Abstract
Purpose
To assess the efficacy and toxicity of chemotherapy consisting of cyclophosphamide, doxorubicin (Adriamycin), vincristine, and dexamethasone (CHOD) plus bis-chloronitrosourea (BCNU), cytosine arabinoside, and methotrexate (BVAM) followed by whole-brain irradiation (WBRT) for patients with primary central nervous system lymphoma (PCNSL).
Methods and Materials
Patients 70 years old and younger with newly diagnosed, biopsy-proven PCNSL received one cycle of CHOD followed by two cycles of BVAM. Patients then received WBRT, 30.6 Gy, if a complete response was evoked, or 50.4 Gy if the response was less than complete; both doses were given in 1.8-Gy daily fractions. The primary efficacy endpoint was 1-year survival.
Results
Thirty-six patients (19 men, 17 women) enrolled between 1995 and 2000. Median age was 60.5 years (range, 34 to 69 years). Thirty (83%) patients had baseline Eastern Cooperative Oncology Group performance scores of 0 to 1. All 36 patients were eligible for survival and response evaluations. Median time to progression was 12.3 months, and median survival was 18.5 months. The percentages of patients alive at 1, 2, and 3 years were 64%, 36%, and 33%, respectively. The best response was complete response in 10 patients and immediate progression in 7 patients. Ten (28%) patients had at least one grade 3 or higher neurologic toxicity.
Conclusions
This regimen did improve the survival of PCNSL patients but also caused substantial toxicity. The improvement in survival is less than that reported with high-dose methotrexate-based therapies.
Keywords: Primary central nervous system lymphoma, Chemotherapy, Whole-brain radiotherapy
INTRODUCTION
Primary central nervous system non-Hodgkin’s lymphoma (PCNSL) is increasing in incidence and targets especially vulnerable populations (1). Current treatments are extending survival but are not increasing the number of cured patients. Furthermore, the quality of such survival is often poor (2).
PCNSL clinical trials have evolved over time and can be described by three general approaches. The first approach, whole-brain radiotherapy (WBRT), resulted in a median survival of 11.6 months, only 6.6 months for patients over the age of 60 years (3). The second approach uses combined modality therapies (CMT) using conventional systemic non-Hodgkin’s lymphoma (NHL) therapies. The results were no different than those reported from patients treated with WBRT alone (4). In the third evolution of PCNSL treatment, newer CMTs use systemic NHL therapies modified for the central nervous system. One such regimen consists of cyclophosphamide, doxorubicin, vincristine, and dexamethasone (CHOD)/bis-chloronitrosourea (BCNU), cytosine arabinoside, and methotrexate (BVAM), use of which was piloted by Bessell et al. (5). This regimen combined an effective NHL regimen for bulky disease (CHOD) plus “penetrating” agents such as BCNU, methotrexate (MTX), and histone de-acetylases in dosages sufficient to produce tumoricidal levels in brain parenchyma.
Based on the favorable results reported in the pilot trial of this regimen (5), the North Central Cancer Treatment Group (NCCTG), developed a North American version of the CHOD/BVAM protocol (NCCTG protocol 93-73-51). This report summarizes the effectiveness of the treatment regimen in terms of objective response, toxicity, survival, and patterns of failure, and assesses factors that appear to be associated with outcome.
METHODS AND MATERIALS
Patient eligibility
All eligible patients had one or more intracranial space-occupying lesions based on clinical grounds confirmed by neuroimaging (computed tomography [CT] or magnetic resonance imaging [MRI], preferably MRI). Pathology was confirmed by central review at Mayo Clinic, Rochester. Patients were also eligible if they had characteristic clinical and imaging findings and had cytology findings consistent with malignant lymphoma, obtained either from vitrectomy or lumbar puncture. Because of toxicity concerns regarding the role of intensive chemotherapy in elderly patients, a complementary trial was in development for patients over age 70, results of which have been reported previously (6). Thus, eligibility for this trial was limited to patients 70 years old or younger.
