzed for cell viability and cell proliferation assays. Briefly, cells were seed at 5×104 per mL and they were treated with single-chain anti-EphA2 antibody. After 24 h, 48 h and 72 h of treatment cell apoptosis and proliferation was assessed using Guava ViaCount (cat. 4000-0041).
Crystallization and data collection
For crystallization, the D2 scFv antibody was mixed with the ligand-binding domain of EphA2 in a unimolar ratio and the complex was purified on a Superdex-200 HiLoad 16/80 (GE Healthcare) column. The EphA2/D2 complex was dialyzed against 5mM HEPES (pH 7.2), 10mM KCl, 2mM MgCl2 and concentrated to 10 mg/ml. It was crystallized in a hanging drop against 20mM citrate (pH 5.6), 200mM ammonium sulfate, 20% PEG 4000. The crystals were flash frozen and data were collected at APS (Argonne National Laboratory, Chicago, IL) beamline ID-24. The structure was determined by molecular replacement using CCP4/Balbes (Long et al., 2008) and the model was refined in Refmac5 (Murshudov et al., 1997) followed by an iterative building process in O (Jones et al., 1991).
Results and discussion
Library screening for EphA2 binders
Two rounds of phage display selection were performed to enrich EphA2-specific binders from the mixture of the synthetic antibody libraries scFvP and scFvM (Brockmann, 2011; Huovinen et al., 2013). Phage immunoassay gave a signal-to-background ratio of 22 for the phage pool after the second round indicating clear enrichment of EphA2-binding phages. The scFv genes from the second round were cloned into the pEB06H vector to express soluble scFv-AP fusion proteins for activity screening. ELISA-based screening of 96 individual clones yielded 58 EphA2-positive clones (sg/bg>3). None of the clones showed cross reactivity to the EphB2-Fc control protein. The EphA2-binding activity was confirmed with a secondary screening assay. Sequencing of 12 clones revealed six unique binders. We selected one of the unique binders, “D2”, for further studies. Its sequence is shown in Table 1.
Table 1.
Amino acid sequence of the anti-EphA2 scFv antibody D2, shown in the order of variable-light chain/linker/variable-heavy chain.

|
The linker region is underlined. Amino acid residues in the EphA2/D2 interface are highlighted in grey (see also Figure 3B).
Biochemical characterization of the D2 scFv
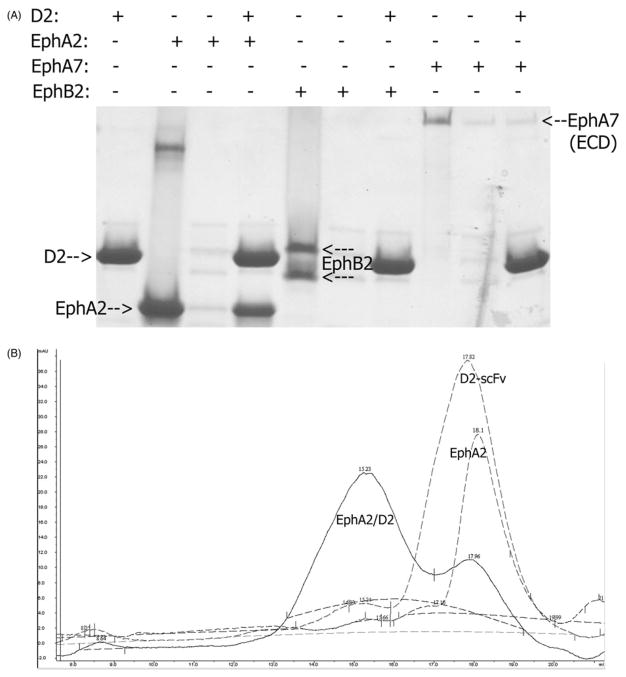
We first carried out pull-down assays to evaluate the interactions of the ligand-binding or extracellular domains of different Eph receptors with the D2 scFv. Antibodies derived from the synthetic libraries scFvM and scFvP have capability to interact with Protein-A as the human VH3-family variable domain is employed as a part of framework of the libraries. We used this interaction to pull-down untagged Eph receptors (Figure 1A). The results confirmed the binding specificity of the D2 for EphA2. As shown in Figure 1(A), the D2 scFv pulls down untagged EphA2, but not EphB2 or EphA7. We used the full extracellular domain of EphA7 to verify that there are no nonspecific epitopes outside the Eph ligand-binding domain.
