Abstract
Individual differences in the intensity of feelings of arousal while viewing emotional pictures have been associated with the magnitude of task‐evoked blood‐oxygen dependent (BOLD) response in the amygdala. Recently, we reported that individual differences in feelings of arousal are associated with task‐free (resting state) connectivity within the salience network. There has not yet been an investigation of whether these two types of functional magnetic resonance imaging (MRI) measures are redundant or independent in their relationships to behavior. Here we tested the hypothesis that a combination of task‐evoked amygdala activation and task‐free amygdala connectivity within the salience network relate to individual differences in feelings of arousal while viewing of negatively potent images. In 25 young adults, results revealed that greater task‐evoked amygdala activation and stronger task‐free amygdala connectivity within the salience network each contributed independently to feelings of arousal, predicting a total of 45% of its variance. Individuals who had both increased task‐evoked amygdala activation and stronger task‐free amygdala connectivity within the salience network had the most heightened levels of arousal. Task‐evoked amygdala activation and task‐free amygdala connectivity within the salience network were not related to each other, suggesting that resting‐state and task‐evoked dynamic brain imaging measures may provide independent and complementary information about affective experience, and likely other kinds of behaviors as well. Hum Brain Mapp 35:5316–5327, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: amygdala, task‐evoked activation, resting‐state connectivity, individual differences, feelings of arousal
INTRODUCTION
The amygdala plays a fundamental role in affective experience. Studies of humans with brain lesions demonstrate the importance of the amygdala for affective behavior ([Feinstein, et al., 2011; but see also [Feinstein, et al., 2011]). Research with healthy individuals has shown that the amygdala processes biologically relevant stimuli [Adolphs, 2008; Davis and Whalen, 2001], especially under conditions of uncertainty, ambiguity, or novelty. In one of the most widely replicated observations in functional neuroimaging, the amygdala's task‐evoked hemodynamic response is greater while processing affectively potent stimuli of various types than while processing neutral stimuli [Breiter et al., 1996; Hsu et al., 2005; Lewis et al., 2007; Morris et al., 1996; Wright et al., 2006]; for a meta‐analytic review see [Kober et al., 2008]. Amygdala activation is consistently observed during the experience of emotions such as anger, sadness, fear, disgust, and happiness [Lindquist et al., 2012; Wilson‐Mendenhall et al., 2013], and the magnitude of amygdala activation relates to individual differences in the intensity of feelings of arousal during affective tasks [Barrett et al., 2007; Colibazzi et al., 2010; Gerdes et al., 2010; Phan et al., 2004; Wilson‐Mendenhall et al., 2013].
A growing body of research also indicates that individual differences in the intensity of negative affect is associated with the strength of task‐free connectivity between particular structures such as the insula and other structures that compose the “salience” network [Seeley et al., 2007; Touroutoglou et al., 2012]. Tract‐tracing and recent resting‐state functional connectivity studies demonstrate that dorsal portions of the amygdala (including the central nucleus) are connected within the salience network [Bickart et al., 2012; Hayes and Northoff, 2011; McDonald 1991b; Ongur and Price, 2000; Price, 2007; Saleem et al., 2008; Seeley et al., 2007; Zhou et al., 2010], which subserves affective responses to salient, ambiguous, or novel negative stimuli.
Despite the importance of these observations, a critical question remains unanswered. Are these two types of functional MRI measures redundant or independent in their relationships to individual differences in affective behavioral responses? Moreover, this question extends well beyond emotion: to our knowledge, there is not yet a study of whether a combination of task‐evoked and task‐free functional neuroimaging measures predicts individual differences in any type of behavior including cognitive functions.
In this article, we examined in a single experiment the contributions of task‐evoked amygdala activation and task‐free dorsal amygdala connectivity within the salience network in predicting individual differences in negative affective experience. Specifically, we hypothesized that the dorsal amgydala's intrinsic connectivity within the salience network would predict individual differences in negative affective experience independently of the contributions of task‐evoked amgydala activation.
To address our hypotheses, we analyzed both resting state and affective reactivity task scans in 25 participants who rated their feelings of arousal while viewing affectively potent images. Affectively potent stimuli such as high arousal negative images can evoke an affective experience that is accompanied by physiological arousal [Russell and Barrett, 1999]. To measure task‐evoked amygdala activation, we calculated the percent BOLD signal change in the amygdala for high arousal negative images (vs. fixation). To assess task‐free connectivity of the dorsal amygdala within the salience network, we used resting state functional connectivity analysis. Using multiple linear regression analysis, we show that individual differences in feelings of arousal arise independently from task‐evoked amygdala activation and task‐free dorsal amygdala connectivity within the salience network.
MATERIALS AND METHODS
Participants
Twenty‐five young adults ranging in age from 19 to 32 (mean age = 24.36 years, SD = 3.12; 9 men) participated in this study, which involved the collection of resting‐state blood oxygenation‐level dependent (BOLD) data as well as task‐evoked BOLD data. The task performed in the scanner was an affective reactivity task in which participants rated their arousal on‐line as they viewed affectively potent images from the International Affective Picture Set (IAPS; [Lang et al., 1997]). All participants were right‐handed, native English speakers and had normal or corrected‐to‐normal vision. No participant reported a history of neurological or psychiatric disorders. Other analyses of data collected as part of a larger project on age‐related differences in affect and novelty processing have previously been published [Andreano et al., 2013; Moriguchi et al., 2011; Weierich et al., 2010].
