Abstract
Despite recent success in melanoma therapy, most patients with metastatic disease still undergo deadly progression. We have identified a novel mechanism of multidrug resistance allowing a small subpopulation of slow-cycling melanoma cells to survive based on elevated oxidative bioenergy metabolism. In this study, we asked whether such slow-cycling cells could be eliminated by co-treatment with the copper-chelator elesclomol. Elesclomol–copper complexes can cause oxidative stress by disruption of the mitochondrial respiration chain or by indirect non-mitochondrial induction of reactive oxygen species. We have found that elesclomol effectively kills the slow-cycling subpopulation and prevents the selective enrichment for slow-cycling cells, which usually results after monotreatment. We hypothesize that elesclomol could overcome the multidrug resistance of slow-cycling melanoma cells and prevent tumor repopulation in melanoma patients in future.
Keywords: melanoma, mitochondria, reactive oxygen species, therapy resistance, tumor heterogeneity
Background and questions addressed
Although melanoma research has made tremendous progress and new successful therapies are approaching the clinics, most patients with advanced melanoma still undergo deadly disease progression. The field has learned, mostly from translational studies on BRAFV600E inhibitors, how melanoma plasticity and heterogeneity bypass therapy. Melanomas apparently undergo a sequential Darwinian evolution under treatment. However, it is still unclear how melanoma cells survive the very first contact with drugs, especially if many of the discovered mechanisms of adaptive and acquired drug resistance need some time to develop (1,2).
Melanomas of various genotypes harbour slow-cycling cells that are determined by expression of the H3K4 demethylase JARID1B and that continuously repopulate the tumor (3). These slow-cycling JARID1Bhigh cells are intrinsically resistant to therapeutic attacks irrespective of the pharmacologic agents used (e.g. vemurafenib or cisplatin). A recent proteomic screen from slow-cycling melanoma cells showed upregulation of mitochondrial proteins with a direct role in the respiratory electron transport (4). Thus, targeting the oxidative metabolism could represent a novel, though toxic strategy to prevent resistance across different melanoma subtypes. First preclinical studies with investigational inhibitors of the oxidative phosphorylation (OXPHOS) such as oligomycin or the complex I inhibitor phenformin have sustained the effectiveness of metabolic targeting in melanoma (4,5).
Experimental rationale
Searching for tolerable inhibitors of the oxidative metabolism, we took notice of elesclomol, a novel, first-in-class copper chelator, which is being tested in clinical trials (6). Elesclomol–copper complexes can induce oxidative stress and cell death by disruption of the mitochondrial respiration chain or by indirect non-mitochondrial formation of reactive oxygen species (ROS) (7–10). Alternatively, (unbound) copper is required for melanoma cell signalling and tumorigenesis (11). In this context, we have made an interesting observation. Endogenous ROS levels seem to be significantly increased in slow-cycling JARID1Bhigh melanoma cells, most likely due to their high dependence on OXPHOS (4). Thus, slow-cycling cells could be more sensitive to further ROS increase than normal tissues, and the intrinsic drug resistance of slow-cycling melanoma cells could be overcome by co-treatment with elesclomol.
Results
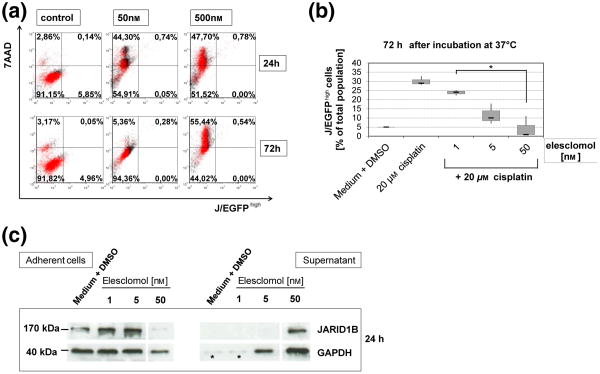
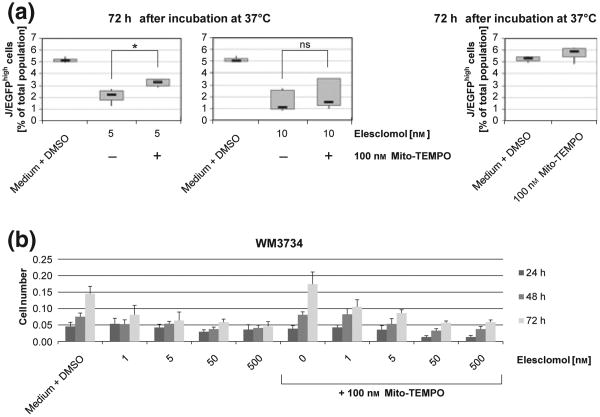
Corresponding to our earlier observations (4) and the literature (10,12), we have confirmed that mitochondrial H2O2 levels are significantly elevated in melanoma cells upon exogenous overexpression of JARID1B (Figure S1a) and that elesclomol induces oxidative stress and cell number reduction irrespective of melanoma cell genotypes (Figures S1b, c and S2). The three-dimensional growth as spheroids and the invasion into collagen were also decreased (Figure S3). For the cell subpopulation-specific evaluation of elesclomol, we performed cytotoxicity assays with melanoma cells stably transduced with a lentiviral JARID1B-promotor EGFP-reporter construct. We found that elesclomol effectively eliminates the slow-cycling JARID1Bhigh subpopulation (J/EGF-Phigh) already after 24 h (Fig. 1a). The addition of elesclomol as co-drug also prevented the selective enrichment for slow-cycling cells, which usually results after monotreatment with common melanoma therapeutics [cisplatin is shown in Figs 1b and S4, (4)]. Subsequent immunoblotting confirmed the killing of JARID1B-expressing cells after elesclomol treatment (Fig. 1c). Thus, our observations confirm a recent study in which