Abstract
The human papilloma virus pseudovirions (HPV-PsVs) approach is an effective gene-delivery system that can prime or boost an immune response in the vaginal tract of non human primates and mice. Intra-vaginal vaccination with HPV-PsVs expressing SIV genes, combined with an intra-muscular gp120 protein injection, induced humoral and cellular SIV-specific responses in macaques. Priming systemic immune responses with intramuscular immunization with ALVAC-SIV vaccines, followed by intra-vaginal HPV-PsV-SIV/gp120 boosting, expanded and/or recruited T-cells in the female genital tract. Using a stringent repeated low dose intra-vaginal challenge with the highly pathogenic SIVmac251, we show that while these regimens did not demonstrate significant protection from virus acquisition, they provided control of viremia in a number of animals. High avidity antibody responses to the envelope gp120 V1/V2 region correlated with delayed SIVmac251 acquisition, while virus levels in mucosal tissues were inversely correlated with anti-envelope CD4+T-cell responses. CD8+T-cell depletion in animals with controlled viremia caused an increase in tissue virus load in some animals, suggesting a role for CD8+T-cells in virus control. This study highlights the importance of CD8+ cells and anti-envelope CD4+ T-cell in curtailing virus replication and anti-envelope V1/V2 antibodies in preventing SIVmac251 acquisition.
Introduction
The development of a vaccine that prevents HIV acquisition remains a formidable challenge. Most currently licensed protective viral vaccines induce neutralizing antibodies that mediate long lasting immunity. However, broadly neutralizing antibodies take an average of 2.5 years to develop during natural HIV infection (1) and often have extensive somatic hyper-mutation (2), a property likely to be difficult to induce via vaccination. In addition, clinical trials using a protein vaccine that primarily induced antibody responses failed to prevent HIV infection(3, 4) and led to increased emphasis on vaccines that induce HIV-specific T-cell responses. However, vaccines that induced robust T-cell responses failed to prevent HIV infection in clinical efficacy trials (Merck STEP trial-HVTN 502, HVTN 503 and HVTN 505) (5-7). In addition, in some of the trials, a higher number of infections occurred in vaccinated individuals than in the placebo arms. All three trials included systemically administered adenovirus vectors, and while the role of vector specific responses remains unclear, the results suggest that systemic CD8 T-cells alone are not sufficient to prevent HIV acquisition.
The RV144 Thai trial was the first HIV vaccine clinical trial to demonstrate measurable protective efficacy. Vaccination significantly reduced the risk of HIV infection, with an estimated efficacy of 31.2%(8). The vaccine regimen consisted of an intramuscular injection of the canarypox vector ALVAC expressing HIV genes, paired with a bivalent envelope protein gp120 boost. This regimen induced mainly non-neutralizing antibodies and CD4+ T-cell responses (8, 9). Antibodies directed to the V1/V2 region of gp120 were found to be a primary correlate of a reduced risk of HIV acquisition, while antibody dependent cellular cytotoxicity (ADCC) was a secondary correlate (9). These findings highlighted the potential of vaccine-induced antibodies in preventing HIV acquisition. Binding non-neutralizing functional antibodies could prevent virus entry and dissemination by impairing virus mobility at the portal of entry, or by destroying newly infected cells by activating the complement pathway, and/or coordinating with macrophages or NK cells (10).
Repeated low-dose mucosal challenge with SHIV or SIV viruses in macaques are reasonable models of HIV sexual transmission (11). The SIVmac251 challenge used in this study is a pathogenic CCR5 user that is resistant to neutralization, similar to most HIV primary isolates. To date, HIV vaccine candidates tested in this macaque model using mucosal repeated low doses of SIVmac251, have recapitulated the results of HIV clinical trials in humans (12-14).
Preventing HIV transmission remains the primary goal of HIV vaccines; however, once infection has occurred the reduction of chronic phase viremia and disease progression are also important objectives. Increasing evidence suggests that while a vaccine induced humoral response is important for protection from virus acquisition (15-17), CD8+ T-cell responses contribute to virus control after lentiviral transmission (16, 18-20) . In the RV144 Thai trial, the ALVAC-HIV/gp120 regimen induced negligible CD8+ T-cell responses and vaccinees that became infected had virus levels and CD4+ T-cell counts similar to the placebo group, requiring the initiation of antiretroviral therapy (21). Multiple lines of evidence implicate CD8+ T-cells in the control of HIV/SIV replication, e.g. CD8+ T-cell depletion of macaques during SIV infection causes a rapid increase in viral burden(22, 23). In addition, during primary HIV infection, the post peak decline in viremia is temporally associated with the induction of CD8+ T-cell responses (24, 25). The immunologic pressure imposed by CD8+ T-cells on HIV is evidenced by the emergence of MHC I restricted escape mutations (26-28). Intriguingly, recent studies have demonstrated potent control of SIV infection by broadly distributed T-cell responses, induced by rhesus CMV vaccine vectors that generate unusual MHC class II restricted CD8+ T-cells targeting promiscuous SIV epitopes (29, 30).
Our goal was to develop a novel vaccine regimen that induces mucosal CD8+ T-cells together with binding functional antibodies and ask whether this vaccine regimen alone could protect, or whether prior priming with systemic immunization could further improve protection. Human papilloma viruses (HPVs) are small non-enveloped DNA viruses that naturally infect epithelial cells within the genital tract; we used HPV-based vectors as a delivery system to specifically target antigen expression to the vaginal epithelium. We created HPV-pseudovirions (PsVs) that express SIV genes and delivered them to basal epithelial cells in the female genital tract. Infection in the vaginal tract is facilitated by micro-trauma that allows access to the basal epithelial layers(31). HPV PsV-mediated gene expression in the female genital tract has been shown to be transient, lasting for approximately 5 days, during which priming of T-cells in the genital draining lymph-nodes and antigen-recall in the genital mucosa has been reported in murine models(32, 33). Initiation of an adaptive response is likely enhanced by the adjuvant-like potential of the HPV capsid, with its ordered protein arrangement(34). HPV-virus like particles have been shown to induce maturation of dendritic cells, resulting in the production of IL6, IL12, and TNF-α, and may be recognized by toll-like receptors on mucosal cells engaging pathogen-associated molecular patterns on the HPV capsid (33, 35, 36). In previous studies, we demonstrated the feasibility of this vaccine approach using model antigens and the ability of HPV-PsVs to express foreign genes in the vaginal tract and induce HPV capsid specific antibodies in serum to each HPV serotype (37).
In this study, we evaluated whether the local mucosal immune responses, induced by HPV PsV vaccines paired with a gp120 protein boost, might prevent SIVmac251 intra-vaginal transmission. In addition, because ALVAC/gp120 regimens demonstrate limited but significant protection from infection in humans as well as in non-human primates (8, 13, 20, 38, 39), we examined whether an ALVAC-SIV systemic prime, paired with a HPV-PsV-SIV/gp120 boost, by inducing also higher systemic response could increase vaccine efficacy.
Materials and Methods
Animals, Immunization, and SIV Challenge
Thirty-six female rhesus macaques of Indian origin, aged 3.5-7 years were used in this study. All animals were housed and cared for under the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care, and the study was conducted with the approval of the Institutional Animal Care and Use Committee at Advanced BioSciences Laboratories in Rockville, MD. The animals were divided into three groups of twelve animals, each based on their MHC alleles. Twelve animals were vaccinated with 108 PFU of ALVAC-SIV encoding SIVmac251 gag, pol and env (gp160) in the thigh at weeks 0 and 4 by intramuscular (i.m.) injection. The ALVAC-SIV vector was made as previously described (40). The twelve ALVAC-SIV vaccinated animals and twelve additional macaques were vaccinated intra-vaginally with HPV Pseudovirions expressing SIVmac251 genes (HPV-PsV-SIV) at weeks 6, 10 and 24. HPV-PsVs were produced as previously described (37, 41). Briefly, DNA constructs encoding the capsids of HPV serotypes 16, 45, 58 and DNA constructs encoding SIV gag-pro, gp120 or the reassortant genes rev, tat and nef were co-transfected into 293T-cells. The resulting PsVs were purified, propagated, and titered. Twenty-eight days prior to each HPV-PsV-SIV vaccination, macaques were given 30 mg/kg Depo-Provera i.m. to thin the vaginal epithelia, and 1 week prior to vaccination, macaques were treated with antibiotics to prevent vaginosis. At 6 and 24 hours pre vaccination, a vaginal application of nonoxynol 9 (N9), a nonionic detergent, was administered as a 10% gel mixed with 4% carboxymethyl cellulose (Sigma-Aldrich, St. Louis, MO). N9 induces microtrauma in the epithelia which facilitates HPV-PsV vaccination. Six hours after the last N9 treatment, a standard 500μl inoculum, consisting of 1010 infectious units (IU) of HPV PsVs and carboxymethyl cellulose, was instilled into the vaginal vault using a positive displacement pipette. In addition, all twenty-four vaccinated macaques were given 2 i.m. injections with 200ug of gp120 protein, as done previously(39). The protein was mixed with the adjuvants alum and monophosphoryl lipid A (MPL) and administered in the thigh muscle at weeks 10 and 24. Twelve macaques were used as controls. Control animals were given the ALVAC vector that did not express SIV genes, HPV-PsV that expressed luciferase, and the adjuvants alum and MPL at similar doses and times as the vaccinated animals.
