Abstract
Functional magnetic resonance imaging (fMRI) was used to study memory-associated activation of medial temporal lobe (MTL) regions in 32 nondemented elderly individuals with mild cognitive impairment (MCI). Subjects performed a visual encoding task during fMRI scanning and were tested for recognition of stimuli afterward. MTL regions of interest were identified from each individual’s structural MRI, and activation was quantified within each region. Greater extent of activation within the hippocampal formation and parahippocampal gyrus (PHG) was correlated with better memory performance. There was, however, a paradoxical relationship between extent of activation and clinical status at both baseline and follow-up evaluations. Subjects with greater clinical impairment, based on the Clinical Dementia Rating Sum of Boxes, recruited a larger extent of the right PHG during encoding, even after accounting for atrophy. Moreover, those who subsequently declined over the 2.5 years of clinical follow-up (44% of the subjects) activated a significantly greater extent of the right PHG during encoding, despite equivalent memory performance. We hypothesize that increased activation in MTL regions reflects a compensatory response to accumulating AD pathology and may serve as a marker for impending clinical decline.
Medial temporal lobe (MTL) structures are essential for memory function. These regions, particularly the hippocampal formation and entorhinal cortex, bear a heavy neuropathological burden very early in the course of Alzheimer’s disease (AD), even before clinical diagnostic criteria for dementia are met.1,2 Using volumetric magnetic resonance imaging (MRI), we can detect MTL atrophy in vivo in elderly individuals with memory impairment, and these measures correlate with memory task performance3,4 and are useful for the identification of subgroups of persons who will progress to a clinical diagnosis of AD within a few years.5–10 However, despite considerable data on the structural correlates of memory impairment in prodromal and very early AD, less is known about the effects of the neurodegenerative process on the functional capacity of these brain regions as measured by functional MRI (fMRI).
fMRI paradigms have been developed that reliably activate MTL regions during memory tasks.11–15 Most fMRI studies of AD have been performed in clinically diagnosed patients with mild-to-moderate dementia and have found decreased MTL activation when subjects attempt to learn new information.16–21
It is not yet clear when in the course of prodromal AD functional activity in the MTL declines, or whether the slope of decline is linear across the range of impairment among individuals at risk for AD. A recent fMRI study of clinic patients with mild cognitive impairment (MCI) showed decreased MTL activation during a memory encoding task.21 However, Small and colleagues16 found that only a subgroup of subjects with “isolated memory decline” demonstrated decreased hippocampal activation during encoding, whereas Bookheimer and colleagues22 reported increased MTL activation in cognitively intact individuals genetically at risk for AD.
These fMRI results may vary because the groups being studied were composed of subjects with differing degrees of impairment. It is increasingly clear that elderly individuals with mild memory impairments represent a relatively heterogeneous group, with a broad range of functional and cognitive difficulties. For example, in a prospective study of memory-impaired nondemented elders, Daly and colleagues23 found that the likelihood that subjects would be diagnosed with AD within 3 years ranged widely, from 0 to 67%, and was highly correlated with the degree of functional impairment in daily life at initial evaluation (based on the summary measure from a standardized clinical scale, the Clinical Dementia Rating Sum of Boxes [CDR-SB]24). This variability in outcome is likely a manifestation of differences in the severity of neuropathology among subjects. It is not yet known whether, within such a subject group, fMRI can be used to detect variability in regional brain activation that is meaningfully associated with degree of impairment or clinical outcome.
Another potential contributor to differences in the results of fMRI studies of prodromal AD is task performance. It appears that the degree of activation detected by fMRI within MTL regions during encoding strongly relates to the subjects’ subsequent ability to remember the items encoded.12–14,25,26 Decreased MTL activation in MCI and AD patients has been associated with relatively poor performance on postscan memory testing.17,18,20,21 In contrast, subjects who were genetically at risk for AD, but could successfully perform the fMRI encoding task, showed increased MTL activation; it has been hypothesized that this may represent a compensatory response that allows for relatively normal memory function (and task performance) in the face of developing pathological change.22 It is not yet known whether there may be an increased MTL response, associated with relatively preserved fMRI memory task performance, in elderly subjects at risk for AD due to mild memory problems.
To address these questions, we performed an fMRI study of visual memory in nondemented elderly individuals clinically at risk for AD based on evidence of functional difficulty in daily life. The purposes of the study were to determine whether variability in activation of the MTL during a picture-encoding task showed systematic relationships to (1) the degree of impairment in daily life, as measured by the CDR-SB; (2) performance on a postscan recognition memory task; or (3) hippocampal and/or parahippocampal volume. Brain regions of interest (ROIs) were identified on structural MRI scans to quantify the extent of fMRI activity within each ROI and determine its relationship to the volume of the region. In addition, longitudinal clinical follow-up data were available on the subjects, enabling a comparison of the MTL activation in individuals who demonstrated progressive clinical decline over time versus those who remained stable.
Subjects and Methods
Subjects
The subjects in the study consisted of 32 older individuals, all of whom provided informed consent in accordance with the Human Research Committee guidelines of the Massachusetts General Hospital. They were drawn from participants in a longitudinal study examining preclinical predictors of AD8,23,27,28 and were selected with the goal of studying nondemented subjects demonstrating a range of cognitive and functional impairment.
Recruitment and Selection Criteria
The longitudinal study participants were recruited through the print media (rather than from a clinical or other medical referral source). Volunteers underwent an extensive clinical, neuropsychological, and laboratory (including apolipoprotein E (APOE) genotyping) evaluation. To be included in this study, participants had to be 65 years or older, free of significant underlying medical, neurological, or psychiatric illness, and meet criteria for MCI: (1) demonstrate a memory complaint corroborated by an informant, and (2) be nondemented by having generally normal cognitive function and intact activities of daily living. Objective evidence of memory impairment was not required, but many participants had memory test scores that were 1.5 standard deviations (SDs) below the mean of their age peers.