Behavioral Data Acquisition
The affective reactivity task included 132 full‐color images, selected from the IAPS [Lang et al., 1997]. Of these 132 images, equal numbers of each of three levels of arousal (high, mid, and low) and types of valence (negative, neutral, and positive) were included, creating six combinations of arousal and valence (high arousal negative, high arousal positive, mid arousal negative, mid arousal positive, mid arousal neutral and low arousal neutral). The affective reactivity task was run using E‐Prime experimental software (Psychology Software Tools, Pittsburgh, PA) on a PC, from which images were projected onto a screen in the magnet bore. Participants viewed images via a mirror mounted on the head coil. The task consisted of five event‐related fMRI runs, the first of which was a familiarization run where participants were familiarized to two images in each stimulus category; during the latter four test runs, participants viewed these familiarized images randomly intermixed with 120 novel images that were shown only once (20 novel images in each of the six valence‐arousal combinations). Each run contained 100 events, of which 40 were fixation events and 60 were stimulus events. Each run was 340 s in length and each image was presented for 3.5 s, with a stimulus onset asynchrony that varied from 4 to 16 s. The fixation lasted 2 s.
Participants used a three‐button response to rate how aroused each image made them feel (1 = low, 2 = mid, 3 = high). As in previous work [Touroutoglou et al., 2012], the subjective arousal ratings were then averaged to create composite measures of feelings of arousal for use in behavioral correlation analyses. Specifically, for each participant, ratings of subjective arousal in response to all of the novel and familiarized images that were rated as high arousal negative based on normative data were averaged to create a composite measure of feelings of arousal in response to negative images. The same procedure was performed for high arousal positive images.
MRI Data Acquisition and Processing Procedures
Structural and functional MRI data were acquired using a Siemens Magnetom Trio Tim 3T whole body high‐speed imaging device equipped for echo planar imaging (EPI) (Siemens Medical Systems, Iselin, NJ) with a 12 channel gradient head coil. Head motion was minimized using head restraints, including a pillow and foam padding. Noise was attenuated with ear plugs. High resolution 3D MPRAGE sequences (TR/TE/flip angle = 2.53 s/3.39 ms/7°) with an in‐plane resolution of 1.3 × 1.0 mm2 and 1.3‐mm slice thickness were collected for spatial normalization and for positioning the slice prescription of the subsequent sequences. Next, T1‐EPI (TR/TE/flip angle = 10 s/39 ms/90°) and T2‐weighted (TR/TE/flip angle = 5.21 s/94 ms/150°) sequences were collected (using the same geometry as the T2*‐weighted functional images) to assist in registration of the functional data to the high‐resolution anatomical scan.
Whole brain task‐evoked fMRI data were acquired using a gradient‐echo T2*‐weighted sequence (TR/TE/flip angle = 2 s/30 ms/90°; 33 coronal slices angled perpendicular to the AC/PC line), with a slice thickness of 5 mm, for a voxel size of 3.12 × 3.12 × 5 mm3, interleaved acquisition order and foot‐to‐head phase encoding. Prior to each scan, four time points were acquired and discarded to allow longitudinal magnetization to reach equilibrium.
Whole‐brain resting state fMRI data were acquired with echo‐planar sequence (TR = 2,000 ms; TE = 30 ms; FA = 90°; 3.1 × 3.1 × 5.0 mm3 voxels, 33 slices). The data involved one (n = 5) or two (n = 20) runs of 128 time points. During the resting state fMRI runs, participants were instructed to keep their eyes open.
Because task‐evoked fMRI data and task‐free (or resting state) fMRI data are two different types of fMRI measures of brain activity, we processed them independently using procedures optimized for each data type. We used the optimal and standard processing procedures for each type of data as previously published by our lab [for the task‐evoked fMRI see Andreano et al., 2013; Moriguchi et al., 2011; Weierich et al., 2010; for the task‐free fMRI see Bickart et al., 2012; Touroutoglou et al., 2012].
Task‐Evoked fMRI Analysis
Preprocessing of the task‐evoked fMRI data involved a series of steps, using the standard processing stream of the Martinos Center for Biomedical Imaging (http://surfer.nmr.mgh.harvard.edu). Task‐evoked fMRI data were motion corrected and inspected for gross motion. Slices were discarded if the total motion vector exceeded 5 mm. Data in each run were intensity normalized and spatially smoothed (full‐width halfmaximum = 8 mm) using a 3D Gaussian filter. To remove temporal autocorrelation noise, we also included polynomial drift correction with two nuisance regressors to account for low‐frequency drift and whitening based on a single autocorrelation function estimated across all brain voxels [Burock and Dale, 2000]. Following preprocessing, task fMRI images for each participant were registered to that participant's anatomical image in native space (a 3D T1‐weighted volume created by averaging that participant's two high‐resolution 3D MPRAGE images together). We estimated the duration of the hemodynamic response to be 20 s. Task fMRI data for each condition were modeled using a finite impulse response (FIR) model beginning at 4 s prestimulus and utilizing 2‐s bins. We included the 4 s prestimulus in our FIR model to make sure that the response to a previous stimulus had returned to baseline.