Four weeks after the last HPV-PsV vaccination at week 28, all thirty six rhesus macaques were challenged intra-vaginally with 250 TCID50 of SIVmac251. The virus was kindly provided by Nancy Miller in the Division of AIDS. Blood was collected and SIV RNA was quantified in plasma seven days post challenge; animals with virus loads less than 50 copies/ml were re-challenged. Animals with two successive viral determinations over 104 were considered persistently SIV infected, repeated SIV challenges were stopped, and virus loads were monitored weekly in the acute phase and monthly in the chronic phase. Animals with virus loads between 50 and 104 copies were retested at day 10. If the virus load increased at day 10 above 104, the animal was considered persistently SIV infected, the challenge phase stopped, and virus load in plasma was monitored thereafter. If however, the virus load at day 10 was less than 50 copies/ml, the animal was considered transiently infected and repeated low dose challenges were resumed. A maximum of 9 repeated low doses of SIVmac251 was administered at 10 day intervals.
Mucosal Antibodies
Vaginal secretions were collected using absorbent cotton sponges. To elute secretions, the sponges were incubated for 10 min in elution buffer, on ice, transferred into a Salivette column (Sarstedt), and then centrifuged at 3000 rpm for 30 min at 4°C. For SIV specific IgA and IgG, serially diluted vaginal secretions were applied to 96-well half-area plates (Greiner Bio-one), previously coated with 50μl (10μg/ml) SIVmac251 gp120 (Advanced Bioscience Laboratories) and blocked with 1% BSA block solution (KPL). After overnight incubation at 4°C, plates were washed with PBS-Tween, reacted with peroxidase-conjugated anti-monkey IgA or IgG antibody (Alpha Diagnostic), and incubated for another hour at room temperature. After washing, 50μl of TMB peroxidase substrate solution was added to each well and incubated for 20 minutes at room temperature. Reactions were stopped by adding 50μl 2M H2SO4, and plates were read at 450 nm within 30 min. Titer was defined as the reciprocal of the dilution at which the absorbance of the test sample was twice that of the negative control sample diluted 1:5. Total IgA and IgG concentrations were similarly determined by incubating serially diluted mucosal samples and a dilution series of a standard normal rhesus macaque serum with known concentrations of IgG and IgA on microplates coated with 1 ug/ml of purified goat anti-monkey IgA or IgG antibody. The Env-specific IgA or IgG titer was divided by the corresponding total IgA or IgG concentration in each secretion and reported as titer/μg total IgG or IgA.
Statistical Analysis
Comparisons between groups were performed using the Mann- Whitney-Wilcoxon test for continuous factors, and paired comparisons between two times were assessed by the Wilcoxon signed rank test. Correlations were performed using the Spearman rank correlation method. The difference between groups in the binding of each of the overlapping gp120 peptides was tested using the exact Mann-Whitney-Wilcoxon test, and the p values were corrected for multiple comparisons by the Hochberg method. Graphical analysis was performed using GraphPad Prism, and error bars on graphs represent the standard error of the means.
IFN-γ ELISPOT
SIV-specific T-cells were assessed using an IFN-γ ELISpot kit from Mabtech as previously described 40. Cryopreserved peripheral blood mononuclear cells (PBMCs) were thawed, rested, and stimulated with either SIVmac251 Gag or gp120 (Env) overlapping 15-mer peptides, Con A, or left unstimulated. PBMCs and stimulants were added to IFN-γ coated plates and incubated for 24 hours. The plates were developed, and the frequency of IFN-γ positive spot-forming cells per 106 PBMC were determined after background subtraction.
Pentamer Staining and Intracellular Cytokine Assays
Ten-color flow cytometric analysis was performed on mononuclear cells from blood, cervico-vaginal, and rectal biopsies. Pinch biopsies obtained from the cervix, vaginal tract, or rectum was washed and incubated for 1 hour with collagenase D at a concentration of 2mg/ml in Iscoves media with antibiotics and amphotericin. Following the incubation, the remaining tissue was mechanically disrupted to obtain a mononuclear cell suspension. Filtered single-cell suspensions of mononuclear cells were used in an intracellular cytokine assay performed as previously described(37). Cells were stimulated with either Env peptides at a concentration of 2μg/ml, PMA and Ionomycin or left unstimulated in the presence of Golgi transport inhibitors, CD107a clone H4A3, anti-CD28ECD clone CD28.2 (eBiosciences), and CD49D clone 9F10 (BD Biosciences) for 6 hours. Cells were then surface stained with CD3 (cloneSP34-2), CD4 (clone L200), CD8 (clone RPA-T8), CD95 (clone DX2), and the live/dead yellow fixable amine dye from Invitrogen. Surface stained samples were washed, permeabilized with Cytofix Cytoperm and stained intracellularly with IFN-γ (clone B27), TNF-α (clone MAB11), and IL-2 (clone MQ1-17H12). Staining reagents were obtained from BD Biosciences unless otherwise stated. Cytokine production after background subtraction from memory (CD95+) CD4+ and CD8+ T-cells and the proportion of mono-functional and poly-functional (simultaneous production of multiple cytokines) responses were determined. For Gag CM9 pentamer detection (obtained from ProImmune), cells were stained for 15 min with the Gag CM9 PE pentamer, washed and then stained with the amine dye and CD3, CD4, CD8, CD28, and CD95 using the same clones as above. Samples were washed, permed with cytofix cytoperm, and stained intracellularly with Ki67 (clone B56, BD Biosciences). All cells were fixed with 1% paraformaldehyde and acquired on an LSR II (BD Biosciences). Data analysis was performed with FlowJo (Tree Star) and with SPICE (NIAID) (42).
CFSE Proliferation assay
The lymphoproliferation assay was performed as previously described(39). Cells were briefly incubated with 5 mM carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen), washed, enumerated, and stimulated with 5 μg/ml of SIV Env, ConA, or left unstimulated for 5 days. Cells were then harvested and stained with CD3, CD4, CD8, CD28, CD95 and the amine dye as described above. Samples were acquired on an LSRII flow cytometer and the frequency of CD3+ CD4+CD95+ or CD3+ CD8+ CD95+ T-cells with diminished expression of CFSE (proliferated) after 5 days of culture was determined and the background subtracted (%CFSE dim in unstimulated cells).
Binding Antibodies and Pepscans
An enzyme-linked immunosorbent assay (ELISA) was used to detect SIVmac251-gp120 binding antibodies in blood as previously described (40) and to detect binding to overlapping peptides spanning gp120. A serial dilution of plasma was added to microtiter plates coated with native purified gp120 Env protein of SIVmac251 or individual peptides, and the antibody titer determined. The absorbance at OD450 nm was reported for peptide mapping. For binding antibodies to gp120, the endpoint titers were defined as 2X the OD450 of the negative control serum.
B cell ELISPOT
SIV Env-specific or total IgG or IgA antibody-secreting cells were analyzed by a B-cell ELISpot as described previously(39). Briefly, MultiScreen 96-well plates (Millipore MAIPS4510) were incubated with 70% ethanol, rinsed, and coated with SIVmac251 gp120 protein or goat anti monkey IgG or IgA (KPL). Coated plates were incubated at 4°C overnight, washed, and blocked for 2 hours at 37°C. Peripheral blood mononuclear cells were stimulated with CpG (ODN-2006; Operon), CD40L, and interleukin 21 (IL-21) (Peprotech) for 3 days at 37°C in 24-well plates. Stimulated PBMCs were then harvested, washed, and 3×105 cells plated and incubated overnight at 37°C. Plates were then washed and incubated with biotinylated goat anti-monkey IgG or IgA (Rockland) and horse-radish peroxidase (HRP)-avidin D conjugate (Vector Laboratories) was added. After several washes plates were developed using 3-amino- 9-ethyl-carbazole (AEC; Sigma). Spot quantitation was performed with an ELISpot reader.
Antibody dependent cellular cytotoxicity assay (ADCC)
ADCC activity mediated by antibodies in plasma samples was detected by the GranToxiLux (GTL) procedure as previously described (20, 43). Briefly, CEM.NKRCCR5 target T-cells were coated with recombinant SIVmac251 gp120 and labeled with a fluorescent target-cell marker and a viability marker. Labeled target cells were washed and plated. Cryopreserved human PBMCs from an HIV-seronegative donor served as effectors and were added to the assay wells at an effector/target ratio of 30:1. Fluorogenic granzyme B (GzB) substrate (OncoImmunin, Inc.) was added to each well. After incubation, serially diluted plasma samples were added to the assay wells. The plates were incubated for 15 min at RT, centrifuged, and incubated for 1 hour at 37°C. The plates were then washed, cells resuspended in PBS, and a minimum of 2,500 events representing viable target T-cells were acquired for each well using an LSRII flow cytometer (BD Biosciences). Data analysis was performed using the FlowJo 8.8.4 software (Tree Star Inc.). The final results are expressed as ADCC titer and maximum Granzyme B activity.
Neutralization Assays
Neutralization was measured as a reduction in luciferase reporter gene expression after a single round of infection in TZM-bl cells, as described previously (20, 44), 53. TZM-bl cells were obtained from the NIH AIDS Research and Reference Reagent Program. Virus was incubated with serial 3-fold dilutions of samples in duplicate. Freshly trypsinized cells were added to each well. One set of control wells received cells and virus (virus control), and another set received cells only (background control). After a 48-hour incubation, cells were transferred to 96-well black solid plates (Costar) for measurements of luminescence. Neutralization titers are the dilution at which relative luminescence units (RLU) were reduced by 50% compared to that in virus control wells after subtraction of background RLUs. Assay stocks of molecularly cloned Env-pseudotyped viruses, SIVmac251.6, SIVmac251.30, was prepared by transfection in 293T-cells and was titrated in TZM-bl cells. The SIVmac251 challenge stock was obtained from Nancy Miller in the Division of AIDS, NIH, expanded on rhesus PBMCs, titered, and used.