Clinical Evaluation and Follow-up
The clinical evaluation, central to the categorization of subjects, was based on the Initial Subject Protocol used in the development of the CDR scale24 and has been described in detail elsewhere.23 The CDR is a structured clinical assessment instrument widely used in the evaluation of this subject population by research groups (eg, the Alzheimer’s Disease Cooperative Study).29 It includes a semistructured history focused on cognitive and functional status, asked of the subject and a collateral informant, and a standardized neurological, psychiatric, and mental status evaluation. In this study, each evaluation was administered by a skilled clinician, took 1 to 2 hours to complete, and was used to generate an overall CDR rating and the CDR-SB score (the sum of the ratings in each of the six CDR subcategories). The mean interrater reliability of the CDR ratings was high (r = 0.99, p < 0.0001), as was that of the six CDR subcategories (r = 0.90).23 For this study, subjects were required to have an overall CDR rating of 0.5, with at least a 0.5 in the memory subcategory.
The clinical evaluation was repeated annually to quantify any progression in functional difficulty, thus generating an overall CDR rating and CDR-SB score annually. For the purposes of this report, “decline” in functional ability was defined as an increase of 1.0 or more points in the CDR-SB score. Subjects who became demented were diagnosed according to standardized criteria.30,31
Magnetic Resonance Imaging Procedures
DATA ACQUISITION
The subjects in the study were scanned on two different 1.5T MRI scanners: a General Electric (GE) Signa (Advanced NMR Systems, Wilmington, MA) scanner and a Siemens Sonata (Siemens Medical Systems, Iselin, NJ) scanner. A “scanner” variable was used in the statistical analyses to determine whether any findings were influenced by differences between the two scanning systems.
First, high-resolution structural data were acquired (GE SPGR sequence: TR/TE, 35/5 milliseconds; field of view, 240; FA, 45 degrees; 124 coronal slices; thickness, 1.5mm; matrix, 256 × 256; Siemens MP-RAGE sequence: TR/TI/TE, 2,730/1,000/3 milliseconds; field of view, 256; FA, 7 degrees; 128 sagittal slices; thickness, 1.33mm; matrix, 192 × 256). Next, blood oxygen level dependent (BOLD) functional data were acquired (GE asymmetric spin echo sequence: TR/TE, 2,500/70 milliseconds; FA, 90 degrees; 20 slices, 7mm thick with 1mm gap; voxel dimensions, 3.125mm2; Siemens gradient echo T2* sequence: TR/TE, 2,500/40 milliseconds; FA, 90 degrees; 29 slices, 5mm thick with 1mm gap; voxel dimensions, 3.125mm2). Functional data were acquired in an oblique coronal orientation beginning at the occipital pole, perpendicular to the anterior-posterior commissure line, to maximize in-plane resolution in the hippocampus. Scanning time for each functional run was 4 minutes and 15 seconds, consisting of 102 time points (4 for T1 stabilization and 98 for data collection).
FUNCTIONAL MAGNETIC RESONANCE IMAGING ACTIVATION TASK
The activation task consisted of three conditions that were alternated in blocks during each scanning run: 1) fixation: subjects viewed a white fixation cross-hair on a black background; 2) novel: subjects viewed 12 novel scenes per block and were asked to try to remember them; 3) repeated: subjects viewed four scenes, previously viewed during a practice trial, repeated in the same order, three times each per block. Each of six scanning runs consisted of the following blocks: fixation (6 seconds), novel (36 seconds), fixation (24 seconds), repeated (36 seconds), fixation (24 seconds), novel (36 seconds), fixation (24 seconds), repeated (36 seconds), fixation (6 seconds). Before these scanning runs, subjects underwent a practice run that was not scanned to assure that they could see the stimuli clearly, and to familiarize them with the scenes that would later be used in the “repeated” condition. The visual scenes, presented for 3 seconds each, consisted of 148 complex color pictures (4 repeated scenes, 144 novel scenes) obtained from a commercial collection of digitized photographs (Corel Corporation, Dallas, TX) and were presented using a standard fMRI projection system.32 This task was based on a paradigm developed by Stern and colleagues.11
Twenty minutes after exiting the scanner, subjects were tested for their memory of the novel scenes in a forced choice 50-item yes/no recognition memory test, using a subset of 25 of the novel scenes (drawn equally from each of the six runs) and an equal number of distractors. Recognition memory performance was calculated as the percentage of previously viewed pictures that were correctly recognized as having been seen before.
Data Analysis
MAGNETIC RESONANCE IMAGING DATA ANALYSIS
Each of the six functional MRI runs was motion-corrected to the first run using AFNI (http://afni.nimh.nih.gov/afni/index.shtml) and then spatially smoothed using a three-dimensional Hanning filter (FWHM = 5mm). The stimulus effects at each voxel were estimated by fitting the amplitudes of two boxcar functions (one for novel and one for repeated conditions) convolved with a gamma function to the BOLD signal across all runs.33 The boxcar was delayed by 5 seconds from block onset to account for the hemodynamic delay. A baseline offset and linear trend also were fit for each run. The residual error was used to estimate the variance of the noise.33
Each subject’s fMRI data set was coregistered to that subject’s structural MRI data set so that each individual’s fMRI data could be localized with reference to their own neuroanatomical space.34 Activation maps were generated for two contrasts: novel versus fixation, which contrasted the encoding of novel complex scenes with visual fixation; and novel versus repeated, which held the visual complexity of the stimuli constant, and thus provided information on the encoding of novel scenes compared with the viewing of familiar scenes.