vemurafenib-resistant, ROShigh melanoma cells could be driven into oxidative cell death in vitro and in vivo by co-administered elesclomol (13). E-lesclomol-mediated killing of JARID1Bhigh cells could be rescued by co-incubation with the mitochondria-specific antioxidant and radical scavenger mito-Tempo (Fig. 2a). However, as demonstrated by crystal violet (Fig. 2b) and MTT assays (not shown), the use of mito-Tempo could not consistently reverse elesclomol's cytotoxic effect on total cell populations. This may indicate a significant role of elevated mitochondrial ROS specifically in elesclomol-mediated killing of JARID1Bhigh melanoma cells. In line with our previous suggestion that loss of JARID1B is associated with deceased tumor repopulation (3), pretreatment of melanoma cells with elesclomol prior to seeding into soft agar significantly inhibited colony formation (Figure S5a). The combination of 1 nM elesclomol together with cisplatin revealed a dramatic loss of cell growth in vitro; however, only in three-dimensional colony formation assays and not in short-term, two-dimensional cell culture (Figure S5b versus S6). This indicates that cells with high tumor/colony formation capacity such as slow-cycling, OXPHOS-dependent cells are more sensitive to elesclomol (particularly when seeded at limited dilution) than cells that grow as rapidly cycling, glycolysis-relying bulk (14,15).
Figure 1.
Elesclomol prevents the survival of intrinsically resistant melanoma cells. (a) Elesclomol treatment eliminates the slow-cycling melanoma cell subpopulation as indicated by the loss of J/EGFPhigh WM3734 cells in flow cytometry (lower right quadrant). 7AAD was used as marker for cell death. (b) The relative percentage of therapy resistant J/EGFPhigh cells among the viable cell fraction increases under treatment with cytotoxic drugs like cisplatin, but decreases again under co-treatment together with elesclomol (P < 0.05). Box plots were calculated from three independently performed flow cytometric experiments. (c) Immunoblots confirm that JARID1B-expressing cells are effectively killed by elesclomol leading to their cell detachment into the culture supernatant. As indicated by the loading controls (see asterisks), there was not enough overall cell killing below 5 nM of elesclomol to load equal protein amounts.
Figure 2.
Scavenging of mitochondrial ROS attenuates the elesclomol-mediated killing of J/EGFPhigh melanoma cells. (a) The decrease of J/EGFPhigh WM3734 cells seen under treatment with elesclomol is reversed in the presence of the mitochondria-targeted antioxidant Mito-Tempo. Box plots were calculated from three independently performed flow cytometric experiments. Asterisk indicates statistical significance with P < 0.05 (ns, non-significant). (b) Crystal violet staining of WM3734 cells (total population) after 24, 48 and 72 h of incubation with elesclomol and mito-Tempo at the indicated concentrations. Shown is one representative example of three independent experiments. Error bars represent the standard deviation.
Discussion
To test elesclomol's suitability as a therapeutic, clinical studies have been previously performed in patients with metastatic stage IV melanoma. After an initial phase IIB study had shown prolonged progression-free survival (PFS) for the combination of elesclomol plus paclitaxel compared to paclitaxel alone (16), the following phase III SYMMETRY study found PFS improvement only in a subgroup of patients with normal baseline lactate dehydrogenase (LDH) (6). A possible explanation could be that high serum LDH levels may reflect a rapidly proliferating, hypoxic tumor burden with increased dependence on glycolysis (7,17). The LDHA gene, for example, is controlled by hypoxia-inducible factor 1a, a key regulator of the hypoxic response in cancer cells (18). Conversely, patients with low LDH would harbour mostly non-hypoxic tumor cells, which rely on OXPHOS and, thus, would be more sensitive to elesclomol (7,19). In addition to this (interindividual) interpretation, we suggest a more refined view taking into consideration metabolic intra-tumoral heterogeneity and its association to intrinsic drug resistance (4). Based on our preclinical observations, we hypothesize that (i) the multidrug resistance of slow-cycling melanoma cells could be overcome by elesclomol and (ii) that combining elesclomol with basic treatment regimens (e.g. vemurafenib), which potently eliminate the rapidly expanding tumor bulk, could prevent tumor repopulation. However, this concept may only work when applied to patients with low tumor burden and low serum LDH levels. In this scenario (or in an adjuvant setting), elimination of slow-cycling, multiresistant cells as a therapeutic mechanism would get enough time to prevent repopulation of the tumor bulk.