Antibody Avidity
Three recombinant SIV envelope proteins, full length gp120, gp120 deleted of the V1V2 region and the V1V2 mini protein, were made from codon-optimized SIVmac239 gp120 and fused to the C-terminal tag of HIV-1 gp120. These proteins were used as an antigen for the capture ELISA to detect SIV Abs against conformational epitopes, as previously described 40. Parallel ELISAs were used to determined antibody avidity. Heat-inactivated plasma samples were serially diluted and applied to a 96-well plate capturing SIVmac239 gp120 proteins in parallel duplicates. After 1 hour of incubation, the plate was washed, and half the samples were treated with Tris-buffered saline (TBS), while the paired samples were treated with 1.5 M sodium thiocyanate (NaSCN; Sigma-Aldrich) for 10 minutes at room temperature. The plate was washed and a goat anti-monkey IgG-detecting Ab (Fitzgerald) was used. The avidity index (%) was calculated by taking the ratio of the NaSCN-treated plasma dilution giving an OD of 0.5 to the TBS-treated plasma dilution giving an OD of 0.5, and multiplying by 100. Plasma of uninfected normal macaques served as negative controls. A high-avidity monkey MAb of 3.11H was included on every plate as the standard.
Viral Load and Transmitted Founder Variants
Plasma SIV RNA was quantified by nucleic acid sequence-based amplification (NASBA), as previously described (44-46). SIV DNA was quantified in mucosal tissues three weeks post SIV infection by a Real-time qPCR assay with sensitivity up to 10 copies/106 cells as previously described (45). Briefly, genomic DNA was extracted from the rectal biopsies with the DNeasy Blood & Tissue kit (Qiagen), according to the manufacturer's protocol except the DNA elution step. The quantity and quality of the DNA were assessed by OD260 measurements using an ND-1000 spectrophotometer (NanoDrop). The TaqMan probe and PCR primers for the real-time PCR were designed within the conserved gag gene of SIVmac239, and probe and primer sequences were used for the monkey albumin gene detection. The reaction conditions are as follows: the 25 μ1 PCR mixture consisted of 500 ng of genomic DNA extracted from tissues; 200 nM primers; 100 nM probe; 2× TaqMan Universal PCR Mastermix (Applied Biosystems) consisting of 10 mM Tris–HCl (pH 8.3); 50 mM KCl; 5 mM MgCl2; 300 μM each of dATP, dCTP, and dGTP; 600 μM dUTP; 0.625 U of AmpliTaq Gold DNA polymerase; and 0.25 U uracil N-glycosylase (UNG). Amplification was performed using 1 cycle at 50°C for 2min and 1 cycle at 95°C for 10 min followed by a two-step PCR procedure consisting of 50 cycles of 15 seconds at 95°C and 1min at 60°C. PCR amplification was performed using the ABI Prism 7500 Sequence Detector System (Applied Biosystems). The normalized value of the SIV proviral DNA load was calculated as SIV DNA copy number/Mac albumin gene copy number×2×106, and expressed as the number of SIV proviral DNA copies per 106 PBMCs or cells. In addition an ultrasensitive nested quantitative real time PCR and RT-PCR approach was also used to identify SIV RNA or DNA in vaginal and rectal tissues before and after CD8+ T-cell depletion, as previously described (18).
Transmitted/founder viruses and their progeny were identified by single-genome amplification (SGA) of SIV RNA from plasma or rectal pinches. SIV RNA was extracted, and limiting-dilution PCR of newly synthesized cDNA was performed. Reverse transcription of RNA to single-stranded cDNA was performed using SuperScript III reverse transcriptase according to manufacturer's recommendations (Invitrogen) using gene specific priming: SIVEnvR1 5′-TGT AAT AAA TCC CTT CCA GTC CCC CC-3′. The envelope gene was then amplified via limiting dilution PCR where only one amplifiable molecule was present in each reaction using a 1× PCR buffer consisting of 2 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate, 0.2 μM of each primer, and 0.025 U/μl Platinum Taq polymerase (Invitrogen) in a 20-μl reaction. First round PCR was performed with sense primer SIVEnvF1 5′-CCT CCC CCT CCA GGA CTA GC-3′ and antisense primer SIVEnvR1 under the following conditions: 1 cycle of 94°C for 2 min, 35 cycles at 94°C for 15 sec, 55°C for 30 sec, and 72°C for 4 min, followed by a final extension of 72°C for 10 min. Nested PCR was performed with primers SIVEnvF2 5′-TAT AAT AGA CAT GGA GAC ACC CTT GAG GGA GC-3′ and SIVEnvR2 5′-ATG AGA CAT RTC TAT TGC CAA TTT GTA-3′ under the same conditions used for first-round PCR, but with a total of 45 cycles. Transmitted/founder virus lineages were determined phylogenetically by identifying all distinct, low-diversity lineages, as described previously (20, 39, 47-49). All 388 sequences are deposited in GenBank under accession number KF646830-KF647217 (http://www.ncbi.nlm.nih.gov/genbank).
CD8+ cell depletion
CD8+ lymphocyte depletion was performed in eleven macaques. Animals were infused with the αCD8 depleting rhesus recombinant antibody M-T807R1, obtained from the Non-Human Primate Reagent Resource. Animals were given three doses of αCD8 antibodies on days 0, 3 and 7. The first dose was administered at a concentration of 10mg/kg, while the other two doses were given at 5mg/kg. The number or frequency of CD3 (cloneSP34-2), CD4 (clone L200), CD8 (clone DK25 Dako), and CD20 (clone B9E9 Beckman Coulter) expressing cells was monitored in the blood, vaginal, and rectal biopsies. The CD8 antibody used for flow cytometry was chosen as it has been shown not to compete with or mask the epitope of the CD8 depleting antibody (50, 51).
Results
Induction of T-cell responses by Intra-vaginal HPV-PsV vaccination
Thirty-six rhesus macaques were distributed into three groups (Figure. 1a): HPV-group 1, ALVAC/HPV-group 2, and controls-group 3. Each group contained 3 MamuA*01 positive animals. The HPV vaccine group was given three intra-vaginal vaccinations with HPV PsVs that expressed the SIV genes gag-pro, gp120, and rev, tat, nef (HPV-PsV-SIV) at weeks 6, 10 and 24. At weeks 10 and 24, animals were also given monomeric gp120 protein adjuvanted in alum and monophosphoryl lipid A (MPL). In the second cohort of animals (ALVAC/HPV group 2), the systemic immune system was primed with ALVAC expressing SIV genes gag, pol and gp160 (40) at weeks 0 and 4 and then boosted intravaginally with HPV-PsV-SIV and intramuscularly with gp120 protein in alum and MPL similar to the group 1 animals. Twelve control macaques (group 3) were vaccinated with the ALVAC empty vector, HPV-PsVs that expressed luciferase, and were given the adjuvants at the same time and dose as the other groups.
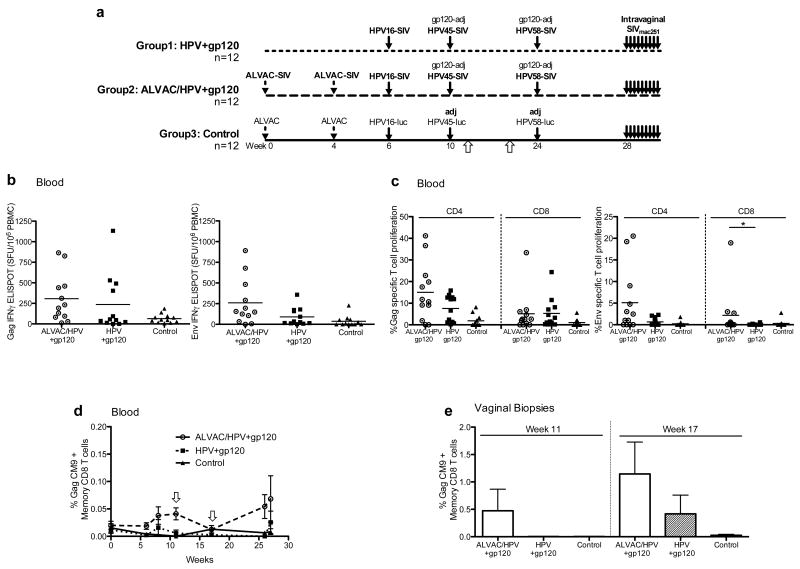
Figure 1. HPV-PsV-SIV vaccines induce cell mediated responses in the blood and female genital tract.
(a) Overview of the vaccination regimen that includes thirty-six macaques, group 1 was given HPV-PsV-SIV+gp120 vaccines, group 2 ALVAC-SIV followed by HPV-PsV-SIV+gp120. The3rd group is the controls that were given the ALVAC-mock vector and HPV-PsV-luciferase. All animals were given alum and MPL adjuvants, represented as adj. Vaccine induced immune responses were measured in blood at various time points throughout the study and in vaginal biopsies at weeks 11 and 17, indicated by white arrows below the regimen. (b) Cell-mediated responses in blood, measured using IFN-γ ELISPOT at week 17 post vaccination. Shown is the number of spot forming units (SFU) per 106 PBMCs after Gag peptide stimulation (left) or Env peptide stimulation (right). Gag/Env specific responses are shown after background subtraction of unstimulated cells. Circles represent animals in the ALVAC/HPV group, squares the HPV group, and control animals are in triangles. (c) The frequency of proliferating CD4+ and CD8+ memory (CD95+) T-cells in blood is shown 1 week after the last vaccination, week 25. Proliferating cells are calculated as the percentage of CFSE dim cells after 5 days of culture with SIVmac251 Gag protein (left) or SIVmac251 Env protein (right). Data presented are after background subtraction of unstimulated cells. A significant difference in CD8+ T-cell Env proliferation was observed between the ALVAC/HPV group and the HPV group, represented by a * using the Mann-Whitney-Wilcoxon test with a p value of 0.039. (d) The frequency of Memory CD95+ Gag CM9+ CD8+ T-cells in blood of MamuA*01+ animals over the course of the vaccination. White arrows indicate weeks 11 and 17 post vaccination (e) Memory CD95+ Gag CM9+ CD8+ T-cells in the vaginal tract measured after the second HPV-PsV vaccination at weeks 11 and 17. The ALVAC/HPV group is in white bars, the HPV group hatched bars, and controls black bars.