The structural MRI data also were used to generate three regions of interest: the hippocampal formation (hippocampus proper, dentate gyrus, and subiculum), parahippocampal gyrus (including the entorhinal cortex), and striate cortex (used as a control). ROIs were drawn manually, by a skilled operator, on multiple slices of the structural MRI in both the right and left hemisphere, for a total of six ROIs. Reliability data for these procedures have been reported previously.8,35 Given the goal of relating each subject’s structural MRI data to his/her functional MRI data, the volumes of the structural ROIs were not normalized to total intracranial volume. Because of the limited resolution of fMRI data and evidence that activation extended over a relatively large portion of the parahippocampal ROI, we opted to include the entorhinal cortex in the parahippocampal ROI.
The extent and magnitude of fMRI activation were examined within each ROI, using a modification of a previously reported method.32 Extent of activation was defined as the number of voxels activated over the significance threshold (p < 0.01) within the structural ROI (ie, number of significantly activated voxels for a given contrast). We did not divide by total number of voxels in the ROI; instead, structural ROI volume was used as a separate variable in statistical analyses.
STATISTICAL ANALYSES
Pearson correlations and partial correlations (adjusting for covariates) were performed to examine relationships among the primary variables of interest. A multiple linear regression model was developed to assess the degree to which these variables were related to clinical status. Analyses of variance (ANOVAs), with post hoc planned comparisons, were performed to evaluate differences between subjects with specific clinical characteristics of interest.
Results
Clinical Status of Subjects
All subjects in the study were nondemented and had an overall CDR rating of 0.5. They nevertheless had a range of degrees of memory impairment and mild functional difficulty in daily life, with CDR-SB scores ranging from 0.5 to 3.0 (at least 0.5 in the memory subcategory). Mini-Mental State Examination36 (MMSE) scores varied from 27 to 30. The mean total learning score for the California Verbal Learning Test37 was 47.4; of the 32 subjects, 14 scored 1.5 SDs lower than the mean of age and education equivalent controls. As noted above, none of the subjects met clinical criteria for dementia at the time of scanning (baseline). The baseline demographic, clinical and psychometric data are presented in Table 1.
Table 1.
Baseline Demographic and Clinical Characteristics of Subjects (n = 32)
| Range | Mean | SD | |
|---|---|---|---|
| Age (yr) | 65–88 | 75 | 5.9 |
| Education (yr) | 7–21 | 16 | 3.0 |
| Sex (M/F) | 16/16 | ||
| APOE-ε4 carriers, n (%) | 7 (22) | ||
| CDR-Sum Boxes | 0.5–3.0 | 1.80 | 0.83 |
| MMSE | 27–30 | 29.4 | 0.82 |
| CVLTa | 29–67 | 47.4 | 10.5 |
Total learning score.
APOE = apolipoprotein E; CDR = Clinical Dementia Rating; MMSE = Mini-Mental State Examination; CVLT = California Verbal Learning Test.
Performance on Functional Magnetic Resonance Imaging Activation Task
Recognition performance on the post scan memory testing ranged from 60% to 92% accuracy (mean correct, 78.6%; SD, 9.3%). Performance on this test was not correlated with the subjects’ age, education, or degree of clinical impairment, as measured by the CDR-SB (p > 0.42)
Relationship of Functional Magnetic Resonance Imaging Activity and Postscan Memory Performance
Performance on the postscan memory test was significantly correlated with the extent of fMRI activity within the ROIs for the hippocampal formation (HF) and parahippocampal gyrus (PHG), but not to that within the striate cortex (ie, the control region; Table 2). For the novel versus fixation contrast, activity in the HF and PHG (bilaterally) was significantly associated with postscan memory performance (r = 0.49–0.63), with the right PHG showing the strongest relationship. Similar findings were demonstrated in the novel versus repeated (NvR) contrast for the HF and PHG, bilaterally (r = 0.46–0.60), with the right PHG again showing the strongest relationship. There was no correlation between California Verbal Learning Test performance and fMRI activity in any ROI. Figures 1 and 2 provide an example of image data from a single subject.
Table 2.
Correlations between Functional and Structural MRI Variables and Postscan Memory Task Performance (r)
| Extent of fMRI Activation |
fMRI Activation (Adjusted for Volume) |
||||
|---|---|---|---|---|---|
| ROI | NvF | NvR | Volume of ROI | NvF | NvR |
| Left HF | 0.53a | 0.47a | 0.38 | 0.48a | 0.42 |
| Right HF | 0.49a | 0.46a | 0.47a | 0.44 | 0.38 |
| Left PHG | 0.61b | 0.48a | 0.39 | 0.53a | 0.42 |
| Right PHG | 0.63b | 0.60b | 0.44 | 0.51a | 0.49a |
| Left striate | 0.29 | 0.29 | 0.14 | 0.26 | 0.26 |
| Right striate | 0.37 | 0.39 | 0.19 | 0.32 | 0.35 |
ROI = region of interest; HF = hippocampal formation; PHG = parahippocampal gyrus; fMRI = functional magnetic resonance imaging; NvF = novel vs fixation; NvR = novel vs repeated.
p < 0.05;
p < 0.01.