To measure the magnitude of task‐evoked amygdala activation, we used an anatomically based approach to conduct an ROI analysis of task‐evoked fMRI data from the amygdala, using FSFAST (http://surfer.nmr.mgh.harvard.edu). We applied FreeSurfer's automated subcortical segmentation method to the native 3D MP‐RAGE structural images for each subject to create an anatomically‐defined amygdala ROI in each hemisphere of each individual [Fischl et al., 2002]. Each ROI was visually inspected; in this dataset, no errors were identified. Using FsFast's func2roi command, the ROIs were used to extract mean BOLD signal within each ROI. We applied an all vs. fixation contrast (all stimuli of all types of arousal and valence vs. fixation) to each amygdala ROI. The data were then extracted from a conjunction of the anatomical label and the all vs. fixation contrast, such that only those voxels within the label that responded to images relative to fixation at P < 0.05 were included. Finally, we extracted signal for the novel high arousal negative vs. fixation contrast from that same functional‐anatomical ROI. These condition‐specific estimates were then used to calculate percent signal change, in this case, for high arousal novel negative stimuli vs. fixation. Signal change estimates from other conditions have been reported in previous papers from our lab [Andreano et al., 2013; Moriguchi et al., 2011; Weierich et al., 2010]. As in our previous work [Moriguchi et al., 2011; Weierich et al., 2010], the percent signal change was calculated for the time window corresponding to 6–8 s post‐stimulus. Thus, for each individual, BOLD percent signal change was measured from the voxels within the combined functional, anatomically defined amygdala ROIs (one in each hemisphere) in which there was activation in the high arousal novel negative condition vs. fixation.
Task‐Free fMRI Analysis
Preprocessing of the resting state fMRI data involved a series of previously established resting state functional connectivity MRI (rs‐fcMRI) procedures [Biswal et al., 1995; Van Dijk et al., 2010; Vincent et al., 2007] including: (1) removal of the four volumes to allow for T1 equilibration effects, (2) slice timing correction (SPM2, Wellcome Department of Cognitive Neurology, London, UK), and (3) head motion correction (FMRIB, Oxford, UK). Data were normalized to the Montreal Neurological Institute (MNI) atlas space (SPM2, Wellcome Department of Cognitive Neurology, London, UK) and re‐sampled to 2‐mm cubic voxels. A low‐pass temporal filter removed frequencies higher than 0.08 Hz. Data were spatially smoothed using a 6 mm full‐width half‐maximum Gaussian filter. Sources of spurious variance and their temporal derivatives were removed through linear regression including: (1) six parameters obtained by rigid‐body correction of head motion correction, (2) the signal averaged over the whole brain, (3) the signal averaged over the ventricles, and (4) the signal averaged over the deep cerebral white matter.
To examine the task‐free functional connectivity of the dorsal amygdala, we used whole brain seed‐based rs‐fcMRI analysis. A hypothesis‐driven approach was taken for this analysis, employing a seed region in the dorsal amygdala (MNI coordinates: −22, −4, −12) as previously identified in Bickart et al. [2012]. This region of the amygdala is connected with a large‐scale network that we originally referred to as an “aversion” network [Bickart et al., 2012], the topography of which corresponds very closely to the so‐called salience network [Seeley et al., 2007]. A spherical ROI was used to interrogate the connectivity strength within this network. For discriminant validity analyses, two additional spherical ROIs were used in the medial amygdala (MNI coordinates: −14, −4, −20) and ventrolateral amygdala (MNI coordinates: −28, −4, −22) whose connectivity networks are preferentially involved in affiliative and perceptual aspects of social perception, respectively [Bickart et al., 2012]. As identified in Bickart et al. [2012], each of the three spherical ROIs had a 2‐mm radius to satisfy two criteria: (a) to be located within the Harvard‐Oxford probabilistic map of the amygdala at 25% or greater probability; and (b) to be separable from one another in space. Each amygdala seed ROI was used to generate a whole‐brain map of Fisher's r‐to‐z correlation coefficients [z(r)] values. Using the three corresponding large‐scale amygdala network map masks as defined independently in a separate sample [Bickart et al., 2012], the strength of task‐free connectivity between each of the three amygdala subregions and the rest of its respective large‐scale network was calculated. We calculated the correlation between low‐frequency BOLD signal fluctuations within each amygdala seed ROI and the mean of the voxel activity in its respective amygdala network mask. This resulted in three amygdala task‐free connectivity strength, z(r), values for each participant: (a) dorsal amygdala task‐free connectivity strength, z(r), values, (b) medial amygdala task‐free connectivity strength, z(r), values and (c) ventrolateral amygdala task‐free connectivity strength, z(r), values. The dorsal amygdala task‐free connectivity strength represented the connectivity strength of interest as contributing to salience processing.
Brain‐Behavior Regression Analyses
We examined the independent and interactive contributions of task‐evoked amygdala activation and task‐free dorsal amygdala connectivity within the salience network to feelings of arousal in the following way. We conducted a linear regression analysis using both task‐evoked BOLD amygdala activation (percent signal change in response to high arousal negative novel images versus fixation) and task‐free dorsal amygdala connectivity strength as independent variables and the arousal ratings while viewing high arousal negative (or positive) images as the dependent variable. We performed this analysis to determine whether both neural measures contributed redundantly or synergistically to the prediction of individual differences in behavior. Brain‐behavior analyses were conducted using PASW Statistics 18, Release Version 18.0.0 (SPSS, 2009, Chicago, IL, http://www.spss.com). Results were considered statistically significant at P < 0.05.
To control for participants' tendency to rate images highly across negative and neutral images, we also tested whether our two fMRI measures of interest predicted variance in the intensity of mean difference score of ratings while viewing high arousal negative images versus low arousal images. For this analysis, we used both task‐evoked BOLD dorsal amygdala activation (percent signal change in response to high arousal negative novel images versus fixation) and task‐free dorsal amygdala connectivity strength as independent variables and the mean difference arousal rating (while viewing high arousal negative images versus low arousal images) as the dependent variable.