Previously, we demonstrated that intra-vaginal delivery of HPV-PsVs expressing SIV Gag recruited CD4+ and CD8+ T-cells to the female genital tract, and resulted in SIV specific responses in the cervico-vaginal lamina propria(37). In this study, we extend and confirm those findings and measured T-cell responses in the blood throughout the study, however cervico-vaginal biopsies were limited to baseline (before vaccination), weeks 11 and 17 (1 and 6 weeks after the second HPV vaccination) Figure 1a. This was done to allow sufficient time for healing before the intra-vaginal SIV challenge at week 28. Vaccination induced similar SIV Gag and Env-specific immune responses in the blood of both vaccine groups measured by IFN-γ ELISPOT at 17 weeks post vaccination (Figure 1b). Proliferative responses were measured one week after the last vaccination (Figure 1c). ALVAC primed HPV+gp120 boosted animals had higher levels of CD4+ Gag and Env T-cell proliferation compared with the HPV group, although the difference was not significant (Figure 1c). Env specific CD8 T-cell proliferative responses were higher in the ALVAC/HPV group compared to the HPV group (p=0.039). Gag CM9 staining in Mamu-A*01 positive animals revealed no Gag-specific CD8+ T-cells in the blood of animals from the HPV or control groups (Figure 1d). As expected, the ALVAC/HPV group developed a systemic CD8+ Gag response that was boosted by intra-vaginal HPV-PsV-SIV vaccinations. The frequency of GagCM9 CD8+ T-cells was also determined in the vaginal tract after the second HPV vaccination at weeks 11 and 17. Similar to the blood, Gag-specific CD8+ T-cells were detected in the ALVAC/HPV group at week 11 but not in the HPV group (Figure 1e). HPV PsV entry in wounded keratinocytes is a slow process taking many hours, with peak expression on days 2- 3(31). Thus, 7 days post vaccination may not have been sufficient time for antigen presentation and T-cell expansion. However, 6 weeks later, T-cell responses were detected in the HPV group and were expanded in the ALVAC/HPV group. The frequency of Gag-specific T-cells was 14 to 16 fold greater in the vaginal tract compared to the blood, and 2 to 3 fold higher in the vaginal tract of the ALVAC/HPV group compared to the HPV group. Gag-specific CD8+ T-cells were not detected in the rectum of vaccinated animals at week 17 (data not shown). The increased T-cell response, observed in the vaginal tract of the ALVAC/HPV group, and the absence of Gag-specific T-cells in the rectum of ALVAC-SIV primed animals suggests that systemic priming followed by intra-vaginal boosting recruits and/or expands cell-mediated responses in the female genital tract.
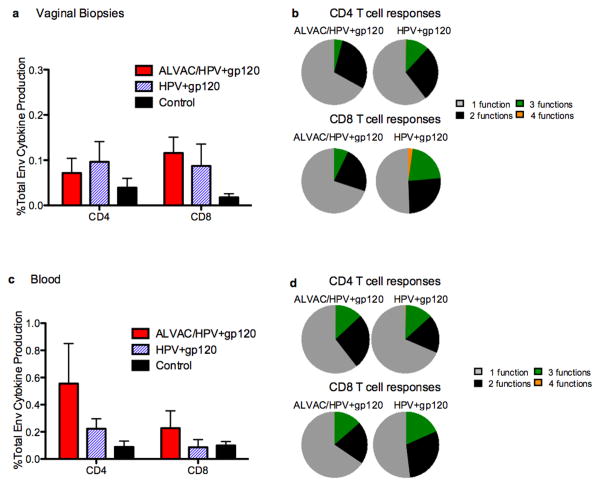
Pinch biopsies of cervico-vaginal tissues yield a limited number of mononuclear cells. Mononuclear cells isolated from the Mamu-A*01 positive animals were used to measure the frequency of GagCM9+CD8+ T-cells, while cells from the remaining twenty-seven Mamu-A*01 negative animals were used to measure functional mucosal responses to envelope peptides at week 17 (Figure 2a). Intracellular cytokine staining for IFN-γ, TNF-α, IL2, as well as the expression of CD107, was determined following 6-hour stimulation with overlapping Env peptides. Vaccination induced mainly mono-functional CD4+ and CD8+ T-cell responses that secreted IFN-γ, TNF-α, or CD107 (Figure 2b). The frequency of env specific T-cells was similar in the two vaccination regimens. Two weeks before the first SIV challenge (week 26), a similar analysis of cytokine profile was performed in the blood of all vaccinated animals (Figure 2c). ALVAC/HPV vaccinated animals had a greater frequency of blood CD4+ T-cell responses compared to the HPV group. Similar to the vaginal tract, primarily monofunctional memory responses were induced in blood (Figure 2d), however TNF-α was the dominating cytokine response in the ALVAC/HPV group, while either TNF-α, IFN-γ or IL2 was produced in the HPV group.
Figure 2. Vaccination induced mainly monofunctional responses in the blood and vaginal tract.
(a) Cytokine production following env peptide stimulation of mononuclear cells from vaginal biopsies obtained at week 17. Shown is the sum of IFN-γ, TNF-α, IL2 and CD107 production after background subtraction in CD95+CD4+ and CD95+CD8+ T-cells. (b) The functional capacity of the SIV specific response is represented by the pie charts, they show the proportion of cells that responded to stimulation by producing either: IFN-γ, TNF-α, IL2 or CD107 or a combination thereof. The fraction of cells that responded to stimulation by producing 1 cytokine is shown in grey, two cytokines black, three cytokines green, or four cytokines orange. CD4 responses are in the top pie panel and CD8 responses in the lower pie panel (c) Total env specific cytokine production in blood after the last vaccination week 26 in CD95+CD4+ and CD95+CD8+ T-cells. Shown is the sum of IFN-γ, TNF-α, IL2 and CD107 production. Pies show the fraction of cells that responded to stimulation by producing 1 cytokine (grey), two cytokines (black), three cytokines (green), or four cytokines (orange). CD4 responses are in the top pie panel and CD8 responses in the lower pie panel.
Systemic and mucosal gp120 specific antibodies induced by vaccination
ALVAC-SIV priming induced gp120 specific IgG in the blood, but by the end of the vaccination regimen both groups had similar levels of high titer binding antibodies (Figure 3a). To assess antibodies in mucosal secretions, we collected vaginal swabs after the last vaccination. Equivalent levels of gp120-specific IgG were found in the ALVAC/HPV and HPV groups presented as titer/ug total IgG to normalize for the levels of total IgG isolated from each animal (Figure 3b). Before normalization by total IgG, we directly compared the gp120 specific titers in each animal's blood and vaginal mucosa and observed that on average env specific IgG were approximately one log lower in the vaginal mucosa than in blood. Low levels of gp120 specific IgA were detected in the vaginal secretions of both vaccinated groups (Figure 3b). A similar frequency of env specific memory B-cells were measured in both groups one week prior to SIV challenge (Figure 3c). While both vaccine regimens induced measurable IgG+ gp120-specific B cells, no IgA+ gp120 specific B cells were detected in blood (data not shown).
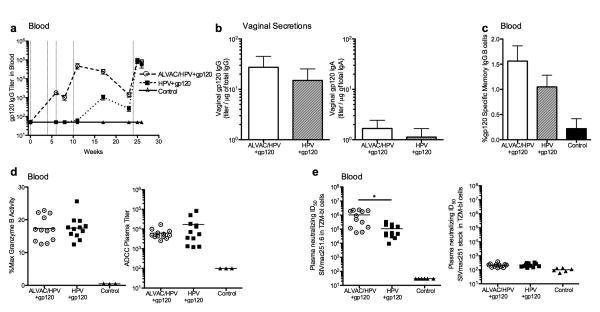
Figure 3. Vaccine induced binding antibodies and their functional capacity.
(a) Glycoprotein 120 specific IgG titer in blood during the vaccination phase with the ALVAC/HPV group represented as circles and with a large dashed line, the HPV group squares with a small dashed line, or controls triangles with a solid line. Vertical lines indicate the time when a vaccine was given: ALVAC weeks 0 and 4, HPV-PsVs weeks 6, 10 and 24 and gp120/adjuvant weeks 10 and 24. (b) Gp120 binding antibody titers in mucosal secretions per μg of total IgG/IgA measured after the last vaccination, week 25. IgG is shown on the left while IgA is on the right. The ALVAC/HPV group is in white bars and the HPV group in hatched bars. (c) Percent gp120 specific IgG memory B-cells measured by B-cell ELISPOT in PBMCs at week 27. (d) ADCC in the blood shown as % Granzyme B activity left, or ADCC Titer right measured at week 26. Circles represent animals in the ALVAC/HPV group, squares the HPV group, and triangles the control group. (e) Neutralization of an easy to neutralize tier 1-like virus SIVmac251.6 (left) and the SIVmac251 challenge stock (right) measured at week 26 after the last vaccination. A significantly higher level of neutralization was observed in the ALVAC/HPV group indicated by the * using the Mann-Whitney-Wilcoxon test p value p=0.0023.
