Abstract
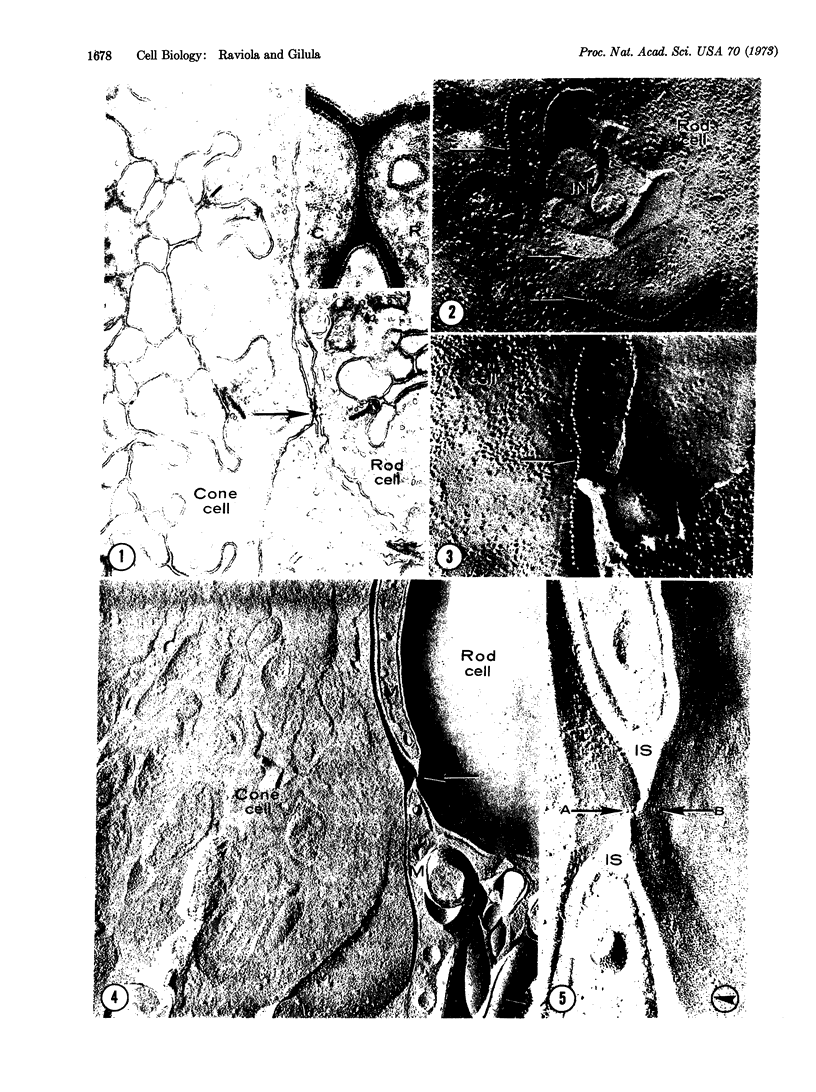
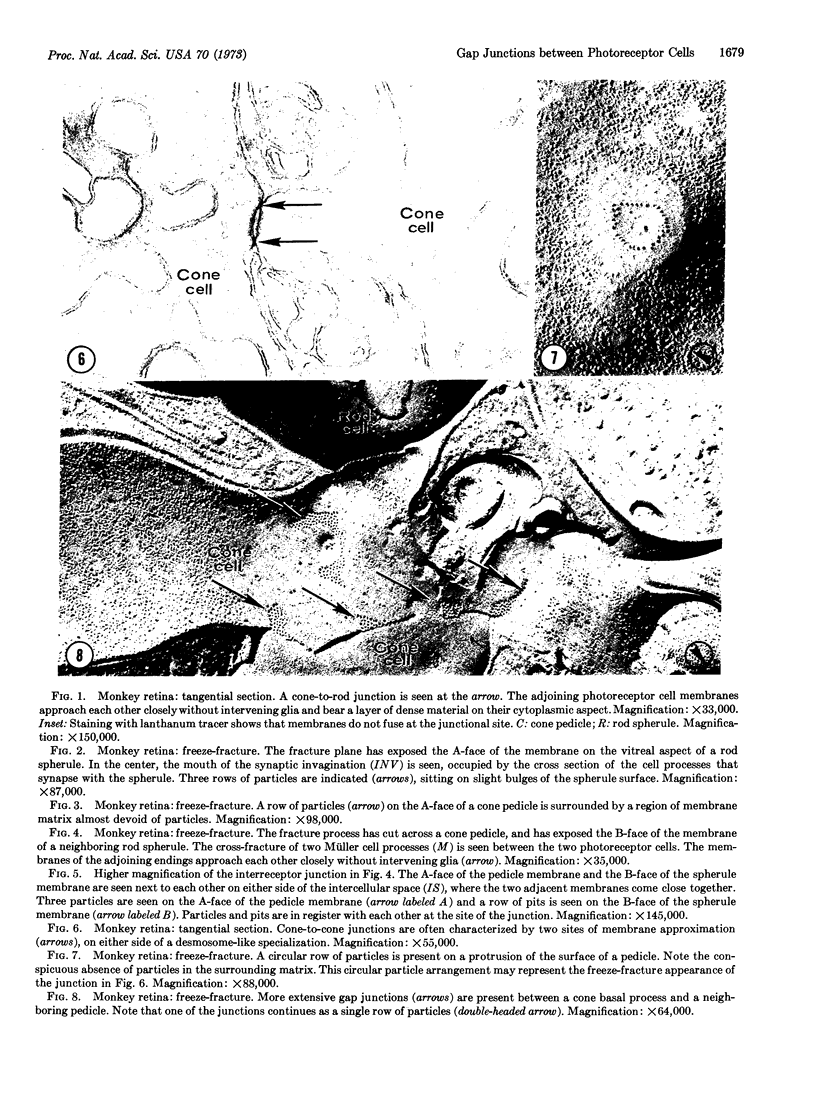
In the outer plexiform layer of the retina the synaptic endings of cone cells make specialized junctions with each other and with the endings of rod cells. The ultrastructure of these interreceptor junctions is described in retinas of monkeys, rabbits, and turtles, in thin sections of embedded specimens and by the freeze-fracturing technique. Cone-to-rod junctions are ribbon-like areas of close membrane approximation. On either side of the narrowing of the intercellular space, the junctional membranes contain a row of particles located on the fracture face A (cytoplasmic leaflet), while the complementary element, a row of single depressions, is located on fracture face B. The particle rows are surrounded by a membrane region that is devoid of particulate inclusions and bears an adherent layer of dense cytoplasmic material. Cone-to-cone junctions in some places are identical to cone-to-rod junctions, while in other places they closely resemble typical gap junctions (nexus). Interreceptor junctions, therefore, represent a morphological variant of the gap junction, and probably mediate electrotonic coupling between neighboring photoreceptor cells.
Keywords: membranes, electron microscopy, freeze-fracturing
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN A. I. SOME OBSERVATIONS ON THE FINE STRUCTURE OF THE RETINAL RECEPTORS OF THE AMERICAN GRAY SQUIRREL. Invest Ophthalmol. 1964 Apr;3:198–216. [PubMed] [Google Scholar]
- Chalcroft J. P., Bullivant S. An interpretation of liver cell membrane and junction structure based on observation of freeze-fracture replicas of both sides of the fracture. J Cell Biol. 1970 Oct;47(1):49–60. doi: 10.1083/jcb.47.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. I. Some electron microscopic observations on inter-receptor contacts in the human and macaque retinae. J Anat. 1965 Jul;99(Pt 3):595–610. [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Dowling J. E. Organization of vertebrate retinas. Invest Ophthalmol. 1970 Sep;9(9):655–680. [PubMed] [Google Scholar]