To assess the discriminant validity of the a priori hypothesized relationship between the task‐free dorsal amygdala connectivity strength within the salience network and feelings of arousal to negative images, we tested whether the strength of task‐free connectivity for the medial and ventrolateral amygdala (thought to be involved in affiliative and perceptual social processes, respectively) predicted variance in the intensity of feelings of arousal in response to negative images. For this analysis, we performed a multiple regression analysis using task‐free dorsal amygdala, medial amygdala and ventrolateral amygdala connectivity strength (as independent variables and the arousal ratings while viewing negative images as the dependent variable.
Finally, since task‐free dorsal amygdala connectivity within the salience network was correlated with feelings of arousal in the hypothesis‐driven brain‐behavior analysis, we also explored the localization of regions within this network that best predicted feelings of arousal in response to negative images. Using FreeSurfer's implementation of general linear model analysis, we entered arousal ratings while viewing high arousal negative images as a covariate of the dorsal amygdala seed activity into a whole brain regression. The resultant whole‐brain map was limited by the dorsal amygdala connectivity mask defined independently in Bickart et al. [2012] and results were considered significant if they met the criteria of p < 0.01 with a cluster size constraint of 10 contiguous voxels.
RESULTS
As predicted, there was significantly greater signal change for novel high arousal negative images versus fixation (p < 10.5) in the dorsal subregion of the amygdala (see Fig. 1 and Fig. S1 in Supporting Information). Consistent with prior evidence on the amygdala's role in affective experience, our analyses revealed that increased left dorsal amygdala task‐evoked activation in response to high arousal negative novel images was related to heightened feelings of arousal. The results demonstrated that participants with more robust task‐evoked dorsal amygdala activation while viewing negative images made higher arousal ratings for those images relative to those individuals with less robust task‐evoked dorsal amygdala activation (Fig. 2). This effect was not observed in the right amygdala (r = 0.16, p < 0.43), consistent with meta‐analytic summaries of neuroimaging studies that have indicated a more laterized emotional processing for amygdala with most studies reporting activation in left amygdala [Baas et al., 2004; Fusar‐Poli et al., 2009].
Figure 1.
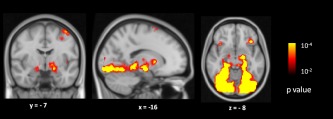
Clusters in bilateral dorsal amygdalae showing significantly greater signal change between novel high arousal negative images versus fixation (red) at p < 0.00001, uncorrected. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 2.
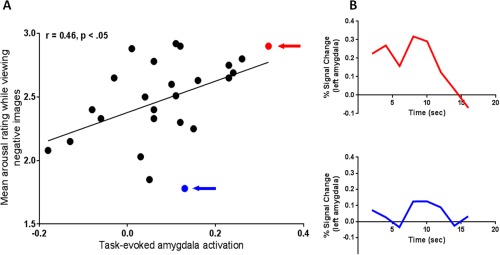
Task‐evoked amygdala activation is related to feelings of arousal. (A) More intense feelings of arousal correlated with greater task‐evoked amygdala activation, r = 0.46, p < 0.05. The task‐evoked left amygdala activation (percent signal change in response to high arousal negative novel images versus fixation) (x axis) is plotted against mean arousal rating while viewing high arousal negative images (y axis). The subjects with the highest and the lowest mean arousal rating while viewing high arousal negative images are highlighted with a red and a blue arrow, respectively; (B) BOLD time course data for these two individuals is shown for the left amygdala activation from the stimulus onset to 16 s post‐stimulus. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
As hypothesized, individual differences in the strength of task‐free dorsal amygdala connectivity within the salience network predicted feelings of arousal. The dorsal amygdala large‐scale connectivity network, binarized at p < 10.5 and overlaid on T1 MNI152 1.0 mm template, is shown in Figure 3. The results demonstrated that participants with stronger dorsal amygdala connectivity at rest made higher arousal ratings when they later viewed negative images relative to those individuals with weaker connectivity (Fig. 4). This effect was observed for both unilateral and bilateral dorsal amygdala connectivity (r = 0.56, p < 0.01). In the remainder of the results, we focus on the left hemisphere. The left dorsal amygdala's level of BOLD activation (high arousal negative novel images vs. fixation) and the task‐free dorsal amygdala connectivity strength within the salience network were not related to each other (r = 0.09, p < 0.68) (see also Fig. S2 in Supporting Information).
Figure 3.
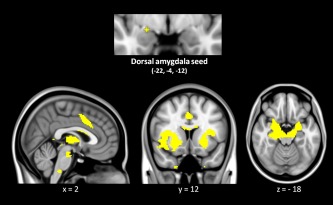
As previously defined in Bickart et al. [2012], the dorsal amygdala seed anchors the dorsal amygdala large‐scale connectivity network (also known as the salience network; see text). The dorsal amygdala map, z < 0.10, is overlaid on T1 MNI152 1.0 mm template brain in radiologic convention to demonstrate the cortical and limbic structures within the dorsal amygdala network (N = 25). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 4.