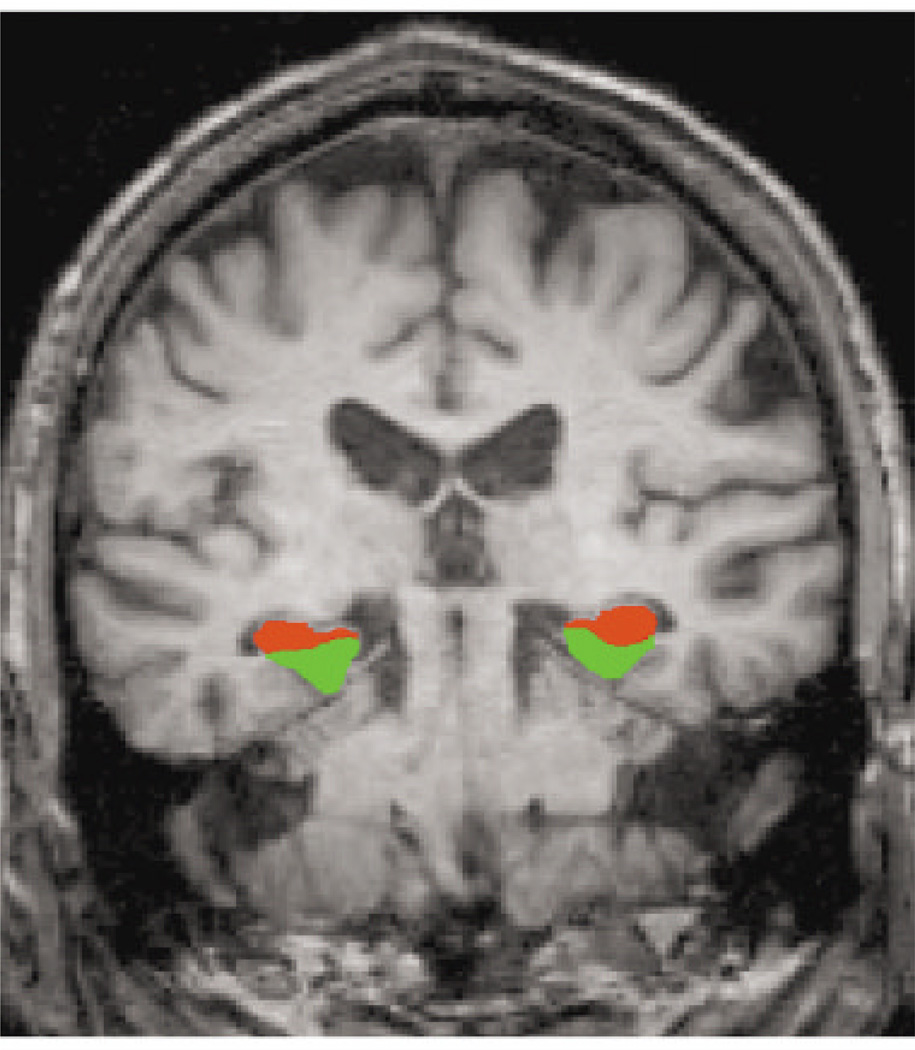
Fig 1.
This coronal magnetic resonance image (MRI) displays the regions of interest (ROIs) for the hippocampal formation (red) and parahippocampal gyrus (green). ROIs were manually delineated from each individual subject’s structural MRI.
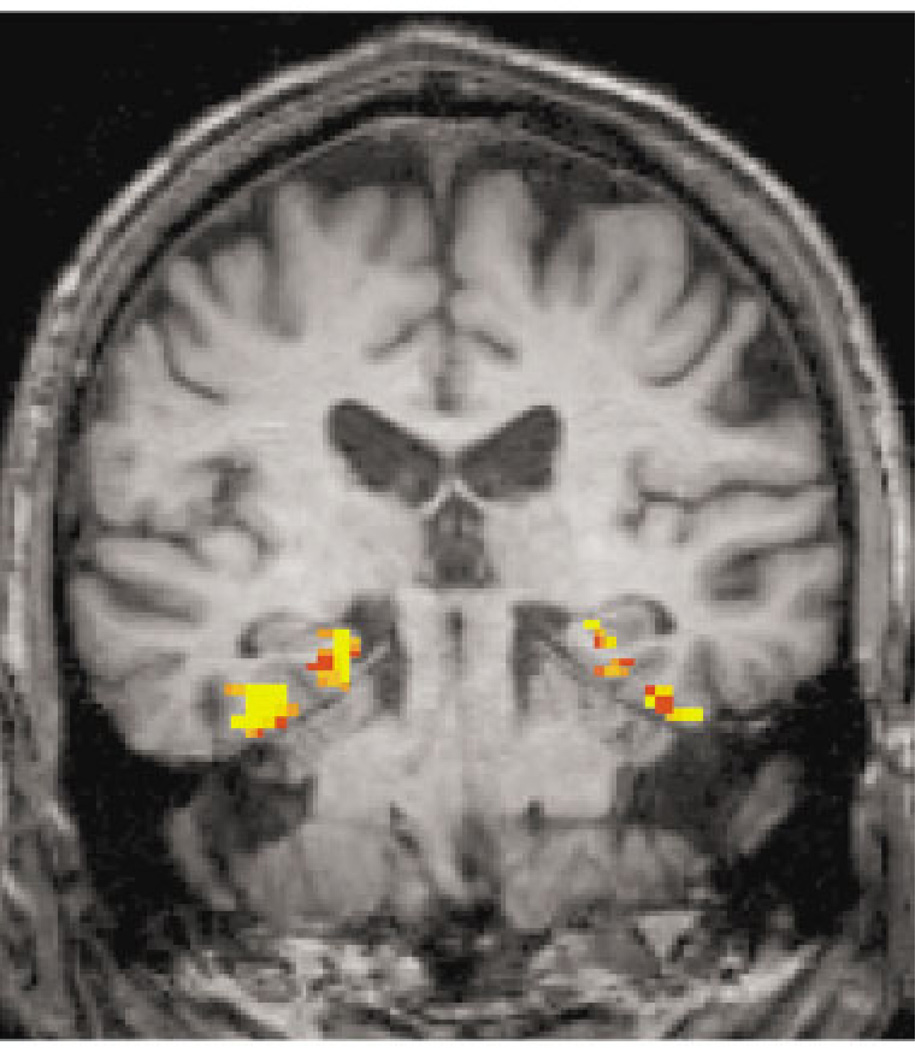
Fig 2.