Figure 1.
(A) Pull-down assay to evaluate the binding of EphA2 to the D2-scFv. D2-scFv is able to pull down untagged EphA2 but not EphB2 or EphA7. The result confirms the binding specificity of the D2 for EphA2. (B) Size-exclusion chromatography of the D2-scFv/EphA2 complex. Monomeric EphA2 and D2 both run close to the 23 kDa molecular weight marker on Superdex-200 (17.8 ml and 18.1 ml, respectively). The peak of their complex shifts to 15.2 ml, indicating a formation of a 1:1 heterodimer, since 67 kDa molecular weight marker runs at 14.3 ml.
We also performed size-exclusion chromatography to evaluate the stoichiometry of the D2-scFv/EphA2 complex. As seen in Figure 1(B), both EphA2 and D2 run around 17–18 ml on a Superdex column, close to the 23 kDa molecular weight marker. After mixing, the complex runs at 15.2 ml as compared to 14.3 ml for the 67 kDa marker. Thus, in size-exclusion chromatography, the D2-scFv/EphA2 complex appears as a 1:1 heterodimer.
The D2 scFv binds to EphA2 but not EphA7 on COS-7 cells
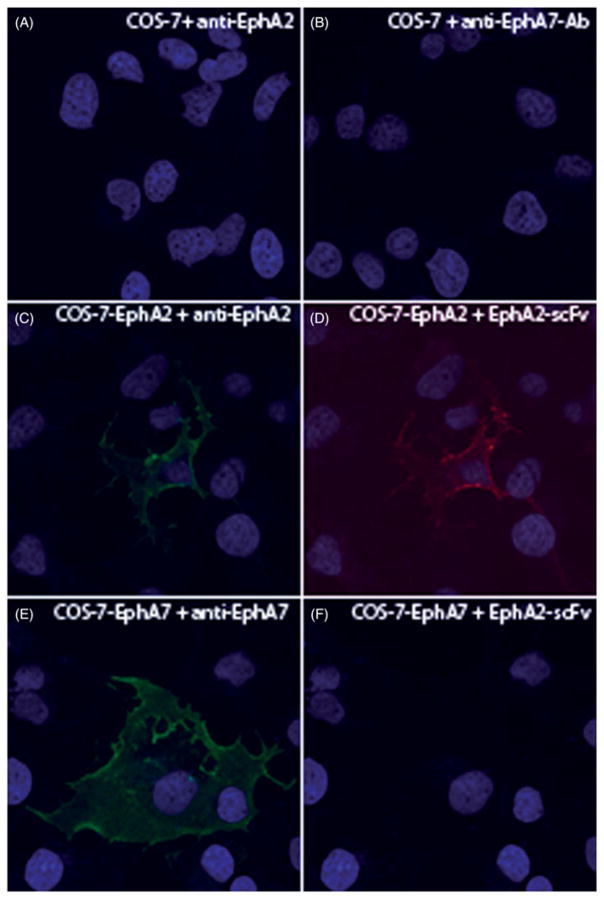
In order to verify the specific binding of D2 in cell culture, COS-7 cells were transfected with plasmids bearing full-length EphA2 or EphA7. Overexpression of the gene products were verified by commercial antibodies. As shown in Figure 2, panels A and B indicate lack of background staining by these antibodies, while EphA2 and EphA7 were detected in the overexpressing cells (panels C and E, respectively). Importantly, the D2 scFv detected EphA2 but not EphA7 in COS-7 cells (panels D and F), demonstrating the specificity of D2 to the EphA2 receptor.