Eligible patients must have had postoperative disease that was evaluable on either enhanced CT or MRI; and patients were required to maintain the same form of imaging as this baseline study throughout the study. Staging to exclude occult systemic NHL was required and was accomplished by following a standard menu of tests (general medical examination; CT of chest, abdomen, and pelvis; and bilateral iliac crest bone marrow aspirate and biopsy) (4). Slit-lamp examination was performed to assess ocular involvement. Patients with evidence of AIDS or a reactive human immunodeficiency virus serology were excluded. This study was reviewed and approved by the institutional research board at each enrolling institution.
Treatment regimen
All treatments were given at a Mayo Clinic facility or at participating NCCTG and Eastern Cooperative Oncology Group (ECOG) member institutions. Therapy began within 3 weeks of PCNSL diagnosis. The schema and details have been reported previously (5) and are summarized in Fig. 1. The day of CHOD administration was defined as Day 1, with dexamethasone continuing for 7 days. MTX (1.5 mg/m2) and ara-C (3 mg/m2) were administered on days 15 and 16, respectively, and continued every 2 weeks for a total of six cycles. Upon completion of CHOD/BVAM chemotherapy, patients began WBRT. Treatment fields included the entire cranial contents and extended inferiorly to the level of C2. Initially, all patients received a total dose of 5,040 cGy. The posterior two-thirds of the eyes were included in the field of radiation if ocular involvement was noted. Due to concerns of neurotoxicity, beginning in March 1998, patients who had a complete response (CR) to chemotherapy received a reduced dose of 30.6-Gy WBRT.
Fig. 1.

Treatment regimen. CHOD = cytoxan (CTX), Adriamycin (ADR), vincristine (VCR), dexamethasone (DXM); BVAM = BCNU, VCR, cytosine arabinoside (ara-C), methotrexate (MTX); NaHCO3 = bicarbonate; CF = leucovorin; VIT B6 = vitamin B6; 1% P-LON = 1% prednisolone; WBRT = whole-brain radiotherapy.
Abbreviations: CHOD = Cytoxan (CTX), Adriamycin (ADR), Vincristine (VCR), Dexamethasone (DXM); BVAM = BCNU, VCR, cytosine arabinoside (ARA-C), methotrexate (MTX); NaHCO3 = bicarbonate; CF = leucovorin; VIT B6 = vitamin B6; 1% P-LON = 1% prednisolone; WBRT = whole brain radiotherapy.
Patient evaluation
Neuroimaging (CT or MRI) was performed at the end of chemotherapy or when clinically indicated. If there was interval progression, patients were removed from the study and were treated at the physician’s discretion. Performance status, mini-mental status examination (MMSE), and toxicity were evaluated at baseline prior to each treatment cycle and with each follow-up evaluation. Toxicity was graded using the National Cancer Institute common toxicity criteria (CTC) version 2.0. Late brain radiotherapy toxicity was graded according to the RTOG/EORTC Late Radiation Morbidity Scoring Schema.
Response evaluation
Objective response to therapy was classified by neuroimaging and patient examination at each of the evaluations. All patients were required to receive regimens of stable or decreasing dosages of corticosteroids to be considered improved. If increasing dosages of corticosteroids were required to maintain neurologic function, the patient was considered to have disease progression and was removed from study. A patient’s CR was defined as complete disappearance of all tumor(s). Response was defined as a reduction of ≥50% in perpendicular diameters on contrast-enhanced scans or mass and no new lesions. For disease that was not bidimensionally measurable, regression was defined as an unequivocal reduction in size on contrast enhancement or a decrease in mass affect with stable or decreasing doses of steroids, as agreed upon independently by the primary physician and the quality control physician; progression was defined as an increase of ≥25% in the product of perpendicular diameters, the appearance of new lesions, an increasing dose of steroids, or an unequivocal increase in size on contrast enhancement of an increase in mass effect, as agreed upon independently by the primary physician and the quality control physician for tumor disease that was not bidimensionally measurable. All other patients were deemed stable. Patients were classified as confirmed responders if the objective response values of CR or response were recorded for them on two or more evaluations performed at least 4 weeks apart.