- Friend D. S., Gilula N. B. Variations in tight and gap junctions in mammalian tissues. J Cell Biol. 1972 Jun;53(3):758–776. doi: 10.1083/jcb.53.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula N. B., Reeves O. R., Steinbach A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972 Feb 4;235(5336):262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A., Revel J. P. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970 May;45(2):272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Cell junctions at the outer synaptic layer of the retina. Invest Ophthalmol. 1972 May;11(5):265–275. [PubMed] [Google Scholar]
- MISSOTTEN L., APPELMANS M., MICHIELS J. L'ULTRA-STRUCTURE DES SYNAPSES DES CELLULES VISUELLES DE LA R'ETINE HUMAINE. Bull Mem Soc Fr Ophtalmol. 1963;76:59–82. [PubMed] [Google Scholar]
- MOOR H., MUHLETHALER K., WALDNER H., FREY-WYSSLING A. A new freezing-ultramicrotome. J Biophys Biochem Cytol. 1961 May;10:1–13. doi: 10.1083/jcb.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Weinstein R. S. The ultrastructure of the nexus. A correlated thin-section and freeze-cleave study. J Cell Biol. 1970 Dec;47(3):666–688. doi: 10.1083/jcb.47.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Karnovsky M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A., Mukherjee T. M., Williams A. W. Freeze-etch appearance of the tight junctions in the epithelium of small and large intestine of mice. Protoplasma. 1969;67(2):165–184. doi: 10.1007/BF01248737. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Marchesi V. T. Demonstration of the outer surface of freeze-etched red blood cell membranes. J Cell Biol. 1970 Jun;45(3):649–653. doi: 10.1083/jcb.45.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]