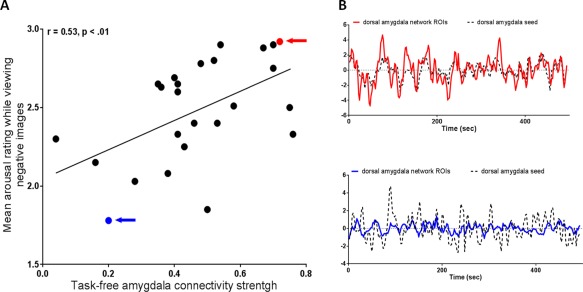
Task‐free dorsal amygdala connectivity within the salience network is related to feelings of arousal. (A) More intense feelings of arousal were predicted by the task‐free dorsal amygdala connectivity strength within the salience network, r = 0.53, p < 0.01. The z‐transformed correlation coefficients of left dorsal amygdala connectivity (x axis) are plotted against mean arousal rating while viewing high arousal negative images (y axis). The subjects with the highest and the lowest level of arousal are highlighted with a red and a blue arrow, respectively; (B) BOLD time course data for these two individuals is shown for the left dorsal amygdala seed and the ROIs of dorsal amygdala connectivity within the salience network. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Using a multiple linear regression analysis, we found that task‐evoked dorsal amygdala activation and task‐free dorsal amygdala connectivity within the salience network each independently predicted the intensity of felt arousal, accounting for a total of 45% of its variance. Those individuals that displayed both the greatest task‐evoked dorsal amygdala activation and the strongest task‐free dorsal amygdala connectivity within the salience network had the most intense feelings of arousal while viewing novel negative images (see Table 1 and Fig. 5). This effect was significant even when participant's tendency to rate more intense arousal experiences across the images were taken into account. Task‐evoked dorsal amygdala activation and task‐free dorsal amygdala connectivity were related to mean difference arousal ratings while viewing high arousal negative images versus low arousal images (r = 0.50, p < 0.05 and r = 0.59, p < 0.01, respectively) (see also Table SI in Supporting Information).
Table 1.
Increased amygdala task‐evoked activation and task‐free connectivity each contributed independently to feelings of arousal while viewing negative images (N = 25)
| Mean arousal rating while viewing negative images | |||
|---|---|---|---|
| B | R 2 change | Total R 2 | |
| Strength of the dorsal amygdala task‐free connectivity within the salience network | 0.49a | 0.28a | 0.45a |
| Amygdala task‐evoked activation | 0.42b | 0.17b | |
Note: This table displays standardized regression coefficients (B) as well as the incremental (R 2 change) and total variance (total R 2) in mean arousal rating predicted by the independent variables entered into a single multiple linear regression model.
p < 0.01.
p < 0.05.
Figure 5.
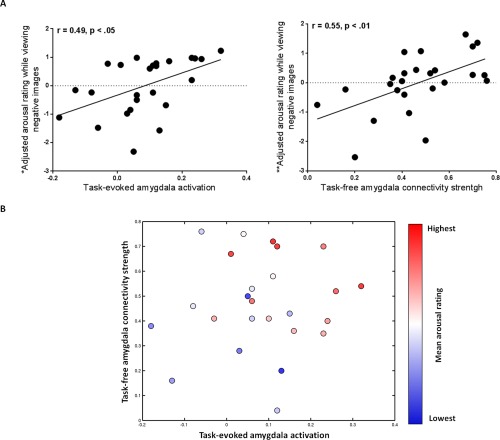
Greater task‐evoked amygdala activation and stronger task‐free dorsal amygdala connectivity within the salience network each contributed independently to feelings of arousal. (A, left graph) *The y axis displays the residual variance in mean arousal rating after the variance due to task‐free dorsal amygdala connectivity within the salience network has been removed; the scatterplot illustrates the relationship between adjusted arousal rating and task‐evoked amygdala activation. (A, right graph) **The y axis displays the residual variance in mean arousal rating after the variance due to task‐evoked amygdala activation has been removed; the scatterplot illustrates the relationship between adjusted arousal rating and task‐free dorsal amygdala connectivity within the salience network. (B) Individuals that displayed both increased task‐evoked amygdala activation and stronger task‐free dorsal amygdala connectivity within the salience network had the highest mean arousal rating while viewing negative images (total variance in mean arousal rating predicted by the independent variables was R 2 = 0.45, p < 0.01). Individuals with the highest level of mean arousal rating are shown in red, those with a medium level of mean arousal rating are shown in white and those with the lowest level of mean arousal rating are shown in blue. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
We next explored the specific regions within the salience network that were driving the relationship between the task‐free dorsal amygdala connectivity and feelings of arousal (Fig. 6). This exploratory analysis allowed us to localize the regions within the salience network that best predicted feelings of arousal in response to negative images. Our findings revealed that connectivity between the dorsal amygdala seed and subcortical regions within the salience network were the best predictors of arousal feelings. Individuals with heightened feelings of arousal had stronger connectivity between the left dorsal amygdala and a relatively large cluster in right anterior and ventromedial posterior thalamus. They also had stronger connectivity between the left dorsal amygdala and right anterior hippocampus as well as bilateral posterior hippocampi, lateral putamen, a region near the ventral tegmental area/substantia nigra bilaterally (see Table 2). Task‐free connectivity between dorsal amygdala and a small cluster in anterior cingulate cortex also predicted feelings of arousal ratings at a more liberal threshold (p < 0.1), however (see Fig. S3 in Supporting Information).
Figure 6.