Study design and statistical methodology
The design chosen for this phase II trial was the Fleming two-stage design (7), because it permits early termination of patient accrual due to evidence of excellent activity as well as of poor activity. The primary efficacy endpoint was defined as 1-year survival. At the time the trial was designed, the median survival of PCNSL patients receiving current therapies was about 10 months, and 1-year survival percentage was 44%, assuming an exponential survival distribution. The 0.05-level Fleming study design was therefore chosen to provide 80% power to detect an increase in 1-year survival from 44% to 63%, which corresponds to an increase in median survival from 10 months to 18 months. Using this design, a maximum of 35 patients would be accrued, 17 in the first cohort and 18 in the second cohort. In addition, an extra accrual suspension was planned after the fifth patient was enrolled in order to look for unacceptable toxicities. If any had been observed among these 5 patients, the study would have been closed. Toxicity in the first 5 patients was acceptable, and accrual continued until 17 patients were enrolled. When it became clear that between 8 and 16 of the first 17 patients would live for at least 365 days after study entry, accrual was resumed to enroll the second cohort of 18 patients.
Exact binomial point estimators and 95% confidence intervals (CI) adjusted for the two-stage design (8) were used to estimate 1-year survival percentages based on 1-year minimum follow-up for all patients. Overall survival and progression-free survival distributions were estimated with Kaplan-Meier curves (9). Response rates were estimated using exact binomial point estimators and 95% CIs. Analysis of variables univariately associated with survival was done with Cox proportional hazards modeling. Multivariable modeling was not performed because the small number of deaths would result in a considerable chance of over-fitting.
RESULTS
The study was activated by NCCTG on July 25, 1995, and by ECOG on February 19, 1999. It was permanently closed on September 27, 2000, when the accrual goal was met.
Patient characteristics
Patient baseline characteristics are shown in Table 1. Of the total, 83% of patients had ECOG performance scores of 0 or 1; 79% of patients had MMSE scores of 27 or above at enrollment, which are considered to be cognitively normal values (10). All patients had evaluable disease.
Table 1.
Patient baseline characteristics
| Characteristic | n patients | % of total (n = 36) |
|---|---|---|
| Gender | ||
| Male | 19 | 53 |
| Female | 17 | 47 |
| Performance score | ||
| 0 | 9 | 26 |
| 1 | 21 | 60 |
| 2 | 2 | 6 |
| 3 | 3 | 8 |
| Not recorded | 1 | — |
| Institution | ||
| Mayo | 12 | 33 |
| Other NCCTG | 18 | 50 |
| ECOG | 6 | 17 |
| MMSE | ||
| <20 | 1 | 3 |
| 20 to 26 | 6 | 18 |
| 27 to 28 | 9 | 26 |
| 29 to 30 | 18 | 53 |
| Not recorded | 2 | - |
| Age (years) | ||
| Mean ± standard deviation | 57.8 ± 8.9 | 57.8 ± 8.9 |
| Median (min, max) | 60.5 (34, 69) | 60.5 (34, 69) |
| Histology | ||
| Large cell | 34 | 94 |
| Other | 2 | 6 |
| CSF involvement* | 1 | 3 |
| Ocular involvement* | 0 | 0 |
Missing this information for 23 patients.
Therapy completion
Figure 2 summarizes patient completion of the treatment program components. Note that of the 36 patients who began CHOD and the first course of BVAM (denoted CHOD/BVAM-1), 12 (33%) patients did not start the second BVAM cycle, over half of them (58%) due to progressive disease during the first treatment course. Of those who were able to complete the second round of BVAM therapy (BVAM-2), 5 patients did not start radiation: 3 of them due to toxicity, 1 due to progression, and 1 who refused further treatment. A total of 14 patients received 50.4 Gy WBRT, and 5 patients received 30.6 Gy. Thus, only 19 (53%) of the study patients were able to complete the entire regimen.
Fig. 2.
Treatment program summary (n = 36). CHOD-BVAM-1 = CHOD and first cycle of BVAM; BVAM-2 = second cycle of BVAM; CR = complete response; PR = XXX, ≥50% or unequivocal reduction in size; PROG = progression, ≥25% increase in size or unequivocal increase in size, increasing steroid dose, or new lesions, STAB = stable disease; REGR = regression; STAB = stable; Unk/NE = XXX.