Example of a functional magnetic resonance image activation map for the novel versus repeated contrast (comparing the encoding of novel pictures vs repeated pictures; threshold p < 0.01).
We then examined these same relationships, adjusting for the volume of each ROI (see Table 2). For the novel versus fixation contrast, the extent of activation in the left HF and the PHG (bilaterally) remained significantly correlated with memory performance (r = 0.48–0.53) after this adjustment. Likewise, for the NvR contrast, the extent of activation in the right PHG remained significantly correlated with memory performance after adjusting for its volume (r = 0.49). Thus, for a given volume of right PHG tissue, a greater extent of tissue activated during the encoding of novel versus repeated scenes was associated with better performance on postscan memory testing.
Correlations between the volume of each ROI and postscan memory performance also were examined (see Table 2). Recognition memory performance was significantly correlated with right HF volume (r = 0.47; p < 0.05); that is, a larger right HF was associated with better memory performance.
Relationship of Clinical Impairment, Functional Magnetic Resonance Imaging Activation and Region of Interest Volume
To examine this relationship, we performed a stepwise multiple linear regression with the CDR-SB score as the dependent variable. The independent variables included in the analysis were age, education, APOE-ε4 genotype (carrier or noncarrier), the volumes of each of the three ROIs (right and left), the extent of fMRI activation during the novel versus repeated contrast for each of the three ROIs (right and left), and scanner type (GE or Siemens). The overall model was statistically significant (R2 = 0.56; F = 11.99; p < 0.001). Three variables entered the regression model: age, right PHG fMRI activation, and left HF structural volume. The beta weights for these variables were age (β = 0.35; p < 0.01), right PHG fMRI activation (β = 0.33; p < 0.03), and left HF volume (β = −0.71; p < 0.001). The beta weights indicate that greater clinical impairment (as measured by the CDR-SB) was associated with older age, greater extent of fMRI activation in the right PHG, and smaller volume of the left HF.
Longitudinal Follow-up of Participants
Longitudinal clinical follow-up data were available on all 32 of the participants in the study. The mean duration of follow-up of the subjects, after completion of the fMRI scanning procedure, was 2.5 years (SD, 0.9 years). Fourteen subjects declined by 1.0 or more on the CDR-SB and thus met criteria for “decliner” (mean decline, 1.6; SD, 0.7). Of those who declined, four were diagnosed with probable AD. Three of the seven individuals with an APOE-ε4 allele declined. Eighteen of the subjects did not decline by 1.0 or more on the CDR-SB and were categorized as “stable.” Table 3 displays the baseline demographic and clinical data for these two groups.
Table 3.
Baseline Demographic and Clinical Characteristics of Subjects who Declined on the CDR-SB Score (≥1) at Follow-up vs Those Who Remained Stable (Mean ± SD)
| Characteristic | Stable | Decliner |
|---|---|---|
| No. of subjects | 18 | 14 |
| Age (yr) | 74.7 ± 5.0 | 74.8 ± 7.2 |
| Education (yr) | 16.0 ± 3.9 | 16.1 ± 1.3 |
| Sex (M/F) | 10/8 | 6/8 |
| APOE-ε4 carriers, n (%) | 4 (22) | 3 (21) |
| CDR–Sum of Boxes | 1.7 ± 0.9 | 1.9 ± 0.7 |
| MMSE | 29.7 ± 0.5 | 28.9 ± 0.9a |
| CVLTb | 47.7 ± 10.8 | 47.0 ± 10.5 |
| Postscan memoryc | 76 ± 10% | 82 ± 8% |
SD = standard deviation; APOE = apolipoprotein E; CDR = Clinical Dementia Rating; MMSE = Mini-Mental State Examination; CVLT = California Verbal Learning Test.
p < 0.005.
Total learning score.
Postscan recognition memory test performance, % correct.
Relationship of Baseline Clinical Variables to Follow-up Status
A two-way ANOVA was used to compare the baseline clinical variables for the decliner and stable subjects, with group (stable vs decliner) and APOE-ε4 genotype as the factors. The decliner group had lower mean scores on the MMSE at baseline (F[1,30] = 11.5; p < 0.005). There was no difference between the two groups’ performance on the postscan recognition memory test or the California Verbal Learning Test, or between their baseline CDR-SB scores.