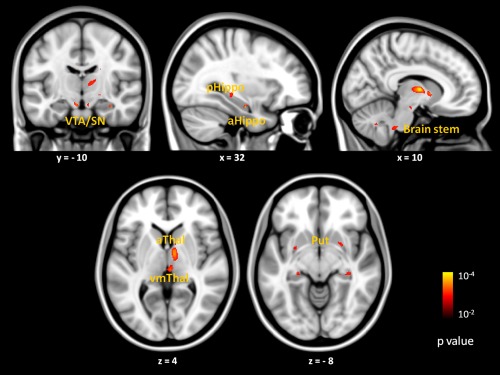
Exploratory analyses revealed that task‐free connectivity between the left dorsal amygdala and subcortical regions within the salience network (dorsal amygdala network previously defined in Bickart et al. [2012]) were the best predictors of feelings of arousal. Brain images show location of voxels where connectivity with dorsal amygdala at rest correlated with mean arousal rating at p < 0.01, uncorrected with a cluster size constraint of 10 voxels. Color bars indicate the p values (10−2 to 10−4) of correlated voxels, which are overlaid on slices of a T1 MNI152 1.0 mm template brain in radiologic convention. Abbreviations: VTA/SN, ventral tegmental area/substantia nigra; pHippo, posterior hippocampus; aHippo, anterior hippocampus; aThal, anterior thalamus; vmThal, ventromedial thalamus; Put, putamen. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Regions within the dorsal amygdala connectivity within the salience network (defined in Bickart et al. [2012] that correlated with feelings of arousal in an exploratory analysis in our brain‐behavior sample (N = 25)
| MNI coordinates of peak voxels | |||
|---|---|---|---|
| x | y | z | |
| Anterior thalamus | 10 | −6 | 6 |
| −6 | −10 | 8 | |
| Ventromedial thalamus | 6 | −22 | 6 |
| −6 | −22 | 8 | |
| Anterior hippocampus | 30 | −10 | −20 |
| −30 | −8 | −22 | |
| Posterior hippocampus | 30 | −30 | −8 |
| −30 | −32 | −6 | |
| Putamen | 22 | 6 | −8 |
| −26 | 0 | −6 | |
| Ventral tegmental area/substantia nigra | 8 | −12 | −18 |
| −8 | −12 | −16 | |
| Brain stem | 10 | −18 | −16 |
| −4 | −16 | −16 | |
Note. This table displays MNI coordinates for voxels within the dorsal amygdala connectivity network in which connectivity strength correlated with mean arousal rating at p < 0.01, uncorrected with a cluster size constraint of 10 voxels.
A final set of analyses characterized the anatomical and behavioral specificity of the results reported thus far. Task‐free connectivity of the medial and ventrolateral amygdala was not related to feelings of arousal while viewing novel negative images (r = 0.25, p < 0.23 and r = −0.03, p < 0.89, respectively). Multiple regression analysis that compared the relative contributions of task‐free connectivity of the dorsal, medial, and ventrolateral amygdala networks in predicting feelings of arousal showed that task‐free connectivity within the dorsal amygdala network was the best predictor of arousal ratings (see Table SII in Supporting Information). Task‐evoked amygdala activation and task‐free connectivity were not related to arousal ratings to positive images (r = 0.10, p < 0.63 and r = −0.15, p < 0.48, respectively).
DISCUSSION
The most novel contribution of this study is the observation that both task‐evoked amygdala activation and task‐free amygdala connectivity independently contribute to explaining individual differences in emotional arousal. To our knowledge, the complementarity of these imaging measures has not yet been reported for any brain network or behavior referable to that network. Previous work has shown that task‐evoked amygdala activity relates to individual differences in feelings of arousal [Barrett et al., 2007; Colibazzi et al., 2010; Gerdes et al., 2010; Phan et al., 2004; Wilson‐Mendenhall et al., 2013] and separately that task‐free amygdala connectivity relates to individual differences in arousal [Kim et al., 2010; Seeley et al., 2007]. We extended this work to show that task‐evoked amygdala activity and task‐free amygdala connectivity are not redundant measures, but rather that both contribute uniquely to the intensity of reported feelings of arousal. That is, people who feel the emotions evoked by negative pictures most intensely have the greatest amygdala reactivity to the pictures and the strongest task‐free amygdala connectivity within the salience network. Our findings indicate that in addition to its reactivity, the amygdala's connectivity to other regions within the salience network plays an independent, additive role in subserving feelings of arousal.
The amygdala functions within a broad network of regions that share both anatomical connectivity and a common role in detecting and guiding reactions to salient stimuli. Tract‐tracing studies in macaques demonstrate that nuclei within the dorsal amygdala and their cellular extensions into the substantia innominata share convergent anatomical connections with the middle cingulate cortex and anterior insula, projecting to interoceptive and pain‐related targets in the insula and the rostrally adjacent orbitofrontal cortex, somatosensory operculum, ventrolateral striatum, caudolateral hypothalamus, as well as autonomic and dopaminergic nuclei of the brainstem [An et al., 1998; Carmichael and Price, 1996; Ferry et al., 2000; Fudge et al., 2002; Haber and Calzavara, 2009; Haber and Knutson, 2010; Haber et al., 2006; Hsu and Price, 2007; Kondo et al., 2003; Kondo et al., 2005; Kunishio and Haber, 1994; McDonald, 1991a,b; Ongur and Price, 2000; Ongur et al., 1998, 2003; Price and Drevets, 2010; Price, 2007; Saleem et al., 2008]. These regions also share a common role in detecting, learning about, and responding to salient, often aversive stimuli [Balleine and O'Doherty, 2010; Craig, 2009; Hayes and Northoff, 2011; Knapska et al., 2007; Murray, 2007; Waraczynski, 2006]. Perhaps the strength of the amygdala's connectivity within the salience network, as measured with task‐free functional neuroimaging, reflects how efficiently it communicates the salience of stimuli to the other regions in the network. It is also possible that the amygdala's connectivity strength at rest contributes to the basal tone of the autonomic nervous system, which biases the system to respond more or less vigorously to salient stimuli.