Survival and therapeutic activity
At the time of this analysis, 30 (83%) patients had died, and follow-up for the remaining 6 living patients ranged from 5.0 to 11.0 years. Figure 3 shows the Kaplan-Meier survival curve for all 36 patients enrolled in the trial. Median survival is 18.5 months, and the percentages of patients alive at 1, 2, and 3 years are 64%, 36%, and 33%, respectively.
Fig. 3.
Kaplan-Meier survival curve with 95% confidence interval (36 evaluable patients; 30 dead).
Univariate association with survival was evaluated. Only the performance score (0/1 versus 2/3) was determined to be significant (p = 0.002). A statistically significant association between radiation dose (30.6Gy vs. 50.4Gy) and survival was not observed (hazard ratio, 0.225; 95% CI, 0.028–1.788; p = 0.16).
Response
The best response observed over the entire course of treatment for 10 patients was a CR of 36 (28%), a response (≥50% or unequivocal reduction in size plus regression) for 10 (28%) patients, and 7 (19%) patients progressed during the first cycle of chemotherapy; the remaining patients experienced stable disease. Thus, 20 (56%) study participants were classified as responders. Of these, 16 (80%) patients were classified as confirmed responders. There were 3 patients who had a CR after WBRT and 7 patients who achieved a CR prior to WBRT. The primary site was the most common site of failure in the 24 patients whose disease progressed (6 patients). Three patients failed in a remote brain site only, 1 patient failed in the primary and a secondary brain site, 1 patient experienced an ocular failure, 1 patient failed in both a remote brain site and the spinal cord, and in 12 patients, the site of failure was not recorded.
The pattern of failure did not appear to correlate with the radiation dose received. Thirteen of the 24 patients who progressed had completed WBRT; of those, 3 patients received 30.6 Gy, and 10 patients received 50.4 Gy. Of the 7 patients for whom therapy failed after CR, therapy failed for 2 patients at the primary site alone, for 1 patient at a secondary brain site, for 1 patient at the eye, and for 1 patient at a secondary brain site and the spinal cord. One patient for whom therapy failed at the primary site had received 30.6 Gy and the other 50.4 Gy; of those patients for whom therapy failed at other sites, 3 patients had received 50.4 Gy WBRT, and 2 patients had received 30.6 Gy.
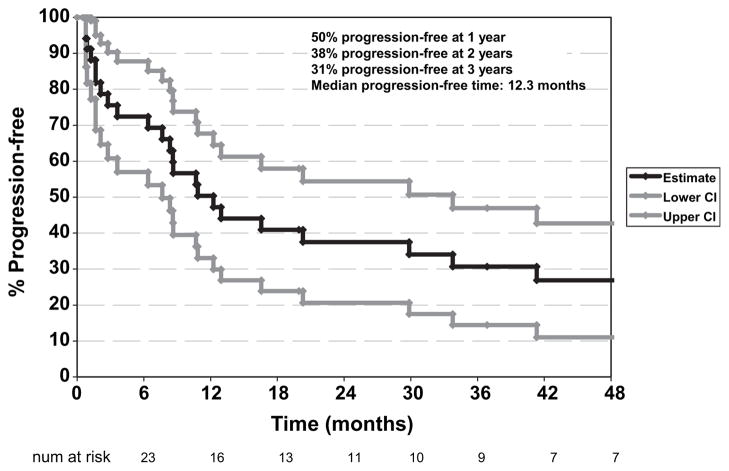
Figure 4 shows the Kaplan-Meier time to progression (TTP) estimate; 24 of the 36 patients (67%) have progressed. Median TTP is 12.3 months, and the percentages of progression-free at 1, 2, and 3 years are 50%, 38%, and 31%, respectively.
Fig. 4.
Kaplan-Meier curve for progression-free survival and 95% confidence intervals.