Figure 2.
Reactivities of EphA2-specific scFv antibodies to COS-7 cell lines overexpressing EphA2 and EphA7 receptors. COS-7 cells were transfected with constructs overexpressing EphA2 and EphA7 receptors. The receptors on the surface of nonpermeabilized cells were detected by type-specific (panels C and E) and scFv (panels D and F) antibodies, respectively. Antibody-binding was visualized by secondary Alexa Fluor 488/568 antibodies.
Crystal structure of the D2/EphA2 complex
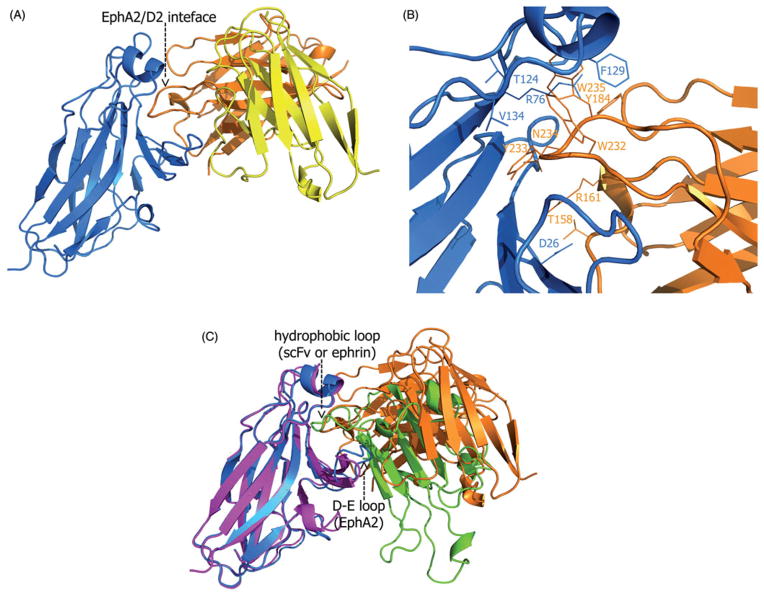
We next determined the high-resolution crystal structure of the complex between the D2 scFv antibody and the EphA2 ligand-binding domain (LBD; Figure 3A). Our model is refined at 2.5Å resolution and has an R-factor of 20.7%. As all immunoglobulin variable domains (Bhat et al., 1990; Zdanov et al., 1994), the variable-light and variable-heavy chains of D2 have two antiparallel beta-sheets each, one consisting of four and the other of five beta-stands. In addition, the heavy chain has two short alpha helices. The beta-strands are connected with loops of varying length, including a 16-amino acids long CDR3 in the heavy chain that appears as the main determinant for the specificity of D2 (see below), as well as five shorter CDR-loops. The twenty amino acid linker, connecting the C-terminus of VL to the N-terminus of VH, is unstructured and is not visible in the electron density map. Crystallographic statistics are presented in Table 2.
Figure 3.
(A) The overall structure of the EphA2/D2-scFv receptor/antibody complex. The scFv variable-light chain is yellow, the variable-heavy chain is orange and the EphA2 receptor is blue. The EphA2/D2 interface mediates antigen recognition and specificity. (B) Interacting amino acid residues in the interface between the EphA2 receptor (blue) and D2-scFv antibody (orange). Most of the interacting residues are hydrophobic and reside within the CDR-H3 loop of the scFv heavy chain, a common feature for majority of the known antibodies-antigen complexes. (C) Superimposition of the ligand-binding domain of EphA2 bound to either the D2-scFv antibody (orange) or ephrin-A1 (green). EphA2 in complex with scFv is shown in blue and in complex with ephrin, in purple. The large protein-protein interfaces centers around the same hydrophobic loop (scFv or ephrin) and cavity (receptor) in both structures. The structure of EphA2 in both complexes is very similar; the only important difference is the D-E loop that is shifted towards the center of the EphA2 hydrophobic surface cavity when in complex with scFv.
