Abstract
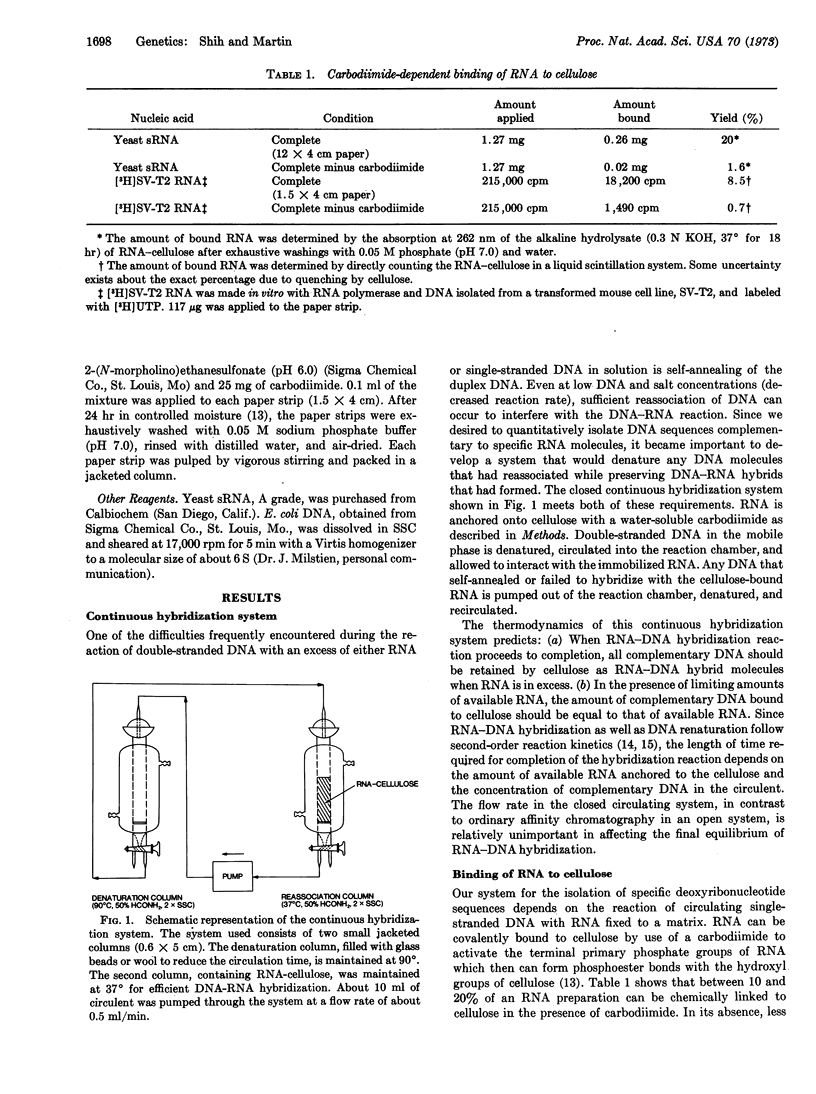
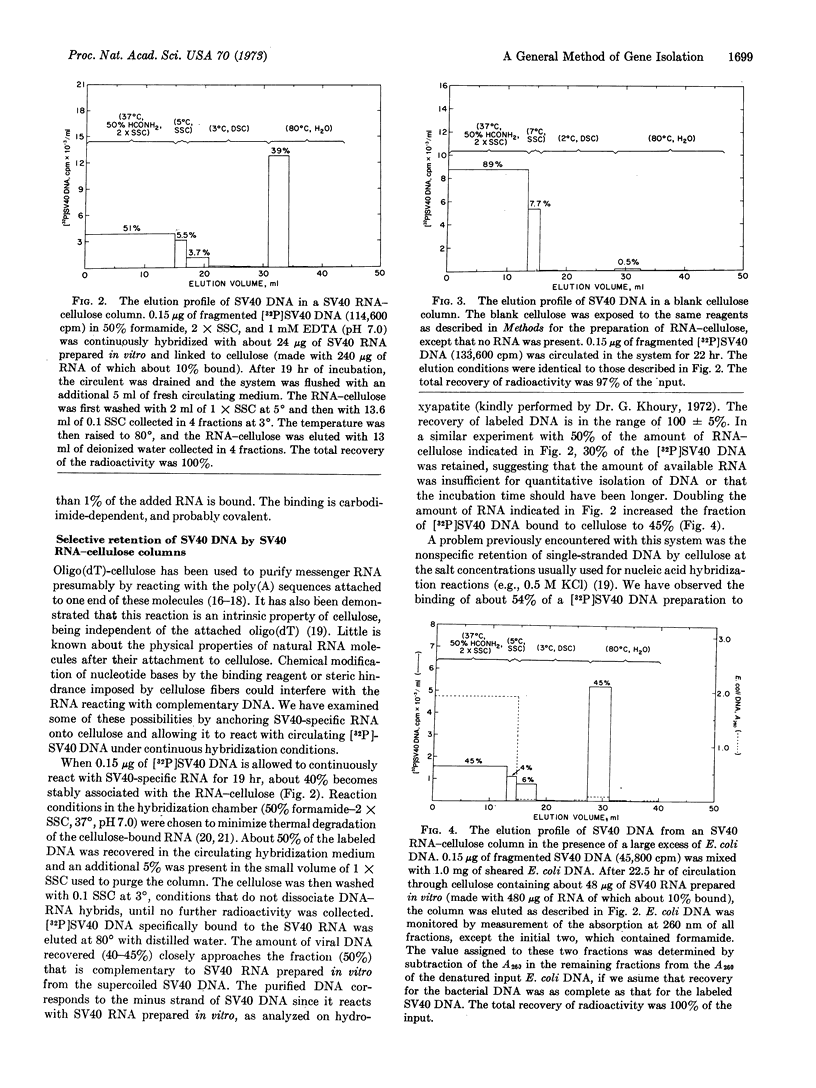
A general method of gene isolation has been developed that involves the chemical linkage of RNA to cellulose by a water-soluble carbodiimide, and the continuous circulation of DNA containing specific sequences complementary to the RNA. The temperature of the cellulose matrix is maintained at 37° (50% formamide, 0.3 M NaCl-0.03 M Na3 citrate) to allow efficient DNA-RNA interaction in the stationary phase, while unreacted and any reassociated DNA is denatured at 90° and then recirculated into the hybridization chamber. Between 40 and 45% of fragmented 32P-labeled simian virus (SV)40 DNA was removed from the circulating solution when cellulosebound SV40-specific RNA, assymmetrically transcribed in vitro with Escherichia coli RNA polymerase, was used. In the presence of 104-fold excess of sheared E. coli DNA, nearly half of the [32P]SV40 DNA was recovered from the mixture as a DNA-RNA hybrid with negligible contamination by bacterial DNA. The isolation procedure is almost quantitative for the complementary DNA. The efficiency and selectivity of this method permit the isolation of a defined DNA sequence from a large and complex genome.
Keywords: RNA-cellulose, simian virus 40, Escherichia coli, gene integration, RNA-DNA hybridization
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C., Smith M. Thermal elution of complementary sequences of nucleic acids from cellulose columns with covalently attached oligonucleotides of known length and sequence. J Biol Chem. 1971 Mar 25;246(6):1944–1946. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J., Kung G., Bekhor I. A method for the hybridization of nucleic acid molecules at low temperature. Biochemistry. 1967 Dec;6(12):3650–3653. doi: 10.1021/bi00864a005. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fournier M. J., Doctor B. P. Isolation and partial characterization of the transfer ribonucleic acid cistrons from Escherichia coli. Nature. 1970 Aug 1;227(5257):448–451. doi: 10.1038/227448a0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- Daniel V., Beckmann J. S., Sarid S., Grimberg J. I., Herzberg M., Littauer U. Z. Purification and in vitro transcription of a transfer RNA gene. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2268–2272. doi: 10.1073/pnas.68.9.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Caramela M. G. The isolation and characterization of adenosine monophosphate-rich polynucleotides synthesized by Ehrlich ascites cells. J Biol Chem. 1969 Mar 10;244(5):1314–1324. [PubMed] [Google Scholar]