Toxicity
Table 2 summarizes the severe (CTC 2.0 grade 3–4) nonhematologic toxicities recorded for all 36 study patients. No treatment-related deaths were noted. Any grade of neurologic toxicity was seen in 30 (83%) patients. Of these 30 patients, 10 (33%) patients reported at least one grade 3+ neurotoxicity; 3 of these patients had received WBRT (2 patients received 30.6 Gy, and 1 patient received 50.4 Gy). The most common toxicities were motor weakness/neuropathy (7 patients), cognitive dysfunction/confusion (3), and seizures (2). Although no statistically significant changes in MMSE or performance score were seen over time, these data are limited by the high number of patients who progressed and were no longer evaluated. By the completion of WBRT, only 8 patients completed an MMSE, and PS data were available for 13 patients.
Table 2.
Nonhematologic toxicities
| Toxicity | Total no. of events | No. of patients |
|---|---|---|
| None | 4 | |
| Grade 1 | 104 | 26 |
| Grade 2 | 51 | 22 |
| Grade 3 | 26 | 16 |
| Grade 4 | 4 | 4 |
| Grade 3 and 4 toxicities | ||
| Vision | 1 | |
| Neuropathy, motor | 7 | |
| Neurocortical (cognitive/confusion) | 3 | |
| Neurologic, other CNS | 1 | |
| Neurocerebellar (ataxia) | 1 | |
| Lethargy | 1 | |
| Seizure | 2 | |
| Headache | 1 | |
| RTOG/EORTC late RT brain | 1 | |
| Mucositis | 1 | |
| Hepatic | 3 | |
| Stomatitis | 3 | |
| Infection | 2 | |
| Nausea | 1 | |
| Constipation | 1 | |
| Other | 1 | |
Table 3 details the hematologic toxicity recorded for the study patients over all cycles of study therapy. Of the 34 patients with hematologic nadir data, 25 (74%) patients had grade 3 to 4 leukopenia, and 9 (26%) patients had grade 3 to 4 thrombocytopenia. Significant infections were recorded for 2 (8%) of the 25 patients with severe leukopenia, and no significant bleeding was observed in the 9 patients with severe thrombocytopenia.
Table 3.
Hematologic toxicities (all cycles)
| Characteristic | No. of patients (% of total n = 34) |
|---|---|
| Leukopenia | |
| Missing | 2 (5.6) |
| Grade 2 | 9 (26.5) |
| Grade 3 | 18 (52.9) |
| Grade 4 | 7 (20.5) |
| Thrombocytopenia | |
| Missing | 2 (5.6) |
| Normal | 14 (41.2) |
| Grade 1 | 5 (14.7) |
| Grade 2 | 6 (17.6) |
| Grade 3 | 6 (17.6) |
| Grade 4 | 3 (8.8) |
DISCUSSION
This phase II trial achieved a moderate initial response rate of 44% (after chemotherapy alone) and a final response rate of 56%, which is consistent with other reported series. These data indicate that PCNSL is responsive to a variety of therapies. Its lethality must be due, in part, to the age of the patient, the patient’s inability to tolerate therapy, and the unique properties of central nervous system (11), as well as the biology of the tumor. We and other investigators have noted that at least 10% of patients do not respond to any therapy (12). In this study, 21% of treated patients did not appear to respond to any form of treatment.
At the time we designed our study, median survival in the PCNSL population was approximately 10 months, with nearly all of the therapies then available (4, 5). Optimistically, we designed our trial to look for substantially improved survival, i.e., an increase in median survival to 18 months. This goal was achieved: the median survival of the 36 patients treated with our regimen (CHOD plus BVAM plus WBRT) was 18 months, and 64% of them were alive at 1 year.
In the current study, only 12 of the 36 (30%) patients enrolled were able to complete the study regimen. Neurologic toxicity was seen in 30 (83%) patients. Of these 30 patients, 10 (33%) patients reported at least one grade 3+ neurotoxicity. Unfortunately, these results are comparable to the neurotoxicity seen in other combined modality studies for PCNSL. O’Brien et al. (13) reported a 58% rate of neurotoxicity in patients over 60 years old who received MTX and WBRT as a part of a phase II trial. Those authors concluded that the risk of neurotoxicity for patients over 60 years old was unacceptable with their regimen. In two reports of different CMT regimens from single-institution trials, Abrey and colleagues (14, 15) reported an incidence of neurotoxicity greater than 80% for patients over 60 years old. The incidence of neurotoxicity is generally considered to be lower for younger patients (6%–27%) (13–17). However, formal cognitive testing of long-term survivors treated with a European Organization for Research and Treatment of Cancer prospective trial (EORTC protocol 20962) revealed an incidence of cognitive impairment of 63%, with one-third of those patients experiencing severe cognitive deficits. Mean age of survivors in that report was 44 years, with a range of 30 to 56 years (18).