The functional capacity of antibodies induced by the two vaccine regimens was determined in the blood, due to the limited quantity of protein extracted from vaginal swabs. The two vaccine regimens induced serum antibodies that mediated similar levels of antibody dependent cellular cytotoxicity (ADCC), measured as % maximum granzyme activity and ADCC titer (Figure 3d). In contrast, the ALVAC/HPV group had significantly greater neutralization titers for the tier-1-like SIVmac251.6 virus compared to the HPV group p=0.0023 (Figure 3e). Neither vaccine regimen induced antibodies that neutralized the tier-2-like SIVmac251.30 isolate (data not shown) or the SIVmac251 challenge stock (Figure 3e).
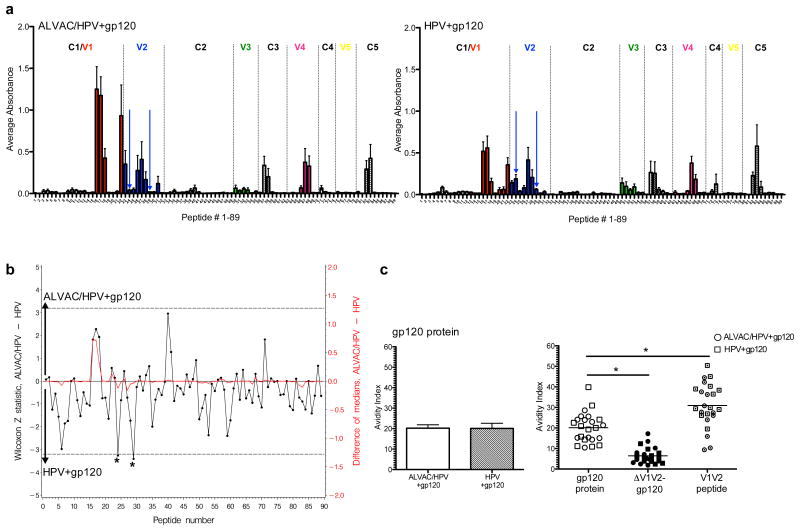
Antibodies to the V1/V2 loop of gp120 were found to be a correlate of reduced HIV risk in the RV144 Thai trial using ALVAC-HIV and gp120 immunogens (9). In another study involving ALVAC-SIV/gp120 vaccination, we found that animals that resisted SIVmac251 infection had high avidity antibodies directed to the V1/V2 region (39). We therefore measured vaccine-induced antibody binding to overlapping linear peptides that spanned gp120, including the V1/V2 region, and antibody avidity. Both regimens had an overall similar recognition of overlapping peptides spanning the constant and variable regions of gp120 (Figure 4a). We compared the average binding of each peptide in the two vaccination regimens using the Z statistic of the Mann-Whitney-Wilcoxon test (Figure 4b). After the correction for multiple comparisons by the Hochberg method, two peptides 24 and 29 in the V2 loops had significantly greater antibody recognition in the HPV group (Figure 4b). The ALVAC/HPV group showed increased binding to peptides 16 and 17 within the C1/V1 region and to peptide 40 in the C2 regions of gp120, but the difference was not statistically significant. The avidity of antibodies to the whole gp120 protein of SIVmac239 was evaluated after sodium thiocyanate treatment. On average, antibodies from both vaccine regimens had a similar avidity index (Figure 4c).
Figure 4. Both vaccination regimens induce antibodies that target the V1/V2 region of gp120.
(a) Average antibody binding to 89 overlapping peptides that span gp120 with the ALVAC/HPV group on the left and the HPV group on the right. Variable regions are in colored bars and dashed vertical lines designate the boundaries of the constant and variable regions. Blue arrows indicate peptides 24 and 29 in the V2 loop. (b) Comparative recognition of overlapping peptides presented as the difference between the median absorbance in the ALVAC/HPV group, relative to the HPV group, shown in red on the right y axis. The statistical significance of the differences is shown by the Z statistic of the Mann-Whitney-Wilcoxon test in black, on the left y axis, with the dashed lines marking significance at the p=0.05 level after the correction for multiple comparisons by the Hochberg method. Increased recognition by the ALVAC group is presented as positive (0 to 5) on the top half of the graph, while increased recognition in the HPV group is presented as negative (0 to -5) on the lower half of the graph. Two peptides in the V2 loop 24 and 29 demonstrated significantly greater antibody recognition in the HPV group relative to the ALVAC group, denoted by asterisks. (c) Avidity, shown as the avidity index of antibodies to the entire gp120 of SIVmac239 on the left. The ALVAC/HPV group is in the white bars and the HPV group in hatched bars. On the right is the comparison of the avidity of antibodies induced by both vaccine regimens to the entire gp120 of SIVmac239, a gp120 protein deleted of the V1/V2 loop (ΔV1/V2), and a conformational mini protein containing the entire V1/V2 step loop of SIVmac239. A significant difference in the avidity index is observed between gp120 and ΔV1/V2-gp120 p<0.0001 and between gp120 and the V1/V2 mini protein p=0.0011, indicated by the * using the Wilcoxon signed rank test.
To determine the contribution of the V1/V2 region of gp120, the avidity index of vaccine induced antibodies was assessed using a gp120 protein in which the V1/V2 loop was deleted (ΔV1/V2) and a conformational protein containing the entire V1/V2 stem loop of SIVmac239, linked to a tag from the C-terminal of HIVgp120. A significant reduction in the avidity index was observed when the V1/V2 region of gp120 was deleted p<0.0001 (Figure 4c). The average avidity to the entire gp120 was 20.1, while the ΔV1/V2 avidity index was 6.4. Furthermore, when the avidity index of antibodies to the V1/V2 mini protein was assessed, an average avidity of 30.9 was observed, a significant increase when compared to gp120 protein p=0.0011 (Figure 4c). In some animals, the V1/V2 avidity was greater than 40. Interestingly, an avidity index of 35-45 has been observed in other vaccination regimens in macaques protected from SIVmac251 and SIVsmE660 infection(15, 39).
Vaccination with ALVAC-SIV/HPV-PsV-SIV/gp120 influence persistent viremia
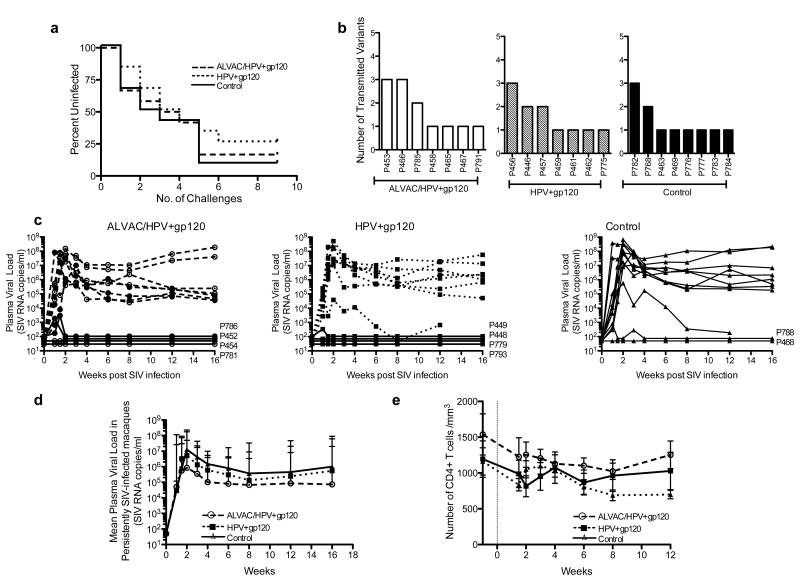
The efficacy of each vaccination regimen was assessed by challenging animals with up to nine intra-vaginal low doses of SIVmac251 (250TCID50), given every 10 days, beginning four weeks after the last vaccination. The level of SIV RNA was determined in plasma by NASBA, 7 days after each challenge; animals that tested negative (<50 copies/ml) were re-challenged. All three groups acquired SIV at a similar rate (Figure 5a), and at the end of the challenge phase five vaccinated animals, two in the ALVAC/HPV group, and three in the HPV group, remained SIV negative in plasma, while one control animal remained negative. Since most HIV infections are initiated with a single or few viral variants, we aimed to model this outcome in our mucosal challenge experiment in macaques. The number of transmitted viral variants is an independent analysis of a limiting dose challenge (47). Thus, we quantified the number of variants in all SIV infected animals that had at least two viral load measurements greater than 104 RNA copies/ml and created neighbor joining trees for each group (Supplemental Figure 1). No difference in the number of transmitted variants was observed between the three groups. Each group had a median of one viral variant and a maximum of three (Figure 5b and Supplemental Figure 1). This suggests that our intra-vaginal SIVmac251 was given at a dose that models HIV heterosexual transmission. Furthermore, neither the intra-vaginal vaccination nor the progesterone/N9 treatment caused a significant increase in the number of transmitted variants. A similar number of variants (median 1) were also observed in naïve macaques that were given a low dose challenge by the vaginal or rectal route (39) (N. Miller NIAID, unpublished observations). No significant associations were observed between vaccine-induced immune responses and the number of transmitted variants.
Figure 5. Vaccine efficacy and plasma viral loads post SIV infection.