Whereas prior work has demonstrated a relationship between task‐free connectivity and task‐evoked activity of regions of the sensorimotor cortex [Fox et al., 2007] or within the frontoparietal network [Mennes et al., 2010, 2011] we found no relationship between the task‐free connectivity and task‐evoked activation of the amygdala within the salience network. This suggests regional variation in the degree to which task‐evoked dynamic responses relate to spontaneous BOLD signal fluctuations. This interpretation would be consistent with recent findings showing that correspondence between task‐evoked coactivation patterns and resting‐state connectivity patterns is low for limbic subcortical regions but high for association areas that span the frontoparietal cortex [Mennes et al., 2013].
Our findings show that although task‐free connectivity and task‐evoked activity of the amygdala are independent from each other, both types of brain activity combine to realize affective experience. Because dynamic stimulus‐driven neural responses and basal spontaneous signal fluctuations each provide only one neural correlate of individual differences in behavior, it would seem valuable for future task‐related fMRI studies of such differences to also collect resting state data when possible. Although our study employed an affective paradigm and compared the task‐free connectivity versus task‐evoked activations of regions within the salience network, it is possible that our findings would generalize to other networks.
One limitation of this study is that the present design focused on functional MRI measures alone. Recent studies, however, suggest that variation in the amygdala volume and its anatomical coupling to medial prefrontal cortex also relate to individual differences in negative affect [Holmes et al., 2012; Kim and Whalen, 2009]. Whether structural MRI and diffusion‐based tractography measures predict individual differences in feelings of arousal over and above task‐evoked activity and task‐free connectivity remains an important issue to pursue in subsequent studies. Furthermore, our paradigm included only a state‐level measure of affective feelings of arousal. Task‐evoked fMRI and task‐free functional connectivity MRI, however, reflect state‐dependent as well as trait‐level sources of variance [Buckner, 2010; Buckner et al., 2013]. Future studies should examine the relative contributions of state and trait on task‐evoked activation versus task‐free functional connectivity. Additionally, there has been some evidence that attending to subjective experience during the viewing of evocative material increases the activity of the affective circuitry [Taylor et al., 2003]. However, other studies have found no effect of attending to ones subjective experience [McClure et al., 2007]. Future studies should examine the effects of judgments of arousal on task‐evoked amygdala responses to emotionally evocative stimuli. Finally, as this study included only healthy young adults, most of whom (16 of 25) were women, further research will be needed to assess whether these findings generalize to more diverse populations.
CONCLUSION
Individual differences in the intensity of feelings of arousal while viewing affectively potent images is associated with two types of fMRI signals in the amygdala, the magnitude of task‐evoked amygdala BOLD response and the task‐free amygdala connectivity strength within the salience network. We demonstrate that greater task‐evoked amygdala activation and stronger task‐free amygdala connectivity within the salience network each contribute independently to feelings of arousal to negative images. Importantly, we show that individuals who have both increased task‐evoked amygdala activation and stronger task‐free amygdala connectivity within the salience network have the most heightened levels of arousal.
Supporting information
Supplementary Information
Supplementary Information
ACKNOWLEDGMENTS
The authors thank Randy Buckner for providing the preprocessing/rs‐fcMRI tools and Ian Kleckner for his assistance in constructing Figure 4. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, or the National Institute on Aging.
REFERENCES
- Adolphs R (2008): Fear, faces, and the human amygdala. Curr Opin Neurobiol 18:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X, Bandler R, Ongur D, Price JL (1998): Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol 401:455–479. [PubMed] [Google Scholar]
- Andreano JM, Dickerson BC, Barrett LF (2013): Sex differences in the persistence of the amygdala response to negative material. Soc Cogn Affect Neurosci. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS (2004): Lateralization of amygdala activation: A systematic review of functional neuroimaging studies. Brain Res Brain Res Rev 45:96–103. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP (2010): Human and rodent homologies in action control: Corticostriatal determinants of goal‐directed and habitual action. Neuropsychopharmacology 35:48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Bliss‐Moreau E, Duncan SL, Rauch SL, Wright CI (2007): The amygdala and the experience of affect. Soc Cogn Affect Neurosci 2:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC (2012): Intrinsic amygdala‐cortical functional connectivity predicts social network size in humans. J Neurosci 32:14729–14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR (1996): Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17:875–887. [DOI] [PubMed] [Google Scholar]
- Buckner RL (2010): Human functional connectivity: New tools, unresolved questions. Proc Natl Acad Sci USA 107:10769–10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BT (2013): Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 16:832–837. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL (1996): Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 371:179–207. [DOI] [PubMed] [Google Scholar]
- Colibazzi T, Posner J, Wang Z, Gorman D, Gerber A, Yu S, Zhu H, Kangarlu A, Duan Y, Russell JA, Peterson BS (2010): Neural systems subserving valence and arousal during the experience of induced emotions. Emotion 10:377–389. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009): How do you feel—Now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ (2001): The amygdala: Vigilance and emotion. Mol Psychiatry 6:13–34. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, Tranel D (2011): The human amygdala and the induction and experience of fear. Curr Biol 21:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, Welsh MJ, Tranel D, Wemmie JA (2013): Fear and panic in humans with bilateral amygdala damage. Nat Neurosci 16:270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry AT, Ongur D, An X, Price JL (2000): Prefrontal cortical projections to the striatum in macaque monkeys: Evidence for an organization related to prefrontal networks. J Comp Neurol 425:447–470. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME (2007): Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56:171–184. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN (2002): Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience 110:257–275. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli P, Placentino A, Carletti F, Allen P, Landi P, Abbamonte M, Barale F, Perez J, McGuire P, Politi PL (2009): Laterality effect on emotional faces processing: ALE meta‐analysis of evidence. Neurosci Lett 452:262–267. [DOI] [PubMed] [Google Scholar]