Our results are inferior to those reported by Bessell and colleagues (5,19), using the same regimen. Careful review of their published articles does not show substantive differences between their described patient population and ours, although the interval from diagnosis to trial onset was not reported in their studies. Radiotherapy dose was different in the two trials. In the trial by Bessel and colleagues (5, 19), WBRT was administered at 45 Gy in 1.8 Gy fractions, with a boost to a single lesion of 10 Gy in 5 fractions. Craniospinal radiation (35 Gy in 25 fractions) was also administered to patients with positive cerebrospinal fluid (CSF) cytology. The authors performed a second study using the same chemotherapy regimen but with a lower WBRT, in an attempt to reduce neurotoxicity (19). In the authors’ analysis of radiation dose and survival in two consecutive CHOD/BVAM trials, patients under 60 years old who received lower-dose WBRT (30.6 Gy vs. 45 Gy) experienced an increased risk of relapse and a lower overall survival. We began our study with a WBRT dose of 50.4 Gy, but after 1998, patients with a CR received 30.6 Gy, based on the early results of the European trial. This reduced radiation dose did not appear to impact the risk of relapse or survival in our study. However, 5 of 19 (26%) patients in our trial who could have started radiotherapy did not undergo WBRT. Bessell et al. (5) reported only 2 of 24 (8%) patients eligible for radiotherapy did not receive treatment. Although it is difficult to compare these small numbers between trials, it is possible that the decreased proportion of patients receiving WBRT may also contribute to the differences in results.
The survival benefits accrued by the patients in this trial are overshadowed by the toxicity of the treatment, especially in light of other studies reported since this trial commenced (11, 14, 15, 20–25). In addition, our study was limited to patients younger than 70 years of age. As age is the most important prognostic factor in PCNSL, selection bias may have also contributed to the improvement in overall survival in this study population. Our analysis of the results and review of literature published since the initiation of this study in 1995 yields at least three possible explanations. Based on the analysis of our initial trial, NCCTG protocol 86-72-52, and the report by Lachance and colleagues (26), the standard lymphoma regimens of CHOP (cyclophosphamide, hydroxydaunorubicin [doxorubicin], vincristine [Oncovin], and prednisone/prednisolone) and CHOD likely address bulky disease, where the blood–brain barrier is incomplete or functionally nonexistent, but does not “reach” infiltrative disease, where progression appears to occur. In addition, the MTX dose chosen may have been too low to clear the CSF. At the time the study was written and begun, data supported the fact that our dosage of MTX (1.5 g/m2) was sufficient to treat disease in brain parenchyma. However, it was only in 1998 that the report by Glantz and colleagues (27) demonstrated that higher systemically administered doses of MTX were required to achieve therapeutic concentrations in the CSF. Other evidence suggests our MTX dose should have been effective for PCNSL. In a recent report from the Trans-Tasman Radiation Oncology Group, O’Brien et al. (13) updated their experience with PCNSL patients treated with 1 g/m2 intravenously given for two cycles prior to WBRT (which commenced on day 15). Median survival for the 46 patients in that study was 36 months, which is comparable to the results of studies using higher doses of MTX (20–22, 25, 28–31).
Our regimen also may have given patients insufficient cycles of MTX, independent of the systemically administered dosage. From New Advances in Brain Tumor Treatment (NABTT) protocol 96-07, it appears that four to six cycles of high-dose MTX (defined as 8 g/m2 administered intravenously) need to be administered before a final judgment is made about utility (21). Thus, our regimen may have used too little in both concentration and total dosing. We cannot address the possibility that the patient cohort was inherently more aggressive, as international prognostic index factors were not recorded (32).