Relationship of Functional Magnetic Resonance Imaging Activation, Region of Interest Volume, and Follow-up Status
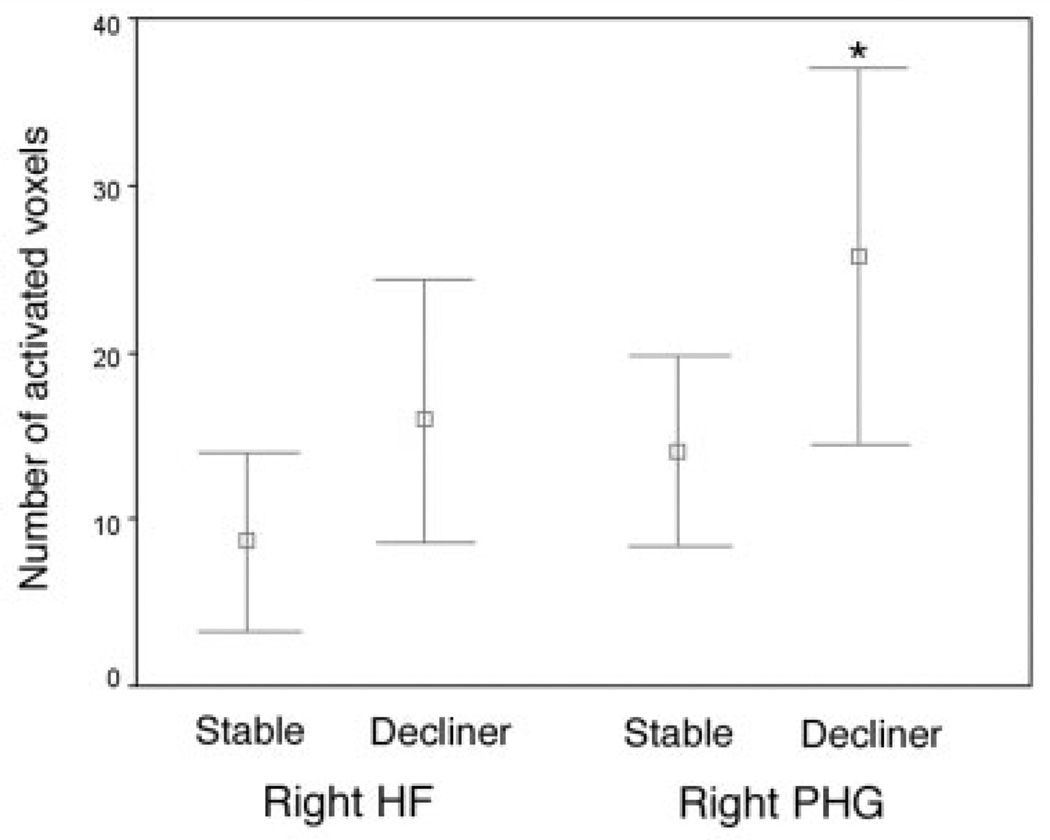
To evaluate differences in fMRI activation and ROI volume in stable versus decliner, we conducted separate two-way repeated-measures ANOVA tests for each ROI, with group and APOE-ε4 genotype as the factors. The two groups differed in extent of fMRI activation in the right PHG (F[1,30] = 4.5; p < 0.05) on the NvR contrast (Fig 3), with the decliner group having a greater extent of fMRI activation. In this group, a trend toward greater extent of activation in the right HF was also present (F[1,30] = 3.4; p = 0.07). The groups did not differ in extent of activation in other ROIs in the NvR contrast, or in any ROIs on the novel versus fixation contrast. There were no group differences in the volumes of the ROIs (p > 0.15). In all of the above ANOVAs, there was no significant effect of APOE-ε4 or an interaction between group and genotype.
Fig 3.
Mean extent of right hippocampal (HF) and parahippocampal (PHG) activation for the subject group that remained stable after longitudinal clinical follow-up (stable) versus those with clinical decline (decliner); *p < 0.05. Error bars indicate 95% confidence intervals.
Discussion
In this study, we used fMRI to investigate memory-related activation of MTL regions among nondemented elderly individuals with MCI. We found that the extent of activation within MTL regions during encoding was systematically associated with postscan recognition memory test performance, and with the clinical status of the subjects at both baseline (ie, at the time of scanning) and follow-up evaluations. As anticipated, extent of MTL activation was correlated with postscan memory test performance, even after adjustment for ROI volume. However, the relationship of fMRI activation to clinical status was “paradoxical” in that greater clinical impairment (as measured by the CDR-SB) was associated with greater extent of PHG activation. This latter finding was observed in a multivariate model that adjusted for hippocampal volume. That is, individuals with greater clinical impairment and hippocampal atrophy were able to perform this memory task but activated a larger extent of parahippocampal tissue.
This paradoxical relationship was also seen for the clinical course of the subjects. After an average of 2.5 years of follow-up, 44% of subjects demonstrated clinical decline, including 12.5% who were diagnosed with probable AD. The decliner group activated a significantly greater extent of the right PHG compared with those who remained stable, signifying that individuals with impending clinical decline activated more parahippocampal tissue when attempting to encode novel scenes during the scanning session. Thus, greater extent of activation within the PHG appeared to herald subsequent clinical decline and/or a diagnosis of AD. Moreover, the volume of the PHG did not differ between the decliner and the stable subject groups, suggesting that functional changes within the MTL may be evident even when the neuropathological burden is relatively circumscribed, as has been observed in transgenic mouse models of AD.38,39
These findings were regionally selective in that similar increases in extent of fMRI activation were not observed in the control region, the striate cortex. It is likely that this relative specificity relates to both the consistent activation of parahippocampal regions when subjects perform visual encoding tasks11,12,14,40 and the selective neuropathological vulnerability of the MTL during questionable AD.1,2 However, given structural MRI data indicating the involvement of superior temporal, anterior cingulate, and other brain regions in prodromal AD,8 further fMRI studies are planned to investigate functional changes in other areas of the brain.
To our knowledge, this is the first report of a selective relationship between regional fMRI activation, degree of functional impairment, and subsequent clinical decline among MCI subjects. These findings are in accord with recent neurochemical evidence of hippocampal cholinergic upregulation in MCI patients41 and are consistent with the hypothesis that increased activation in the MTL and neocortical regions during memory task performance reflects a compensatory response to accumulating AD pathology.22,42,43 Alternatively, increased regional brain activation may be a marker of the pathophysiological process of AD itself, such as aberrant sprouting of cholinergic fibers.44–46 It is important, however, to acknowledge that multiple nonneural factors may confound the interpretation of changes in the hemodynamic response measured by BOLD fMRI, such as age- and disease-related changes in neurovascular coupling,47,48 AD-specific alterations in vascular physiology,49,50 and resting hypoperfusion and metabolism in MCI and AD,51–53 which may result in an amplified BOLD fMRI signal during activation.54,55
Although our primary measure of interest in this study was extent of activation, we also examined the magnitude of activation within each ROI (data not shown), using a method previously reported.32 Magnitude of activation was defined as the average percentage of signal change from baseline during a given condition (ie, novel or repeated) within voxels showing significant task-related activity (as identified from an omnibus analysis). For all ROIs and task conditions, there was no relationship between magnitude of activation and postscan memory performance, clinical impairment, or decline. One possible explanation for this observation, which is consistent with other reports,56 relates to the physiological basis of the fMRI signal, which generally correlates with neuronal firing rates.57 We speculate that pathological alterations within MTL regions may reduce the density of neurons that are able to rapidly fire in response to a stimulus (and thus the magnitude of fMRI response) and may induce the recruitment of adjoining areas (and thus increase extent of response). This observation also suggests that a reduction in resting MTL perfusion is unlikely to account for our findings, because such a reduction would be expected to affect the magnitude of change in fMRI signal, rather than its extent.
