Abstract
Glutamine synthetase (EC 6.3.1.2) in embryonic neural retina in culture is rapidly induced by hydrocortisone. Retina polysomes involved in translation of this enzyme were precipitated with a high degree of specificity by the gammaglobulin isolated from antiserum against the enzyme (anti-enzyme gammaglobulin). Using immunoprecipitation procedures, we determined that the amount of polysome-bound nascent enzyme was maximal in polysomes comprising 9-14 ribosomes and was about 3-fold higher in the induced than in the noninduced retina. Within this size group of polysomes, those comprising 11-13 ribosomes showed consistently greater binding of anti-enzyme [125I]gammaglobulin than of normal [125I]-gammaglobulin. This size of polysomes corresponds to that calculated for a monocistronic messenger RNA for the subunit of this enzyme, which has a molecular weight of 42,000. The application of immunochemical techniques to identification of templates for synthesis of an enzyme in embryonic cells that constitutes less than 1% of the total cellular proteins indicates the usefulness of this method for detailed studies on regulation of other quantitatively minor products significant in cell differentiation.
Keywords: differentiation, enzyme induction, immunoprecipitation, nascent enzyme, messenger RNA
Full text
PDF

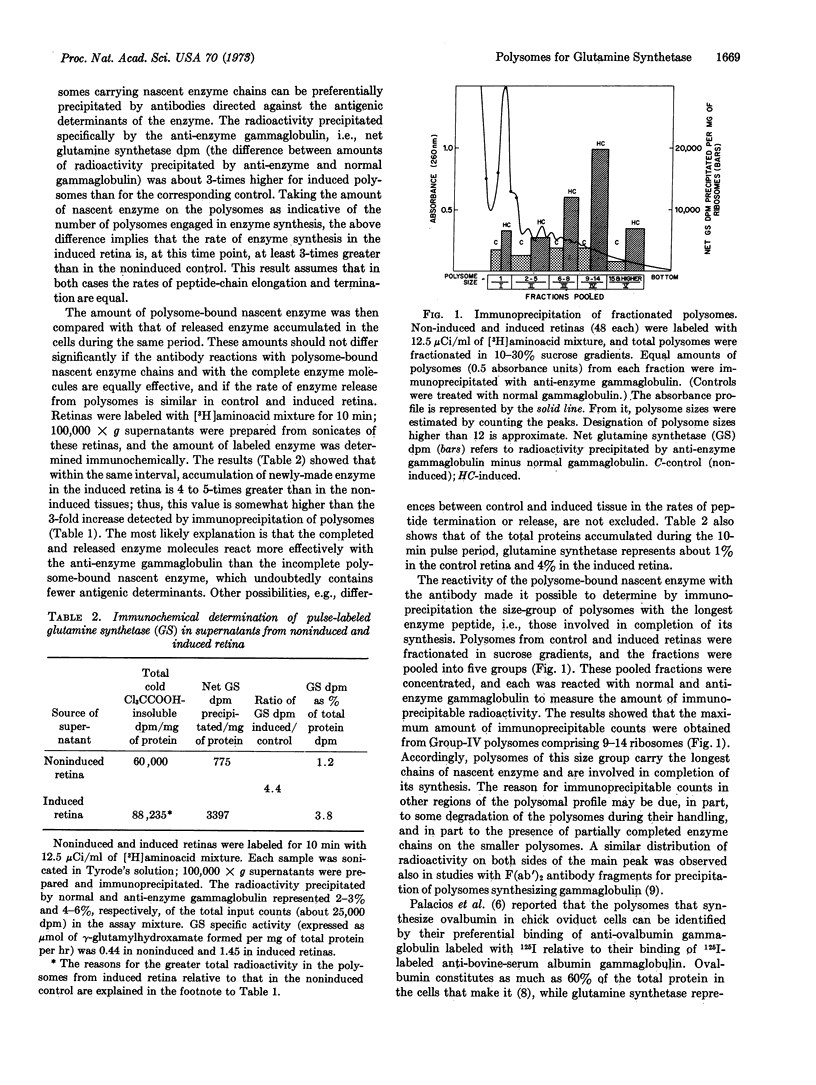
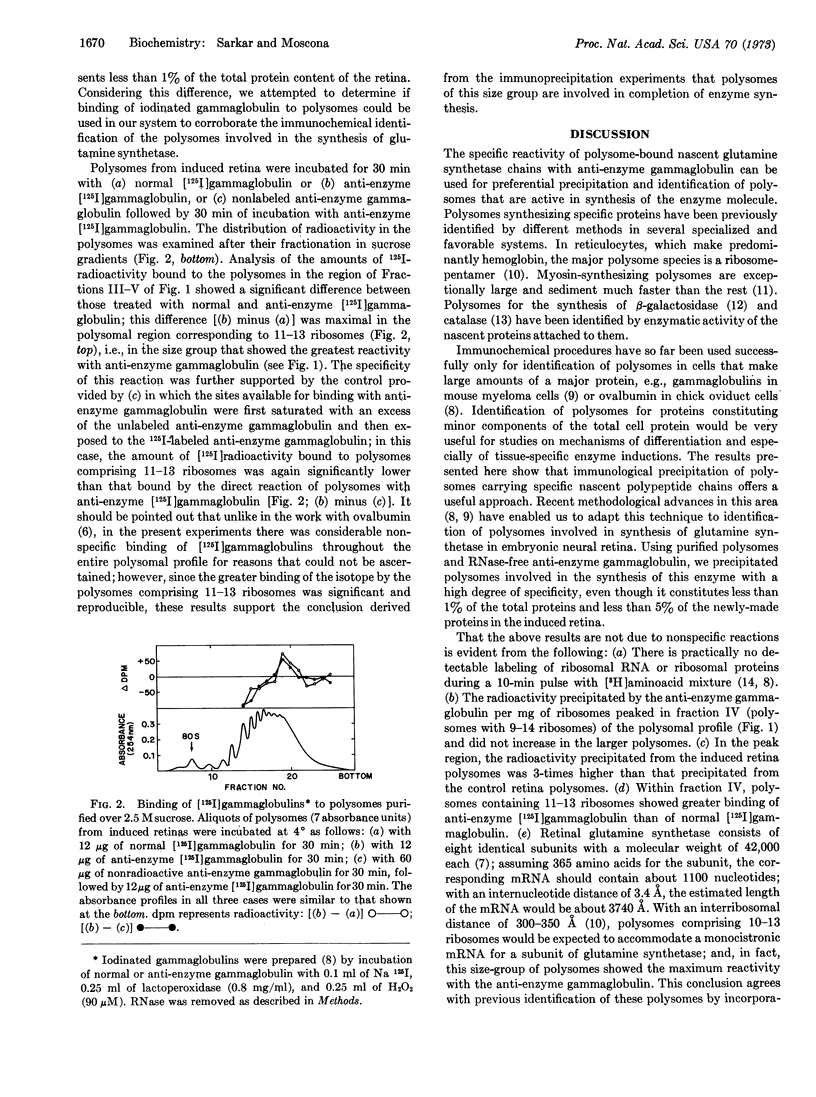

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alescio T., Moscona A. A. Immunochemical evidence for enzyme synthesis in the hormonal induction of glutamine synthetase in embryonic retina in culture. Biochem Biophys Res Commun. 1969 Jan 27;34(2):176–182. doi: 10.1016/0006-291x(69)90628-7. [DOI] [PubMed] [Google Scholar]
- Delovitch T. L., Davis B. K., Holme G., Sehon A. H. Isolation of messenger-like RNA from immunochemically separated polyribosomes. J Mol Biol. 1972 Aug 28;69(3):373–386. doi: 10.1016/0022-2836(72)90251-3. [DOI] [PubMed] [Google Scholar]