CONCLUSIONS
In conclusion, this regimen did improve the survival of our PCNSL patients but at the cost of substantial toxicity. The improvement in survival is less than that reported with MTX-based therapies that use higher dosing. Further research into causation is necessary in order to develop pathogenesis-based prevention and treatment strategies.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service Grants CA-25224, CA-35101, CA-60276, CA-35113, CA-35272, CA-35269, CA-35195, CA-52352, CA-35103, CA-35415, CA-35431, and CA-63849.
Additional participating members include: Daniel A. Nikcevich, M.D., Duluth Community Clinical Oncology Program (CCOP), Duluth, MN; Kendrith M. Rowland, Jr, M.D., Carle Cancer Center CCOP, Urbana, IL; Martin Wiesenfeld, M.D., Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA; Tudor Dentchev, M.D., Altru Health Systems, Grand Forks, ND; Loren K. Tschetter, M.D., Sioux Community Cancer Consortium, Sioux Falls, SD; Paul L. Schaefer, M.D., Toledo Community Hospital Oncology Program CCOP, Toledo, OH; Gamini S. Soori, M.D., Missouri Valley Cancer Consortium, Omaha, NE; Shaker R. Dakhil, M.D., Wichita Community Clinical Oncology Program, Wichtia, KS; Patrick J. Flynn, M.D., Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN.
Footnotes
Conflict of interest: none.
References
- 1.Olson JE, Janney CA, Rao RD, et al. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: A surveillance, epidemiology, and end results analysis. Cancer. 2002;95:1504–1510. doi: 10.1002/cncr.10851. [DOI] [PubMed] [Google Scholar]
- 2.O’Neill BP, Wang CH, O’Fallon JR, et al. for the North Central Cancer Treatment Group. The consequences of treatment and disease in patients with primary central nervous system non-Hodgkin’s lymphoma: Cognitive function and performance status. Neuro Oncol. 1999;1:196–203. doi: 10.1093/neuonc/1.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23:9–17. doi: 10.1016/0360-3016(92)90538-s. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill BP, Wang CH, O’Fallon JR, et al. Primary central nervous system non-Hodgkin’s lymphoma (PCNSL): survival advantages with combined initial therapy? A final report of the North Central Cancer Treatment Group (NCCTG) Study 86-72-52. Int J Radiat Oncol Biol Phys. 1999;43:559–563. doi: 10.1016/s0360-3016(98)00450-7. [DOI] [PubMed] [Google Scholar]
- 5.Bessell EM, Graus F, Lopez-Guillermo A, et al. CHOD/BVAM regimen plus radiotherapy in patients with primary CNS non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2001;50:457–464. doi: 10.1016/s0360-3016(01)01451-1. [DOI] [PubMed] [Google Scholar]
- 6.Laack NN, Ballman KV, Brown PB, et al. Whole-brain radiotherapy and high-dose methylprednisolone for elderly patients with primary central nervous system lymphoma: Results of North Central Cancer Treatment Group (NCCTG) 96-73-51. Int J Radiat Oncol Biol Phys. 2006;65:1429–1439. doi: 10.1016/j.ijrobp.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 7.Fleming T. One-sample multiple testing procedure for phase II trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- 8.Duffy D, Santer T. Confidence intervals for a binomial parameter based on multistage tests. Biometrics. 1987;43:81–93. [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Crum RM, Anthony JC, Bassett SS, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 11.Doolittle ND, Abrey LE, Ferrari N, et al. Targeted delivery in primary and metastatic brain tumors: summary report of the seventh annual meeting of the Blood-Brain Barrier Disruption Consortium. Clin Cancer Res. 2002;8:1702–1709. [PubMed] [Google Scholar]
- 12.Reni M, Ferreri AJ. Therapeutic management of primary CNS lymphoma in immunocompetent patients. Expert Rev Anticancer Ther. 2001;1:382–394. doi: 10.1586/14737140.1.3.382. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien PC, Roos DE, Pratt G, et al. Combined-modality therapy for primary central nervous system lymphoma: Long-term data from a phase II multicenter study (Trans-Tasman Radiation Oncology Group) Int J Radiat Oncol Biol Phys. 2006;64:408–413. doi: 10.1016/j.ijrobp.2005.07.958. [DOI] [PubMed] [Google Scholar]