Our results extend those of other recent fMRI investigations of MTL activation during encoding in mildly impaired, nondemented elders,16,21 but comparisons are complicated by differences in the characteristics of subjects, data analysis methods, and postscan memory test performance. First, the participants in this study were generally similar to those in the other studies, in that they were carefully selected to exclude significant neurological or psychiatric conditions, but our subjects likely represented a broader range along the spectrum of MCI. The subjects in this study were recruited from the community. Machulda and colleagues studied MCI patients who were referred by medical practioners; their nine subjects appeared similar to a mild AD patient group in both MTL activation and performance on a neuropsychological memory task.21 Conversely, Small and colleagues selected subjects from a longitudinal community study who demonstrated a decline in neuropsychological memory test performance rather than a clinical history of decline and excluded individuals with an objective memory deficit.16 MTL activation in the 12 subjects in this study was heterogeneous, with some individuals showing MTL activation that was similar to that of an AD patient group and some with relatively preserved entorhinal activation.
Varying methodological approaches to neuroimaging data analysis also may account for differences in results. Our approach was to examine the extent of fMRI activation within an ROI, based on each subject’s individual anatomy. This allowed for the calculation of both the total number of voxels in a given ROI (a measure of structural volume), as well as the number of voxels in the ROI that met criteria for task-related fMRI activation (a measure of the extent of activation within the ROI). Machulda and colleagues21 also measured the number of activated voxels within an ROI, but their method included bilateral HF, PHG, and fusiform gyri within a single ROI. Our results and those of Small and colleagues16 demonstrate differential activation within hippocampal and parahippocampal regions during encoding, suggesting that measuring activation in these areas separately may be informative. Although these previous studies used ROI methods to localize fMRI activation, they did not report ROI volumes.
Finally, it is worth considering the subjects’ ability to remember items encoded during the fMRI memory paradigm, which is known to correlate with MTL activation.12–14,25,26 It is difficult to compare the postscan memory performance of our subjects with that of Machulda and colleagues21 because of substantial differences in the encoding task itself: their subjects encoded a total of 12 scenes, of which they freely recalled 33%. This performance correlated with degree of activation, whereas forced-choice recognition did not, suggesting that recall was a better reflection of encoding and may, at least in part, explain the observed decreased fMRI activation.
It is important to acknowledge the potential limitations of our study. First, the design of the cognitive task did not enable us to control or measure aspects of cognitive processing performed by the subjects when they encoded stimuli. Although postscan recognition scores approached 80%, and thus indicated that subjects attended to the task, it is not clear whether variation in processing speed or encoding strategy may have contributed to differences in activation.58 In addition, our analytic approach used an arbitrary threshold (p < 0.01) to identify voxels with significant activation. Although this method is commonly used, Machulda and colleagues21 pointed out the value of considering a range of thresholds to attempt to avoid “artifactual false positive activation.” Finally, to quantify extent of activation, we localized functional activation using ROIs identified from each subject’s structural images, which then required the superimposition of structural and functional MRI data. For studies of structure–function relationships in neurodegenerative diseases, this approach clearly has advantages over methods that involve spatial transformations to an average template; yet differences in spatial resolution between the functional and structural data, as well as spatial distortion artifacts produced by echo-planar functional acquisition sequences,59 make this method imperfect, possibly resulting in mislocalization of functional activation. Techniques are currently being developed to correct for geometric distortions in fMRI data60 and will be incorporated in future studies to minimize this problem.
Taken together with other studies, our results suggest that a complex set of factors influence the functional properties of MTL regions in the setting of neurodegenerative disease, including the severity of impairment of the subjects, the degree of brain atrophy, and the level of performance on the memory task. Although further investigation is necessary before it will be clear how these results can be applied, our findings suggest that increased MTL activation among elderly individuals with MCI may precede impending clinical decline.
Acknowledgments
This study was supported by the NIH (National Institute on Aging, PO1-AG04953, D.B., M.S.A.; K23-AG22509, B.C.D.; National Institute of Neurological Disorders and Stroke, K23-NS02189, R.A.S.), and the Clinical Investigator Training Program (Harvard/ MIT Health Sciences and Technology-Beth Israel Deaconess Medical Center, in collaboration with Pfizer Inc, B.C.D.).
We thank Dr. Bruce Rosen and the Martinos Imaging Center, which is supported by grants from the NCRR (P41-RR14075) and the Mental Illness and Neuroscience Discovery (MIND) Institute. We thank the staff of the MGH Gerontology Research Unit for assistance with subject recruitment, evaluation, and data management, and Jennifer Holmes, Mary Foley, and Larry White for assistance with MRI data collection. We express special appreciation to our subjects for their valuable contributions, without which this study would not have been possible.