Table 2.
Data collection and refinement statistics (values in the brackets are for the highest resolution shell).
| Wavelength (Å) | 0.9792 |
| Resolution range (Å) | 30–2.4 (2.5/2.46* – 2.4) |
| Space group | P41212 |
| Cell dimensions | a=b=87.77Å, c=239.88Å |
| Redundancy | 7.2 (7.3) |
| Completeness | 99.9 (100.0) |
| Rsym | 0.057 (0.432) |
| I/σI | 18.5 (2.5) |
| Subunits/ASU | 2 |
| Reflections total | 35740 (2702) |
| Working set | 33860 (2566) |
| Test set | 1880 (136) |
| Rwork/Rfree | 0.207/0.255 (0.328/0.366) |
| Number of atoms | 6683 |
| Protein | 6840 |
| Water | 290 |
| Ion | 10 |
| Mean B value (Å2) | 40.1 |
| RMS deviations | |
| Bond lengths (Å) | 0.013 |
| Bond angles (°) | 1.753 |
| Ramachandran analysis | |
| Favored | 95.7 |
| Allowed | 4.3 |
| Disallowed | 0 |
Resolution limits for data integration and refinement statistics, respectively.
The structure of the EphA2 receptor in the complex is remarkably similar to that in its complex with the ephrin-A1 ligand (Himanen et al., 2009). The two EphA2 structures can be superimposed with root-mean-square deviation (RMSD) between Cα positions of about 0.4Å. The only important difference is the D-E loop that is shifted towards the center of the hydrophobic cavity on the surface of the receptor (Figure 3C). This conformational re-arrangement allows Met32 of the EphA2 to form a Van der Waals bond with the Trp232 of the D2 antibody. Another small difference between the structures lies within the A-C loop of the EphA2, but this structural element is too far from the D2/EphA2 interface to have any influence on the antigen/antibody binding or specificity.
There are two 1:1 EphA2/D2 complexes in the asymmetric unit of the crystals. Both complexes have a nearly identical, extensive EphA2/D2 interaction interface, stretching over 1,300 Å2 on each molecule. The interface has a fairly broad network of hydrogen and Van der Waals bonds and salt bridges that stabilize the complex. The interacting amino acid residues in the interface are shown in Figure 3(B) and Table 1. The majority of the contact-forming residues of scFv reside in the three complementarity determining regions (CDR) of the heavy chain, and only two light chain amino acids in the CDR-2 region directly interact with the antigen. Hence, the heavy chain clearly plays a prominent role in the defining the specificity of D2 to EphA2. Especially the CDR-H3 loop of the heavy chain is tightly interacting with several residues of EphA2. The central role of CDR-H3 in the binding is a common feature for the majority of the known antibodies-antigen complexes (Rock et al., 1994).
The two EphA2/D2 heterodimers in the asymmetric unit pack via another small EphA2/D2 crystal-packing interface (burying approximately 450Å2 on each molecule). This interface has only three D2 residues that are in direct contact with the EphA2 receptor. They are all outside of the CDR loops and 13–19Å apart from each other and thus, not able to form a continuous binding surface. We are currently exploring whether there is any potential biological relevance to these contacts.