- Gelb L. D., Aaronson S. A., Martin M. A. Heterogeneity of murine leukemia virus in vitro DNA; detection of viral DNA in mammalian cells. Science. 1971 Jun 25;172(3990):1353–1355. doi: 10.1126/science.172.3990.1353. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Kitos P. A., Saxon G., Amos H. The isolation of polyadenylate with unreacted cellulose. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1426–1437. doi: 10.1016/0006-291x(72)90232-x. [DOI] [PubMed] [Google Scholar]
- Kohne D. E. Isolation and characterization of bacterial ribosomal RNA cistrons. Biophys J. 1968 Oct;8(10):1104–1118. doi: 10.1016/S0006-3495(68)86542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Axelrod D. SV40 gene activity during lytic infection and in a series of SV40 transformed mouse cells. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1203–1210. doi: 10.1073/pnas.64.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. L., Morowitz H. J. Partial purification of native rRNA and tRNA cistrons from mycoplasma sp. (Kid). Proc Natl Acad Sci U S A. 1969 Aug;63(4):1282–1289. doi: 10.1073/pnas.63.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeckpeper B. J., Smith K. D. Use of formamide in nucleic acid reassociation. Biochemistry. 1972 Mar 28;11(7):1319–1326. doi: 10.1021/bi00757a032. [DOI] [PubMed] [Google Scholar]
- Sgaramella V., Spadari S., Falaschi A. Isolation of the hybrid between ribosomal RNA and DNA of Bacillus subtilis. Cold Spring Harb Symp Quant Biol. 1968;33:839–842. doi: 10.1101/sqb.1968.033.01.095. [DOI] [PubMed] [Google Scholar]
- Shapiro J., Machattie L., Eron L., Ihler G., Ippen K., Beckwith J. Isolation of pure lac operon DNA. Nature. 1969 Nov 22;224(5221):768–774. doi: 10.1038/224768a0. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Bonner J. Template properties of DNA-polypeptide complexes. J Mol Biol. 1970 Jun 14;50(2):333–344. doi: 10.1016/0022-2836(70)90196-8. [DOI] [PubMed] [Google Scholar]
- Trilling D. M., Axelrod D. Encapsidation of free host DNA by simian virus 40: a simian virus 40 pseudovirus. Science. 1970 Apr 10;168(3928):268–271. doi: 10.1126/science.168.3928.268. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]