(a) The rate of SIV infection is shown by the percentage of uninfected animals at each challenge in the control group (black solid line), ALVAC/HPV group (large dashes), and the HPV group (small dashes). (b) The number of transmitted founder viral variants is shown for each vaccine group with the ALVAC/HPV group represented by open bars, the HPV group hatched bars, and the control group by black bars. The number of variants was determined during the first two weeks of infection in animals that had two successive positive tests for SIV RNA in plasma >104 copies/ml. (c) Plasma viral load over time in the ALVAC/HPV group open circles, HPV group squares, and control group triangles. The animal codes of animals with transient plasma viremia, or that tested negative for SIV RNA in plasma, are shown to the right of each graph (d) Geometric mean of plasma viral load in animals that were persistently SIV infected. Persistent infection was defined as two successive positive tests for SIV RNA in plasma >104 copies/ml. (e) The average absolute number of CD4+ T-cells in the blood per cubic mm is shown for persistently SIV infected animals in the ALVAC/HPV group (open circle), the HPV group (squares), and control group (triangles).
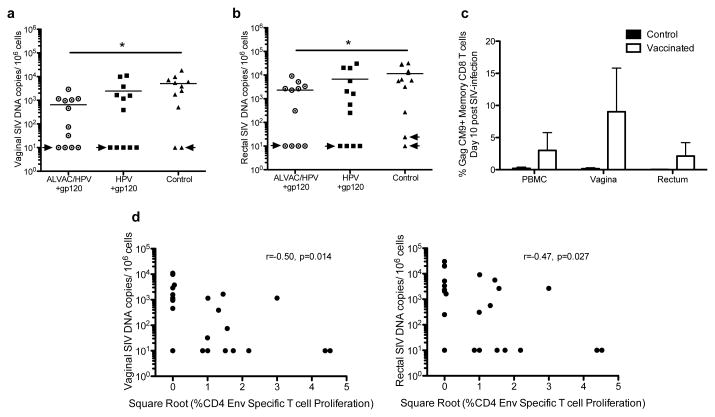
Most infected animals demonstrated high peak (106-108) and set point (105-107) plasma virus (Figure 5c). However, of the vaccinated animals, 8 had no detectable plasma virus or transient plasma viremia that remained below the limit of assay detection: 50 SIV RNA copies/ml (Figure 5c). In total, sixteen of twenty-four vaccinated animals and ten of twelve controls demonstrated persistent SIV viremia. Persistent viremia was defined as at least two successive plasma viral load measurements over 104 SIV RNA copies/ml. We next compared the viremia over the 16 weeks of follow up in the persistently SIV infected animals. No significant differences in either peak or set point viremia in vaccinated animals or controls were observed (Figure 5d). In addition, a similar loss of CD4+ T-cells was observed in the blood of all persistently SIV infected macaques (Figure 5e). In order to assess virus levels in mucosal tissues, pinch biopsies were collected from the vagina and rectum during acute SIV infection, and the levels of SIV DNA determined (Figure 6a and 6b). Unlike our findings for plasma viremia, significantly less SIV DNA was measured in the vaginal and rectal mucosa during the acute phase in the ALVAC/HPV vaccinated animals in comparison to controls p=0.014 and p=0.022 (Figure 6a and 6b). Reduced viral DNA in the mucosa during the acute phase of SIV infection was also temporally associated with the expansion of GagCM9+CD8+ T-cells measured 10 days post SIV infection (Figure 6c), with the vaginal tract having the highest frequency of SIV specific CD8+ T-cells and the lowest virus DNA levels in the acute phase of infection.
Figure 6. Reduced SIV DNA in mucosal tissues of ALVAC/HPV vaccinated animals.
(a) Vaginal SIV DNA levels per 106 cells, circles represent the ALVAC/HPV group, squares the HPV group, and triangles the controls. Significantly less SIV DNA was present in the vaginal tissues of the ALVAC/HPV group compared to the control group, denoted by an * using the Mann-Whitney-Wilcoxon test with a p value of p=0.014. Arrows indicate animals that had either transient plasma viremia or tested negative for SIV RNA in plasma (b) Rectal SIV DNA per 106 cells in vaccinated macaques and controls. Significantly less SIV DNA was present in the rectal tissues of the ALVAC/HPV group compared to controls denoted by an * using the Mann-Whitney-Wilcoxon test with a p value of p=0.022 (c) Mononuclear cells from the blood, vaginal and rectal biopsies were obtained ten days post SIV infection. The frequency of memory (CD95+) Gag CM9 specific CD8+ T-cells is shown in the vaccinated animals (white bars) and controls (black bars). (d) An inverse correlation was observed between the levels of SIV DNA in the vaginal tract (left), rectum (right), and the env specific proliferating memory (CD95+) CD4+ T-cells presented as the square root of the data. The correlation was assessed using a non-parametric Spearman test with r-values of -0.5 and -0.47 and p values of 0.014 and 0.027 for the vaginal and rectal tract respectively.
CD4+T-cells and antibodies to V1/V2 correlate with protection from persistent viremia
We investigated potential associations between mucosal SIV DNA levels in the acute phase and vaccine induced responses. We observed an inverse correlation between the level of Env specific CD4+ T-cell proliferation in blood measured two weeks after the last vaccination and SIV DNA in the vaginal and rectal tract post SIV infection r= -0.5, -0.47 and p= 0.014 and 0.027 for vaginal and rectal tissues respectively (Figure 6d). We did not observe a correlation between vaccine induced CD8 T cell responses and SIV viral loads. However the temporal expansion of vaginal CD8 T cell during the acute phase and association of CD4 helper responses with reduced viremia may indicate that the increased CD4+T-cell responses induced by the ALVAC/HPV may have helped the development of a secondary CD8+ T-cell response, which in turn affected virus replication in the mucosa.
Next, we investigated the four animals with transient viremia; these animals had virus loads ranging from 50-104 copies/ml and then controlled viremia during the remaining repeated low dose challenges and for 14 weeks of follow up. We questioned whether their SIV-specific immune responses were boosted during the successive SIV challenges. We observed a reduction in gp120 binding antibodies and IFN-γ ELISPOT responses to Gag in the vaccinated animals comparing responses before the first SIV challenge to samples collected after the 5th challenge (Supplemental data 2a). In addition, we did not detect a Vif specific response in the cervico-vaginal tract at the end of the challenge phase (Supplemental data 2b). Vif is not in any of the vaccines administered, but is abundant in the challenge virus. Furthermore, the levels of gp120 binding antibody titers in the vaginal secretions had also declined following the 9th and final SIV challenge, when compared to pre SIV levels, consistent with the findings in blood (Supplemental data 2c).
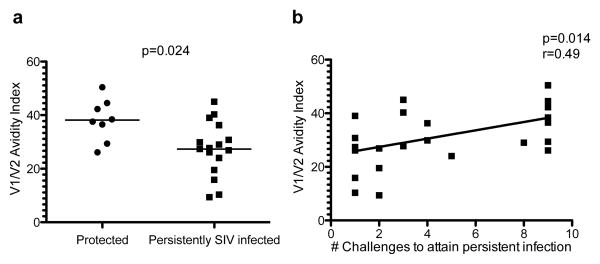
The two distinct outcomes observed in this study, i.e. persistent SIV infection versus protection from infection or high virus replication, gave us the opportunity to investigate any associations between the measured immune responses and outcome. A comparison of immune responses in protected animals and persistently SIVmac251 infected macaques yielded a significant difference in the levels of antibodies with high avidity to the V1/V2 region (Figure 7a). Furthermore, we found a significant correlation between the number of challenges to attain persistent infection and the avidity index of V1/V2 antibodies in blood (Figure 7b). In all, these data highlight the importance of Env-specific CD4+T-cells in the containment of virus replication at mucosal sites and of high avidity antibodies targeted to the V1/V2 region of gp120 in the prevention of SIVmac251 acquisition.
Figure 7. High avidity antibodies to the V1/V2 region associated with delayed persistent SIV infection.
(a) Avidity to V1/V2 in the plasma of vaccinated animals grouped by infection status. The animals with persistent SIV infection were compared to the animals that were either protected from SIV infection or from high viremia. A significant difference was observed using a Mann Whitney-Wilcoxon test with a p value of 0.024 (b) A direct correlation was observed between the V1V2 antibody avidity and the number of SIV challenges needed to attain persistent SIV infection, assessed by a Spearman test r=0.49 and p=0.014. Animals that were either protected from infection or had only transient viremia are represented at challenge 9.
CD8 T-cells contribute to protection from persistent viremia
Three weeks after the 9th SIV challenge, SIV-negative animals and animals with transient viremia had less than 50 copies of SIV RNA in plasma and were tested for SIV DNA in the mucosa (Figure 6a and 6b arrows). With the exception of one of the control animals that had 24 copies of SIV DNA/106 cells in the rectum, the remaining nine animals tested negative for SIV DNA in the vaginal and rectal tract, using an assay that detects greater than 10 SIV DNA copies/106 cells(45). We followed these ten animals for 14 weeks, testing for SIV RNA in blood, and all remained SIV negative.
In order to investigate more in detail the immune control of viremia, we performed CD8 depletion in nine of the ten animals (one animal was excluded due to surgical complications), as well as in two persistently viremic macaques, as a control. Treatment with anti-CD8 antibody rapidly depleted CD8+ cells in the blood (Supplemental Figure 2d) and caused a significant decline in the frequency of CD8+ cells in the lymph nodes (57%) and rectum (62%) (Supplemental Figure 2e). As expected, in the two animals with persistent viremia, plasma virus levels further increased following CD8 depletion (Supplemental Figure 2f). We collected vaginal and rectal biopsies before CD8 depletion (pre), 9 days post depletion (during) when CD8+ T-cells were undetectable in the blood, and 42 days post treatment (post) when CD8+ T-cells had rebounded, and quantified SIV DNA and RNA using a low copy ultrasensitive assay (18). Both unimmunized animals tested positive at mucosal sites either pre, during, or post CD8+T-cell depletion (Table 1). Of the vaccinated animals, four remained negative, and three of them belonged to the HPV/gp120 group (Table 1), suggesting early and long term control of virus replication. Collectively, these results indicate that undetectable plasma virus does not exclude local infection and highlight the importance of CD8+T-cells in the local control of virus.