References
- 1.Gomez-Isla T, Price JL, McKeel DW, Jr, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kordower JH, Chu Y, Stebbins GT, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- 3.de Toledo-Morrell L, Dickerson B, Sullivan MP, et al. Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer’s disease. Hippocampus. 2000;10:136–142. doi: 10.1002/(SICI)1098-1063(2000)10:2<136::AID-HIPO2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Jack CR, Jr, Xu YC, et al. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- 5.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visser PJ, Scheltens P, Verhey FR, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 7.Convit A, de Asis J, de Leon MJ, et al. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in nondemented elderly predict decline to Alzheimer’s disease. Neurobiol Aging. 2000;21:19–26. doi: 10.1016/s0197-4580(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 8.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 9.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 10.Mungas D, Reed BR, Jagust WJ, et al. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology. 2002;59:867–873. doi: 10.1212/wnl.59.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern CE, Corkin S, Gonzalez RG, et al. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brewer JB, Zhao Z, Desmond JE, et al. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- 13.Wagner AD, Schacter DL, Rotte M, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperling RA, Bates JF, Cocchiarella AJ, et al. Encoding novel face-name associations: a functional MRI study. Hum Brain Mapp. 2001;14:129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small SA, Perera GM, DeLaPaz R, et al. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Ann Neurol. 1999;45:466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Rombouts SA, Barkhof F, Veltman DJ, et al. Functional MR imaging in Alzheimer’s disease during memory encoding. AJNR Am J Neuroradiol. 2000;21:1869–1875. [PMC free article] [PubMed] [Google Scholar]
- 18.Kato T, Knopman D, Liu H. Dissociation of regional activation in mild AD during visual encoding: a functional MRI study. Neurology. 2001;57:812–816. doi: 10.1212/wnl.57.5.812. [DOI] [PubMed] [Google Scholar]
- 19.Gron G, Bittner D, Schmitz B, et al. Subjective memory complaints: objective neural markers in patients with Alzheimer’s disease and major depressive disorder. Ann Neurol. 2002;51:491–498. doi: 10.1002/ana.10157. [DOI] [PubMed] [Google Scholar]
- 20.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machulda MM, Ward HA, Borowski B, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61:500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daly E, Zaitchik D, Copeland M, et al. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000;57:675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- 24.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 25.Strange BA, Otten LJ, Josephs O, et al. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J Neurosci. 2002;22:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sperling R, Chua E, Cocchiarella A, et al. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 28.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 29.Grundman M, Petersen RC, Ferris SH, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 30.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 31.McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 32.Sperling R, Greve D, Dale A, et al. Functional MRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci USA. 2002;99:455–460. doi: 10.1073/pnas.012467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burock MA, Dale AM. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum Brain Mapp. 2000;11:249–260. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 35.Killiany RJ, Moss MB, Albert MS, et al. Temporal lobe regions on magnetic resonance imaging identify patients with early Alzheimer’s disease. Arch Neurol. 1993;50:949–954. doi: 10.1001/archneur.1993.00540090052010. [DOI] [PubMed] [Google Scholar]
- 36.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 37.Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test, research edition, manual. San Antonio, TX: The Psychological Corporation, Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 38.Chapman PF, White GL, Jones MW, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 39.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 40.Kelley WM, Miezin FM, McDermott KB, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- 41.DeKosky ST, Ikonomovic MD, Styren SD, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- 42.Becker JT, Mintun MA, Aleva K, et al. Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer’s disease. Neurology. 1996;46:692–700. doi: 10.1212/wnl.46.3.692. [DOI] [PubMed] [Google Scholar]
- 43.Grady CL, McIntosh AR, Beig S, et al. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer’s disease. J Neurosci. 2003;23:986–993. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mesulam MM. Neuroplasticity failure in Alzheimer’s disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 45.Hashimoto M, Masliah E. Cycles of aberrant synaptic sprouting and neurodegeneration in Alzheimer’s and dementia with Lewy bodies. Neurochem Res. 2003;28:1743–1756. doi: 10.1023/a:1026073324672. [DOI] [PubMed] [Google Scholar]
- 46.Masliah E, Alford M, Adame A, et al. Abeta1–42 promotes cholinergic sprouting in patients with AD and Lewy body variant of AD. Neurology. 2003;61:206–211. doi: 10.1212/01.wnl.0000073987.79060.4b. [DOI] [PubMed] [Google Scholar]
- 47.Buckner RL, Snyder AZ, Sanders AL, et al. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci. 2000;12(suppl 2):24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- 48.D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 49.Johnson SC, Saykin AJ, Baxter LC, et al. The relationship between fMRI activation and cerebral atrophy: comparison of normal aging and Alzheimer disease. Neuroimage. 2000;11:179–187. doi: 10.1006/nimg.1999.0530. [DOI] [PubMed] [Google Scholar]
- 50.Mueggler T, Sturchler-Pierrat C, Baumann D, et al. Compromised hemodynamic response in amyloid precursor protein transgenic mice. J Neurosci. 2002;22:7218–7224. doi: 10.1523/JNEUROSCI.22-16-07218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson KA, Jones K, Holman BL, et al. Preclinical prediction of Alzheimer’s disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 52.De Santi S, de Leon MJ, Rusinek H, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22:529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 53.El Fakhri G, Kijewski MF, Johnson KA, et al. MRI-guided SPECT perfusion measures and volumetric MRI in prodromal Alzheimer disease. Arch Neurol. 2003;60:1066–1072. doi: 10.1001/archneur.60.8.1066. [DOI] [PubMed] [Google Scholar]
- 54.Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Smith CD, Andersen AH, Kryscio RJ, et al. Women at risk for AD show increased parietal activation during a fluency task. Neurology. 2002;58:1197–1202. doi: 10.1212/wnl.58.8.1197. [DOI] [PubMed] [Google Scholar]
- 57.Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum Brain Mapp. 1999;8:102–108. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jezzard P, Clare S. Sources of distortion in functional MRI data. Hum Brain Mapp. 1999;8:80–85. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<80::AID-HBM2>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutton C, Bork A, Josephs O, et al. Image distortion correction in fMRI: a quantitative evaluation. Neuroimage. 2002;16:217–240. doi: 10.1006/nimg.2001.1054. [DOI] [PubMed] [Google Scholar]