- 14.Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16:859–863. doi: 10.1200/JCO.1998.16.3.859. [DOI] [PubMed] [Google Scholar]
- 15.Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: The next step. J Clin Oncol. 2000;18:3144–3150. doi: 10.1200/JCO.2000.18.17.3144. [DOI] [PubMed] [Google Scholar]
- 16.Correa DD, DeAngelis LM, Shi W, et al. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62:548–555. doi: 10.1212/01.wnl.0000109673.75316.d8. [DOI] [PubMed] [Google Scholar]
- 17.Fliessbach K, Urbach H, Helmstaedter C, et al. Cognitive performance and magnetic resonance imaging findings after high-dose systemic and intraventricular chemotherapy for primary central nervous system lymphoma. Arch Neurol. 2003;60:563–568. doi: 10.1001/archneur.60.4.563. [DOI] [PubMed] [Google Scholar]
- 18.Harder H, Holtel H, Bromberg JE, et al. Cognitive status and quality of life after treatment for primary CNS lymphoma. Neurology. 2004;62:544–547. doi: 10.1212/wnl.62.4.544. [DOI] [PubMed] [Google Scholar]
- 19.Bessell EM, Lopez-Guillermo A, Villa S, et al. Importance of radiotherapy in the outcome of patients with primary CNS lymphoma: An analysis of the CHOD/BVAM regimen followed by two different radiotherapy treatments. J Clin Oncol. 2002;20:231–236. doi: 10.1200/JCO.2002.20.1.231. [DOI] [PubMed] [Google Scholar]
- 20.Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: An intent-to-treat analysis. J Clin Oncol. 2003;21:4151–4156. doi: 10.1200/JCO.2003.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Batchelor T, Carson K, O’Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: A report of NABTT 96-07. J Clin Oncol. 2003;21:1044–1049. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 22.DeAngelis LM, Seiferheld W, Schold SC, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Deangelis LM, Hormigo A. Treatment of primary central nervous system lymphoma. Semin Oncol. 2004;31:684–692. doi: 10.1053/j.seminoncol.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Ferreri AJ, Reni M, Pasini F, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58:1513–1520. doi: 10.1212/wnl.58.10.1513. [DOI] [PubMed] [Google Scholar]
- 25.Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003;21:4483–4488. doi: 10.1200/JCO.2003.03.108. [DOI] [PubMed] [Google Scholar]
- 26.Lachance DH, Brizel DM, Gockerman JP, et al. Cyclophosphamide, doxorubicin, vincristine, and prednisone for primary central nervous system lymphoma: Short-duration response and multifocal intracerebral recurrence preceding radiotherapy. Neurology. 1994;44:1721–1727. doi: 10.1212/wnl.44.9.1721. [DOI] [PubMed] [Google Scholar]
- 27.Glantz MJ, Cole BF, Recht L, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: Is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16:1561–1567. doi: 10.1200/JCO.1998.16.4.1561. [DOI] [PubMed] [Google Scholar]
- 28.Abrey LE, Panageas KS. Identifying and addressing barriers to the delivery of optimal therapy for primary central nervous system lymphoma in the broader community. Leuk Lymphoma. 2006;47:2449–2452. doi: 10.1080/10428190600881272. [DOI] [PubMed] [Google Scholar]
- 29.Gavrilovic IT, Hormigo A, Yahalom J, et al. Long-term follow-up of high-dose methotrexate-based therapy with and without whole-brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24:4570–4574. doi: 10.1200/JCO.2006.06.6910. [DOI] [PubMed] [Google Scholar]
- 30.Hodson DJ, Bowles KM, Cooke LJ, et al. Primary central nervous system lymphoma: a single-centre experience of 55 unselected cases. Clin Oncol (R Coll Radiol) 2005;17:185–191. doi: 10.1016/j.clon.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Kiewe P, Fischer L, Martus P, et al. Primary central nervous system lymphoma: monocenter, long-term, intent-to-treat analysis. Cancer. 2008;112:1812–1820. doi: 10.1002/cncr.23377. [DOI] [PubMed] [Google Scholar]
- 32.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]