Supplementary Material
Data S1. Experimental Procedures.
Figure S1. Mitochondrial ROS measurement in melanoma cells.
Figure S2. Elesclomol treatment inhibits melanoma cell growth in vitro.
Figure S3. Elesclomol inhibits growth of melanoma spheroids and invasion into collagen.
Figure S4. Confirmation of cell death in culture supernatant.
Figure S5. Elesclomol treatment reduces the colony formation capacity and long-term growth of melanoma cells.
Figure S6. Addition of elesclomol to cisplatin does not significantly enhance the inhibition of two-dimensional melanoma cell growth in vitro.
Acknowledgments
MC, HC, IB, XZ and AR performed the research; IB and AR designed the experiments; IB, AH, MH, DS, TV and AR analysed and discussed the data; MC, IB and AR wrote the manuscript; all authors proofread the manuscript. IB and XZ thank Markus Hoth for his continuous support. Parts of the work have been supported by the Hiege Foundation, the Monika-Kutzner Foundation, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, DFG grants RO3577/3-1, BO3643/3, SFB1027 (project C4) and NIH grants CA25874, CA047159.
Footnotes
Conflict of interest: AH has been a consultant of Synta Pharmaceuticals. The other authors declare no conflict of interest.
Supporting Information: Additional supporting data may be found in the supplementary information of this article.
References
- 1.Hartsough E, Shao Y, Aplin AE. J Invest Dermalol. 2014;134:319–325. doi: 10.1038/jid.2013.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi H, Hugo W, Kong X, et al. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roesch A, Fukunaga M, Schmidt E, et al. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roesch A, Vultur A, Bogeski I, et al. Cancer Cell. 2013;23:811–825. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan P, Ito K, Perez-Lorenzo R, et al. Proc Natl Acad Sci USA. 2013;110:18226–18231. doi: 10.1073/pnas.1317577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Day SJ, Eggermont AM, Chiarion-Sileni V, et al. J Clin Oncol. 2013;31:1211–1218. doi: 10.1200/JCO.2012.44.5585. [DOI] [PubMed] [Google Scholar]
- 7.Blackman RK, Cheung-Ong K, Gebbia M, et al. PLoS One. 2012;7:e29798. doi: 10.1371/journal.pone.0029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirshner JR, He S, Balasubramanyam V, et al. Mol Cancer Ther. 2008;7:2319–2327. doi: 10.1158/1535-7163.MCT-08-0298. [DOI] [PubMed] [Google Scholar]
- 9.Hasinoff BB, Yadav AA, Patel D, et al. J Inorg Biochem. 2014;137:22–30. doi: 10.1016/j.jinorgbio.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Barbi de Moura M, Vincent G, Fayewicz SL, et al. PLoS One. 2012;7:e40690. doi: 10.1371/journal.pone.0040690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brady DC, Crowe MS, Turski ML, et al. Nature. 2014;509:492–496. doi: 10.1038/nature13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kluza J, Corazao-Rozas P, Touil Y, et al. Cancer Res. 2012;72:5035–5047. doi: 10.1158/0008-5472.CAN-12-0979. [DOI] [PubMed] [Google Scholar]
- 13.Corazao-Rozas P, Guerreschi P, Jendoubi M, et al. Oncotarget. 2013;4:1986–1998. doi: 10.18632/oncotarget.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vander Heiden MG, Cantley LC, Thompson CB. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berridge MV, Herst PM, Tan AS. Mitochondrion. 2010;10:584–588. doi: 10.1016/j.mito.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 16.O’Day S, Gonzalez R, Lawson D, et al. J Clin Oncol. 2009;27:5452–5458. doi: 10.1200/JCO.2008.17.1579. [DOI] [PubMed] [Google Scholar]
- 17.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Br J Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semenza GL. Cancer Metastasis Rev. 2000;19:59–65. doi: 10.1023/a:1026544214667. [DOI] [PubMed] [Google Scholar]
- 19.Ho J, de Moura MB, Lin Y, et al. Mol Cancer. 2012;11:76. doi: 10.1186/1476-4598-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Experimental Procedures.
Figure S1. Mitochondrial ROS measurement in melanoma cells.
Figure S2. Elesclomol treatment inhibits melanoma cell growth in vitro.
Figure S3. Elesclomol inhibits growth of melanoma spheroids and invasion into collagen.
Figure S4. Confirmation of cell death in culture supernatant.
Figure S5. Elesclomol treatment reduces the colony formation capacity and long-term growth of melanoma cells.
Figure S6. Addition of elesclomol to cisplatin does not significantly enhance the inhibition of two-dimensional melanoma cell growth in vitro.