D2 scFv competes with ephrin-A5 for EphA2 binding
Interestingly, the high-affinity, interface between EphA2 and D2 centers around the exact same hydrophobic Eph surface cavity as the interface between EphA2 and ephrin-A1 ((Himanen et al., 2009); Figure 3C). The apex of the lengthy CDR-H3 in D2 protrudes deep into the ligand-binding cavity of EphA2. At the very tip of the loop there are four consecutive residues, W232, Y233, N234 and W235, the side chain of which point into different directions. These residues constitute an anchor-like structure that occupies the ligand-binding pocket forming multiple contacts with the antigen. The three bulky hydrophobic residues on the tip of CDR-H3 are well suited to interact with the hydrophobic ligand-binding cavity of the EphA2 in a way similar to the ephrin G-H loops in the known Eph/ephrin complexes (Himanen et al., 2001, 2009). Thus, Thr124, Phe129 and V134 of EphA2 form a hydrophobic contact area for the CDR-H3 anchor, consisting of W232, Y233 and W235, while a hydrogen bond between Arg76 (EphA2) and Tyr184 (scFv) further stabilizes the complex. It is interesting to note that while the sequence similarity is very high between different members of the Eph family, a careful assessment of the sequences provides an explanation for the D2 scFv specificity. Indeed, although the Arg76 is conserved throughout the family, the interface residues T124, F129 and V134 are evolutionary somewhat diverged: Thr-124 is Phe, Phe-129 is Leu and Val-134 is Met in both EphB2 and EphA7. These subtle differences might, in part, account for the high specificity of D2 scFv for EphA2.
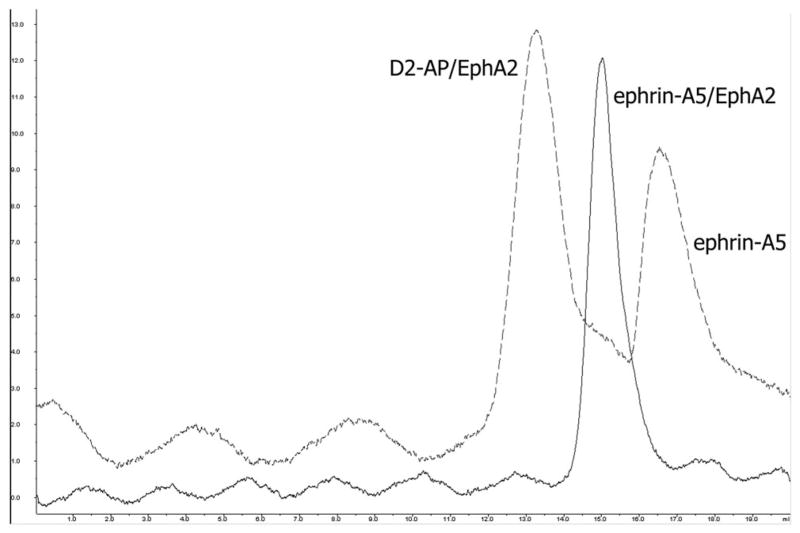
To evaluate the potential consequence of the apparently overlapping binding epitopes, we measured the influence of D2-scFv on the binding of EphA2 with its ephrin ligand. As expected from the structure, the scFv antibody upon binding to EphA2 blocks the binding of ephrin-A5 to the receptor. As shown in Figure 4, ephrin-A5 runs as a monomeric ligand on a size exclusion column when added to a preformed EphA2/D2 complex. However, when mixed with the un-complexed EphA2, ephrin-A5 forms a heterodimeric receptor/ligand complex that runs near the position of the 43 kDa molecular marker.
Figure 4.
D2-scFv blocks the binding of ephrin-A5 to its EphA2 receptor. When added to a D2-scFv/EphA2 complex, ephrin-A5 runs as a monomer on a size exclusion column (dotted line) but forms a heterodimeric receptor/ligand complex when mixed with an un-complexed EphA2 (dotted line).