- Gerdes AB, Wieser MJ, Muhlberger A, Weyers P, Alpers GW, Plichta MM, Breuer F, Pauli P (2010): Brain activations to emotional pictures are differentially associated with valence and arousal ratings. Front Hum Neurosci 4:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Calzavara R (2009): The cortico‐basal ganglia integrative network: The role of the thalamus. Brain Res Bull 78:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R (2006): Reward‐related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive‐based learning. J Neurosci 26:8368–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DJ, Northoff G (2011): Identifying a network of brain regions involved in aversion‐related processing: Across species translational investigation. Front Integr Neurosci 5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, Buckner RL (2012): Individual differences in amygdala‐medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. J Neurosci 32:18087–18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Price JL (2007): Midline and intralaminar thalamic connections with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol 504:89–111. [DOI] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF (2005): Neural systems responding to degrees of uncertainty in human decision‐making. Science (New York, N.Y.) 310:1680–1683. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ (2009): The structural integrity of an amygdala‐prefrontal pathway predicts trait anxiety. J Neurosci 29:11614–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ (2010): Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex 21:1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Radwanska K, Werka T, Kaczmarek L (2007): Functional internal complexity of amygdala: Focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev 87:1113–1173. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss‐Moreau E, Lindquist KA, Wager TD (2008): Functional grouping and cortical‐subcortical interactions in emotion: A meta‐analysis of neuroimaging studies. Neuroimage 42:998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL (2003): Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol 465:499–523. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL (2005): Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol 493:479–509. [DOI] [PubMed] [Google Scholar]
- Kunishio K, Haber SN (1994): Primate cingulostriatal projection: Limbic striatal versus sensorimotor striatal input. J Comp Neurol 350:337–356. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN (1997): International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: NIMH Center for the Study of Emotion and Attention. [Google Scholar]
- Lewis PA, Critchley HD, Rotshtein P, Dolan RJ (2007): Neural correlates of processing valence and arousal in affective words. Cereb Cortex 17:742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss‐Moreau E, Barrett LF (2012): The brain basis of emotion: A meta‐analytic review. Behav Brain Sci 35:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, Fromm S, Charney DS, Leibenluft E, Ernst M, Pine DS (2007): Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry 64:97–106. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1991a): Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience 44:1–14. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1991b): Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal‐like areas of the rat brain. Neuroscience 44:15–33. [DOI] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal B, Castellanos X, Milham MP (2010): Inter‐individual differences in resting state functional connectivity predict task‐induced BOLD activity. NeuroImage 50:1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B, Castellanos FX, Milham MP (2011): Linking inter‐individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage 54:2950–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Colcombe S, Castellanos FX, Milham MP (2013): The extrinsic and intrinsic functional architectures of the human brain are not equivalent. Cereb Cortex 23:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Negreira A, Weierich M, Dautoff R, Dickerson BC, Wright CI, Barrett LF (2011): Differential hemodynamic response in affective circuitry with aging: An fMRI study of novelty, valence, and arousal. J Cogn Neurosci 23:1027–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ (1996): A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383:812–815. [DOI] [PubMed] [Google Scholar]
- Murray EA (2007): The amygdala, reward and emotion. Trends Cogn Sci 11:489–497. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL (2000): The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10:206–206. [DOI] [PubMed] [Google Scholar]
- Ongur D, An X, Price JL (1998): Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol 401:480–505. [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL (2003): Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460:425–449. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I (2004): Neural correlates of individual ratings of emotional salience: A trial‐related fMRI study. Neuroimage 21:768–780. [DOI] [PubMed] [Google Scholar]
- Price JL (2007): Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann N Y Acad Sci 1121:54–71. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC (2010): Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Barrett LF (1999): Core affect, prototypical emotional episodes, and other things called emotion: Dissecting the elephant. J Pers Social Psychol 76:805–819. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL (2008): Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol 506:659–693. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I (2003): Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage 18:650–659. [DOI] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Barrett LF (2012): Dissociable large‐scale networks anchored in the anterior insula subserve affective experience and attention/executive function NeuroImage 60:1947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL (2010): Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME (2007): Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447:83–86. [DOI] [PubMed] [Google Scholar]
- Waraczynski MA (2006): The central extended amygdala network as a proposed circuit underlying reward valuation. Neurosci Biobehav Rev 30:472–496. [DOI] [PubMed] [Google Scholar]
- Weierich MR, Wright CI, Negreira A, Dickerson BC, Barrett LF (2010): Novelty as a dimension in the affective brain. NeuroImage 49:2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson‐Mendenhall CD, Barrett LF, Barsalou L (2013): Neural evidence that human emotions share core affective properties. Psychol Sci 24:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS (2006): Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiol Aging 27:361–374. [DOI] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW (2010): Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain 133:1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information