Table 1. Virus in mucosal tissues before, during or after CD8+ cell depletion.
| Vaginal Biopsies | Rectal Biopsies | |||||||
|---|---|---|---|---|---|---|---|---|
| Animal | Group | SIV | Pre | During | Post | Pre | During | Post |
| Infected | αCD8+ tx | αCD8+ tx | αCD8+ tx | αCD8+ tx | αCD8+ tx | αCD8+ tx | ||
| P448 | HPV | NO | - | - | - | - | - | - |
| P449 | HPV | NO | - | - | - | - | - | - |
| P779 | HPV | NO | - | - | - | - | - | - |
| P793 | HPV | YES | - | - | - | - | pos | - |
| P781 | ALVAC/HPV | NO | - | - | - | - | - | - |
| P454 | ALVAC/HPV | YES | - | - | - | - | - | pos |
| P786 | ALVAC/HPV | YES | - | - | - | pos | pos | - |
| P788 | Control | YES | pos | - | - | pos | pos | - |
| P468 | Control | YES | - | - | pos | - | - | - |
Abbreviations: (-) No SIV DNA or RNA detected, (pos) Positive for SIV RNA or DNA or both
Discussion
SIV infection of rhesus macaques models key aspects of HIV infection. To date, HIV vaccine candidates tested in the macaque model, using repeated low doses of SIV given across mucosal surfaces, have recapitulated the results of HIV clinical trials(12-14). Furthermore, a low-dose mucosal challenge with an uncloned SIV swarm can be tittered to transmit a single or few virus variants in macaques (49), similar to the bottleneck described during most HIV infections (48). Thus, in this study, we used a repeated intra-vaginal challenge with a SIVmac251 swarm to test the efficacy of a novel mucosal vaccination regimen HPV-PsV-SIV, with and without ALVAC-SIV priming. Neither vaccine regimen significantly altered the rate of SIV acquisition compared to controls, although several animals were either protected from SIV infection or had transient viremia. In all, sixteen of twenty-four (∼67%) vaccinated animals developed persistent infection and ten of twelve (∼83%) of controls. Vaccinated animals that became persistently viremic had similar peak and set-point plasma virus levels, not surprisingly given the low levels of systemic CD8+ T-cell responses induced by these vaccines. However, we observed a significant reduction in viral burden in mucosal tissues in vaccinated animals compared to controls and this reduction was significant in the ALVAC/HPV group that had the highest levels of vaginal Gag specific CD8+ T-cell responses. The limited number of mononuclear cells isolated from the vaginal tract precluded our assessment of vaginal Gag specific responses in all animals and of rev, tat and nef responses. In addition, we observed an inverse correlation between proliferating Env specific CD4+ T-cells in blood and the levels of mucosal SIV DNA. A number of vaccinated animals had a long lasting control of viremia. Altogether, these data suggest that T-cell responses induced by HPV-PsV vaccination exerted early mucosal virus control, but virus expansion likely outpaced T-cell expansion, leading to systemic dissemination and uncontrolled viremia. Surprisingly, the mucosal T-cell response induced by the HPV-PsVs/gp120 regimen did not curtail local virus levels or provide sustained virus control, whereas priming with ALVAC-SIV, that induced an overall higher Gag and Env proliferative T-cell response (Figure 1c) and better control of mucosal virus levels (Figure 6a and 6b), suggesting a role of SIV-specific T-cells (higher in the ALVAC-SIV primed group (Figures 1e and 6c), in early protection from virus replication. In a murine model, intra-vaginal HPV-PsV vaccination induces long-lived CD103+ CD8+ T-cells that home to the site of vaccination and intercalate throughout the epithelial layers of the vaginal tract (32). If HPV-PsV vaccination similarly induces tissue resident CD8+ T-cells in humans as it does in mice, these CD8+ T-cells would be well positioned to combat HIV at the site of virus entry. The exposed columnar epithelium in the cervix of young women is potentially susceptible to HPV vaccination and a likely site of HIV transmission (52).
Progesterone treatment was used to facilitate vaccine delivery and may have influenced the vaccine-induced immune response, as progesterone has been shown to reduce antiviral responses (53). Intra-vaginal delivery of vaccines and the disruption of the epithelium used to facilitate delivery are cumbersome, and they introduce several challenges for clinical applications. However, vaccination in the secretory phase may eliminate the need for hormonal treatment, and the collection of a cytology specimen as routinely done during a pap-smear causes sufficient microtrauma to facilitate HPV vaccination (54). Thus, intra-vaginal HPV vaccination could be easily incorporated into a routine gynecological visit and would potentially confer protection against both HPV and HIV.
Both regimens (ALVAC/HPV and HPV) elicited high titer gp120 specific IgG in the blood and vaginal secretions, but neither regimen induced appreciable levels of IgA in the vaginal secretions, nor detectable gp120 specific IgA+ memory B-cells in the blood. Thus, IgA was unlikely to have played a role in the outcome of these studies. We found similar levels of ADCC, and neither vaccine regimen induced antibodies capable of neutralizing the SIVmac251 challenge stock.
A theme is emerging from non-human primate studies that are consistent with the results of the RV144 Thai trial: non-neutralizing antibodies mediate protection from lentiviral infection. Indeed, in a vaccine regimen that efficiently primes CD8+ T-cells using gp96 Ig to express SIV peptides, protection from SIV infection was only achieved when an antibody inducing protein boost was added to the vaccination regimen(55). Our results similarly support this concept, as delayed persistent SIV infection was associated with the avidity of antibodies directed to the V1/V2 region of gp120 and not with T-cells. The functional role of antibodies to the V1/V2 region in the efficacy of HIV vaccines remains to be clearly defined and recent data suggest that monoclonal antibodies to V2 synergize with other envelope region to neutralize the virus (56). The studies described underscore the importance of this immunologic target, as we demonstrated that antibodies targeting the V1/V2 region were associated with delayed virus acquisition as in the case of HIV in RV144. These data suggest the relevance of this animal model for the preclinical evaluation of HIV vaccine candidates.
Supplementary Material
Acknowledgments
We would like to thanks to Dr. Nancy Miller and the Division of AIDS for the SIVmac251 virus stock and supporting the measurement of several antibody responses. We would also like to thank Dr. Jean Charles Grivel for a processing protocol for vaginal biopsies, Robyn Parks for help processing samples and Teresa Habina for editing the manuscript. We thank Cynthia Thompson for helping with HPV PsV's expansion and Kathy McKinnon for flow cytometric support. Additionally we thank Advanced Bioscience Laboratories, specifically Debora Weiss, Jim Treece, Maria Grazia Ferrari, Hye-kyung Chung, and Eun Mi Lee for their animal care, sample collection, and quantification of SIV RNA and DNA. Finally, we thank Keith Reimann and the NIH reagent resource for the CD8 depleting reagent.
Footnotes
This study was supported by the Intramural budget to GF and JS, an Office of AIDS award, and in part with federal funds from the National Cancer Institute, NIH, under contracts HHSN261200800001E and HHSN266200400088C. John Schiller and Barney Graham are name inventors on US, Application No. 12/863,572 filed 19 Jul 2010. HPV Virus-Like Particles for Delivery of Gene-Based Vaccines. Genoveffa Franchini is named inventor on US application No: PCT/US1992/005107 filed Jun 12, 1992, Immunodeficiency virus recombinant poxvirus vaccine.