Effect of EphA2-scFv on lymphoma cells
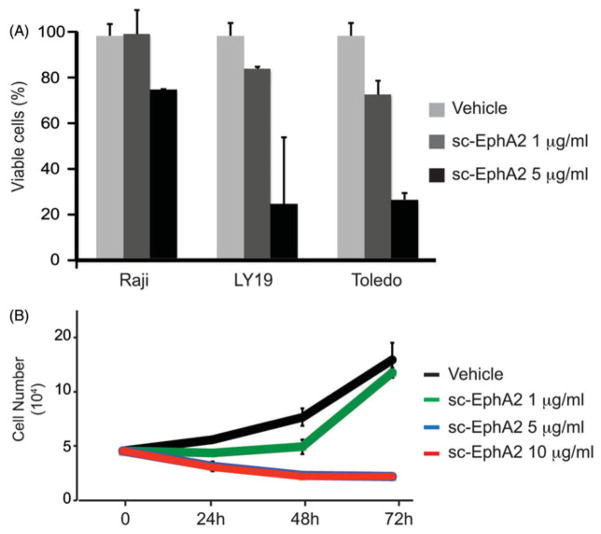
Finally, we tested the effect of D2 scFv on lymphoma cell cultures (Figure 5). Lymphoma cell lines express EphA2, the activity of which can be regulated by a soluble form of EphA7 (Oricchio et al., 2011). We used the EphA2-scFv antibody as an alternative way to block EphA2 activity in lymphoma. Treatment with the EphA2 single-chain antibody indeed induces apoptosis (Figure 5A) and reduces cell proliferation (Figure 5B) in the lymphoma cells lines. We are currently investigating ways to improve the efficacy of D2 by affinity maturation.
Figure 5.
Block and let die: exogenous administration of anti-EphA2 scFv antibody reduces cell proliferation and induces apoptosis in lymphoma cell lines. (A) Apoptosis analysis in Raji, Ly19 and Toledo lymphoma cell lines treated for 24 h with 1 μg/ml or 5 μg/ml of D2 scFv. (B) Cell proliferation analysis was performed every 24 h in Raji lymphoma cell line treated with vehicle as control or 1 μg/ml, 5 μg/ml or 10 μg/ml of D2 scFv.
Conclusions
The results in this paper highlight the possibilities of producing specific antibodies against members of very conserved protein families using synthetic antibody libraries. We show that our single-chain anti-EphA2 antibody, produced by a recombinant antibody phage library technology, shows high specificity, as well as biological activity. It’s worth keeping in mind that the receptors within the Eph family are also extremely highly conserved between species, generally having only 2–3 amino acid differences between the human and mouse proteins. In such instances it is virtually impossible to create antibodies using the classical murine technology. In addition, the synthetic library technology produces antibodies based on human immunoglobulin genes that can easily be manipulated for having, e.g. different tags for research purposes, or developed towards lead compounds for therapeutic interventions. Regarding the latter avenue, our results on the effect of our anti-EphA2 scFv on lymphoma cell lines are especially encouraging.
Acknowledgments
These studies were supported by the NIH (RO1NS038486), Academy of Finland (263255&129156), Cancer Society of Finland, European Union (AIROPico, FP7-PEOPLE-2013- IAPP Grant No. 612308), Turku Graduate School of Biomedical Sciences (TuBS) and Biocenter Finland.
Footnotes
Declaration of interest
The authors declare no conflict of interest.