- Heywood S. M., Dowben R. M., Rich A. The identification of polyribosomes synthesizing myosin. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1002–1009. doi: 10.1073/pnas.57.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIHO Y., RICH A. INDUCED ENZYME FORMED ON BACTERIAL POLYRIBOSOMES. Proc Natl Acad Sci U S A. 1964 Jan;51:111–118. doi: 10.1073/pnas.51.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. A., Moscona M. H., Saenz N. Enzyme induction in embryonic retina: the role of transcription and translation. Proc Natl Acad Sci U S A. 1968 Sep;61(1):160–167. doi: 10.1073/pnas.61.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona M., Frenkel N., Moscona A. A. Regulatory mechanisms in the induction of glutamine synthetase in the embryonic retina: immunochemical studies. Dev Biol. 1972 May;28(1):229–241. doi: 10.1016/0012-1606(72)90140-6. [DOI] [PubMed] [Google Scholar]
- Palacios R., Palmiter R. D., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. I. Specific binding of 125 I-anti-ovalbumin to polysomes. J Biol Chem. 1972 Apr 25;247(8):2316–2321. [PubMed] [Google Scholar]
- Palmiter R. D., Palacios R., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. II. Quantification and immunoprecipitation of polysomes. J Biol Chem. 1972 May 25;247(10):3296–3304. [PubMed] [Google Scholar]
- Sarkar P. K., Fischman D. A., Goldwasser E., Moscona A. A. Isolation and characterization of glutamine synthetase from chicken neural retina. J Biol Chem. 1972 Dec 10;247(23):7743–7749. [PubMed] [Google Scholar]
- Sarkar P. K., Moscona A. A. Changes in macromolecular synthesis associated with the induction of glutamine synthetase in embryonic retina. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2308–2311. doi: 10.1073/pnas.68.9.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P. K., Moscona A. A. The ribonucleoprotein nature of the rapidly labeled RNA in the embryonic retina. Dev Biol. 1971 Oct;26(2):187–200. doi: 10.1016/0012-1606(71)90121-7. [DOI] [PubMed] [Google Scholar]
- Schwartz R. J. Steroid control of genomic expression in embryonic chick retina. Nat New Biol. 1972 May 24;237(73):121–125. doi: 10.1038/newbio237121a0. [DOI] [PubMed] [Google Scholar]
- Uenoyama K., Ono T. Nascent catalase and its messenger RNA on rat liver polyribosomes. J Mol Biol. 1972 Mar 14;65(1):75–89. doi: 10.1016/0022-2836(72)90493-7. [DOI] [PubMed] [Google Scholar]