References
- 1.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 2011;7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van GF, Hu D, Tappero JW, Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 4.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 5.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del RC, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, Mlisana K, Metch B, de BG, Latka MH, Roux S, Mathebula M, Naicker N, Ducar C, Carter DK, Puren A, Eaton N, McElrath MJ, Robertson M, Corey L, Kublin JG. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011;11:507–515. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, Frahm N, Hural J, Anude C, Graham BS, Enama ME, Adams E, DeJesus E, Novak RM, Frank I, Bentley C, Ramirez S, Fu R, Koup RA, Mascola JR, Nabel GJ, Montefiori DC, Kublin J, McElrath MJ, Corey L, Gilbert PB. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013;369:2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de SM, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 9.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hope TJ. Moving ahead an HIV vaccine: to neutralize or not, a key HIV vaccine question. Nat Med. 2011;17:1195–1197. doi: 10.1038/nm.2528. [DOI] [PubMed] [Google Scholar]
- 11.Sui Y, Gordon S, Franchini G, Berzofsky JA. Nonhuman primate models for HIV/AIDS vaccine development. Curr Protoc Immunol. 2013;102 doi: 10.1002/0471142735.im1214s102. Unit 12 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qureshi H, Ma ZM, Huang Y, Hodge G, Thomas MA, DiPasquale J, DeSilva V, Fritts L, Bett AJ, Casimiro DR, Shiver JW, Robert-Guroff M, Robertson MN, McChesney MB, Gilbert PB, Miller CJ. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine. J Virol. 2012;86:2239–2250. doi: 10.1128/JVI.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Rompay KK, Abel K, Lawson JR, Singh RP, Schmidt KA, Evans T, Earl P, Harvey D, Franchini G, Tartaglia J, Montefiori D, Hattangadi S, Moss B, Marthas ML. Attenuated Poxvirus-Based Simian Immunodeficiency Virus (SIV) Vaccines Given in Infancy Partially Protect Infant and Juvenile Macaques Against Repeated Oral Challenge With Virulent SIV. J Acquir Immune Defic Syndr. 2005;38:124–134. doi: 10.1097/00126334-200502010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds MR, Weiler AM, Piaskowski SM, Piatak M, Jr, Robertson HT, Allison DB, Bett AJ, Casimiro DR, Shiver JW, Wilson NA, Lifson JD, Koff WC, Watkins DI. A trivalent recombinant Ad5 gag/pol/nef vaccine fails to protect rhesus macaques from infection or control virus replication after a limiting-dose heterologous SIV challenge. Vaccine. 2012;30:4465–4475. doi: 10.1016/j.vaccine.2012.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai L, Kwa S, Kozlowski PA, Montefiori DC, Ferrari G, Johnson WE, Hirsch V, Villinger F, Chennareddi L, Earl PL, Moss B, Amara RR, Robinson HL. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J Infect Dis. 2011;204:164–173. doi: 10.1093/infdis/jir199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, DiPasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF, Robert-Guroff M. Replicating adenovirus-SIV recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low dose rectal SIVmac251 challenge. J Virol. 2012;86:4644–4657. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel V, Jalah R, Kulkarni V, Valentin A, Rosati M, Alicea C, von GA, Huang W, Guan Y, Keele BF, Bess JW, Jr, Piatak M, Jr, Lifson JD, Williams WT, Shen X, Tomaras GD, Amara RR, Robinson HL, Johnson W, Broderick KE, Sardesai NY, Venzon DJ, Hirsch VM, Felber BK, Pavlakis GN. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous simian immunodeficiency virus challenge. Proc Natl Acad Sci U S A. 2013;110:2975–2980. doi: 10.1073/pnas.1215393110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaccari M, Keele BF, Bosinger SE, Doster MN, Ma ZM, Pollara J, Hryniewicz A, Ferrari G, Guan Y, Forthal DN, Venzon D, Fenizia C, Morgan T, Montefiori D, Lifson JD, Miller CJ, Silvestri G, Rosati M, Felber BK, Pavlakis GN, Tartaglia J, Franchini G. Protection afforded by an HIV vaccine candidate in macaques depends on the dose of SIVmac251 at challenge exposure. J Virol. 2013;87:3538–3548. doi: 10.1128/JVI.02863-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rerks-Ngarm S, Paris RM, Chunsutthiwat S, Premsri N, Namwat C, Bowonwatanuwong C, Li SS, Kaewkungkal J, Trichavaroj R, Churikanont N, de Souza MS, Andrews C, Francis D, Adams E, Flores J, Gurunathan S, Tartaglia J, O'Connell RJ, Eamsila C, Nitayaphan S, Ngauy V, Thongcharoen P, Kunasol P, Michael NL, Robb ML, Gilbert PB, Kim JH. Extended evaluation of the virologic, immunologic, and clinical course of volunteers who acquired HIV-1 infection in a phase III vaccine trial of ALVAC-HIV and AIDSVAX B/E. J Infect Dis. 2013;207:1195–1205. doi: 10.1093/infdis/jis478. [DOI] [PubMed] [Google Scholar]
- 22.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, Kostrikis LG, Zhang L, Perelson AS, Ho DD. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. Journal of Experimental Medicine. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 24.Koup RA, Safrit JT, Cao Y, Andrew CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. Journal of Virology. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price DA, Goulder PJ, Klenerman P, Sewell AK, Easterbrook PJ, Troop M, Bangham CR, Phillips RE. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, Feeney M, Tang Y, Holmes EC, Allen T, Prado JG, Altfeld M, Brander C, Dixon C, Ramduth D, Jeena P, Thomas SA, St JA, Roach TA, Kupfer B, Luzzi G, Edwards A, Taylor G, Lyall H, Tudor-Williams G, Novelli V, Martinez-Picado J, Kiepiela P, Walker BD, Goulder PJ. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004;10:282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- 28.Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, Shaw GM. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 29.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, Scholz I, Gilbride RM, Lewis MS, Gilliam AN, Ventura AB, Malouli D, Xu G, Richards R, Whizin N, Reed JS, Hammond KB, Fischer M, Turner JM, Legasse AW, Axthelm MK, Edlefsen PT, Nelson JA, Lifson JD, Fruh K, Picker LJ. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340:1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Fruh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, Picker LJ. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 32.Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, Lowy DR, Schiller JT. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest. 2012;122:4606–4620. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham BS, Kines RC, Corbett KS, Nicewonger J, Johnson TR, Chen M, LaVigne D, Roberts JN, Cuburu N, Schiller JT, Buck CB. Mucosal delivery of human papillomavirus pseudovirus-encapsidated plasmids improves the potency of DNA vaccination. Mucosal Immunol. 2010;3:475–486. doi: 10.1038/mi.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol. 2012;10:681–692. doi: 10.1038/nrmicro2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudolf MP, Fausch SC, Da Silva DM, Kast WM. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitope-specific human T cell responses in vitro. J Immunol. 2001;166:5917–5924. doi: 10.4049/jimmunol.166.10.5917. [DOI] [PubMed] [Google Scholar]
- 36.Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, Schiller JT. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 37.Gordon SN, Kines RC, Kutsyna G, Ma ZM, Hryniewicz A, Roberts JN, Fenizia C, Hidajat R, Brocca-Cofano E, Cuburu N, Buck CB, Bernardo ML, Robert-Guroff M, Miller CJ, Graham BS, Lowy DR, Schiller JT, Franchini G. Targeting the vaginal mucosa with human papillomavirus pseudovirion vaccines delivering simian immunodeficiency virus DNA. J Immunol. 2012;188:714–723. doi: 10.4049/jimmunol.1101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franchini G, Robert-Guroff M, Tartaglia J, Aggarwal A, Abimiku A, Benson J, Markham P, Limbach K, Hurteau G, Fullen J, et al. Highly attenuated HIV type 2 recombinant poxviruses, but not HIV-2 recombinant Salmonella vaccines, induce long-lasting protection in rhesus macaques. AIDS Res Hum Retroviruses. 1995;11:909–920. doi: 10.1089/aid.1995.11.909. [DOI] [PubMed] [Google Scholar]
- 39.Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, Ferrari G, Pal R, Ferrari MG, Whitney S, Hudacik L, Billings E, Rao M, Montefiori D, Tomaras G, Alam SM, Fenizia C, Lifson JD, Stablein D, Tartaglia J, Michael N, Kim J, Venzon D, Franchini G. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol. 2013;87:1708–1719. doi: 10.1128/JVI.02544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pal R, Venzon D, Letvin NL, Santra S, Montefiori DC, Miller NR, Tryniszewska E, Lewis MG, Vancott TC, Hirsch V, Woodward R, Gibson A, Grace M, Dobratz E, Markham PD, Hel Z, Nacsa J, Klein M, Tartaglia J, Franchini G. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J Virol. 2002;76:292–302. doi: 10.1128/JVI.76.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. Journal of virology. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A. 2011;79:603–612. doi: 10.1002/cyto.a.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol. 2005;Chapter 12 doi: 10.1002/0471142735.im1211s64. Unit. [DOI] [PubMed] [Google Scholar]
- 45.Lee EM, Chung HK, Livesay J, Suschak J, Finke L, Hudacik L, Galmin L, Bowen B, Markham P, Cristillo A, Pal R. Molecular methods for evaluation of virological status of nonhuman primates challenged with simian immunodeficiency or simian-human immunodeficiency viruses. J Virol Methods. 2010;163:287–294. doi: 10.1016/j.jviromet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Romano JW, Williams KG, Shurtliff RN, Ginocchio C, Kaplan M. NASBA technology: isothermal RNA amplification in qualitative and quantitative diagnostics. Immunological Investigations. 1997;26:15–28. doi: 10.3109/08820139709048912. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, Mansfield KG, Tomaras GD, Haynes BF, Kolodkin-Gal D, Letvin NL, Hahn BH, Shaw GM, Barouch DH. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, Mascola JR, Nabel GJ, Haynes BF, Bhattacharya T, Perelson AS, Korber BT, Hahn BH, Shaw GM. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitz JE, Simon MA, Kuroda MJ, Lifton MA, Ollert MW, Vogel CW, Racz P, Tenner-Racz K, Scallon BJ, Dalesandro M, Ghrayeb J, Rieber EP, Sasseville VG, Reimann KA. A nonhuman primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am J Pathol. 1999;154:1923–1932. doi: 10.1016/S0002-9440(10)65450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 53.Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol. 2003;77:9845–9851. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts JN, Kines RC, Katki HA, Lowy DR, Schiller JT. Effect of Pap smear collection and carrageenan on cervicovaginal human papillomavirus-16 infection in a rhesus macaque model. J Natl Cancer Inst. 2011;103:737–743. doi: 10.1093/jnci/djr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strbo N, Vaccari M, Pahwa S, Kolber MA, Doster MN, Fisher E, Gonzalez L, Stablein D, Franchini G, Podack ER. Cutting edge: novel vaccination modality provides significant protection against mucosal infection by highly pathogenic simian immunodeficiency virus. J Immunol. 2013;190:2495–2499. doi: 10.4049/jimmunol.1202655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pollara J, Bonsignori M, Moody MA, Liu P, Alam SM, Hwang KK, Gurley TC, Kozink DM, Armand LC, Marshall DJ, Whitesides JF, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb ML, O'Connell RJ, Kim JH, Michael NL, Montefiori DC, Tomaras GD, Liao HX, Haynes BF, Ferrari G. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. 2014 doi: 10.1128/JVI.00156-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.