References
- Bhat TN, Bentley GA, Fischmann TO, Boulot G, Poljak RJ. Small rearrangements in structures of Fv and Fab fragments of antibody D1.3 on antigen binding. Nature. 1990;347:483–485. doi: 10.1038/347483a0. [DOI] [PubMed] [Google Scholar]
- Brockmann EC, Akter S, Savukoski T, Huovinen T, Lehmusvuori A, Leivo J, Saavalainen O, et al. Synthetic single-framework antibody library integrated with rapid affinity maturation by VL shuffling. Protein Eng Des Sel. 2011;24:691–700. doi: 10.1093/protein/gzr023. [DOI] [PubMed] [Google Scholar]
- Couchman JR. Commercial antibodies: The good, bad, and really ugly. J Histochem Cytochem: J Histochem Soc. 2009;57:7–8. doi: 10.1369/jhc.2008.952820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen JP, Rajashankar KR, Lackmann M, Cowan CA, Henkemeyer M, Nikolov DB. Crystal structure of an Eph receptor-ephrin complex. Nature. 2001;414:933–938. doi: 10.1038/414933a. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, et al. Repelling class discrimination: Ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Goldgur Y, Miao H, Myshkin E, Guo H, Buck M, Nguyen M, et al. Ligand recognition by A-class Eph receptors: Crystal structures of the EphA2 ligand-binding domain and the EphA2/ephrin-A1 complex. EMBO Rep. 2009;10:722–728. doi: 10.1038/embor.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom HR, de Bruine AP, Hufton SE, Hoet RM, Arends JW, Roovers RC. Antibody phage display technology and its applications. Immunotechnol: Int J Immunol Eng. 1998;4:1–20. doi: 10.1016/s1380-2933(98)00007-4. [DOI] [PubMed] [Google Scholar]
- Huovinen T, Syrjanpaa M, Sanmark H, Brockmann EC, Azhayev A, Wang Q, Vehniainen M, Lamminmaki U. Two ScFv antibody libraries derived from identical VL-VH framework with different binding site designs display distinct binding profiles. Protein Eng Des Sel. 2013;26:683–693. doi: 10.1093/protein/gzt037. [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa P, Paavolainen L, Tiitta S, Karjalainen M, Paivarinne J, Nieminen J, Marjomaki V, et al. BioImageXD: An open, general-purpose and high-throughput image-processing platform. Nat Methods. 2012;9:683–689. doi: 10.1038/nmeth.2047. [DOI] [PubMed] [Google Scholar]
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Long F, Vagin AA, Young P, Murshudov GN. BALBES: a molecular-replacement pipeline. Acta Crystallographica D. 2008;64:125–132. doi: 10.1107/S0907444907050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallographica D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Oricchio E, Nanjangud G, Wolfe AL, Schatz JH, Mavrakis KJ, Jiang M, Liu X, et al. The Eph-receptor A7 is a soluble tumor suppressor for follicular lymphoma. Cell. 2011;147:554–564. doi: 10.1016/j.cell.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paino A, Ahlstrand T, Nuutila J, Navickaite I, Lahti M, Tuominen H, Valimaa H, et al. Identification of a novel bacterial outer membrane interleukin-1beta-binding protein from aggregatibacter actinomycetemcomitans. PLoS One. 2013;8:e70509. doi: 10.1371/journal.pone.0070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. An open letter to our readers on the use of antibodies. J Comp Neurol. 2005;493:477–478. doi: 10.1002/cne.20839. [DOI] [PubMed] [Google Scholar]
- Siddiqui MZ. Monoclonal antibodies as diagnostics; an appraisal. Indian J Pharmaceut Sci. 2010;72:12–17. doi: 10.4103/0250-474X.62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192–1198. doi: 10.1126/science.1241145. [DOI] [PubMed] [Google Scholar]
- Stern M, Herrmann R. Overview of monoclonal antibodies in cancer therapy: Present and promise. Crit Rev Oncol/Hematol. 2005;54:11–29. doi: 10.1016/j.critrevonc.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Yagami H, Kato H, Tsumoto K, Tomita M. Monoclonal antibodies based on hybridoma technology. Pharmaceut Pat Anal. 2013;2:249–263. doi: 10.4155/ppa.13.2. [DOI] [PubMed] [Google Scholar]
- Zdanov A, Li Y, Bundle DR, Deng SJ, MacKenzie CR, Narang SA, Young NM, Cygler M. Structure of a single-chain antibody variable domain (Fv) fragment complexed with a carbohydrate antigen at 1.7-A resolution. Proc Natl Acad Sci USA. 1994;91:6423–6427. doi: 10.1073/pnas.91.14.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]