Abstract
Chronic manganese (Mn) exposure produces a neurological syndrome with psychiatric, cognitive, and parkinsonian features. Gene expression profiling in the frontal cortex of Cyno-mologous macaques receiving 3.3–5.0 mg Mn/kg weekly for 10 months showed that 61 genes were increased and four genes were decreased relative to controls from a total of 6766 genes. Gene changes were associated with cell cycle regulation, DNA repair, apoptosis, ubiquitin-proteasome system, protein folding, cholesterol homeostasis, axonal/vesicular transport, and inflammation. Amyloid-β (Aβ) precursor-like protein 1, a member of the amyloid precursor protein family, was the most highly up-regulated gene. Immunohistochemistry confirmed increased amyloid precursor-like protein 1 protein expression and revealed the presence of diffuse Aβ plaques in Mn-exposed frontal cortex. Cortical neurons and white matter fibers from Mn-exposed animals accumulated silver grains indicative of on-going degeneration. Cortical neurons also exhibited nuclear hypertrophy, intracytoplasmic vacuoles, and apoptosis stigmata. p53 immunolabeling was increased in the cytoplasm of neurons and in the nucleus and processes of glial cells in Mn-exposed tissue. In summary, chronic Mn exposure produces a cellular stress response leading to neurodegenerative changes and diffuse Aβ plaques in the frontal cortex. These changes may explain the subtle cognitive deficits previously demonstrated in these same animals.
Keywords: Alzheimer’s disease, amyloid-β, amyloid-β precursor-like protein 1, manganese, neurodegeneration, non-human primates, p53
Manganese (Mn) is an essential metal that is required for the normal function of a multitude of biological processes in humans. However, conditions that increase brain Mn concentrations produce neurological disease (Levy and Nassetta 2003; Aschner et al. 2007). Toxic concentrations of Mn in the brain result from exposure to naturally occurring or anthropogenic sources in the environment (Takser et al. 2003; Rollin et al. 2005; Rodriguez-Agudelo et al. 2006), in occupational settings (Bast-Pettersen et al. 2004; Josephs et al. 2005; Bowler et al. 2007; Kim et al. 2007; Lucchini et al. 2007) or from certain medical conditions related to liver disease (Klos et al. 2006a,b) or parenteral nutrition (Komaki et al. 1999; Iinuma et al. 2003). There are reports of neurological disease in young addicts injecting high doses of Mn as a component of ‘designer’ psychostimulant drugs (Meral et al. 2007; Sikk et al. 2007). Further, there is concern that the long-term use of gasoline containing the Mn additive methylcyclopentadienyl manganese tricarbonyl increases Mn exposure to the general population and consequently the burden of neurological disease (Kaiser 2003; Walsh 2007).
Exposure to high concentrations of Mn is known to induce a parkinsonian syndrome associated with accumulation of Mn in basal ganglia structures, especially in the globus pallidus (Guilarte et al. 2006a; Perl and Olanow 2007). Manganese-induced parkinsonism has received a great deal of attention in the recent scientific literature, and thus a high percentage of recent studies examining Mn-induced neurotoxicity have been focused on Mn-induced changes in the dopaminergic system in the basal ganglia (Guilarte et al. 2006a; Perl and Olanow 2007). Recent reports also indicate that contemporary levels of occupational or environmental exposures to Mn cause problems of attention and cognitive impairment as well as increased expression of neuropsychological symptoms (Josephs et al. 2005; Bouchard et al. 2006; Bowler et al. 2006; Klos et al. 2006b) that may persist long after cessation of exposure (Bouchard et al. 2007). An association between environmental exposure to Mn and deficits in measures of intellectual functioning has also been described in children (Takser et al. 2003; Wasserman et al. 2006). It has been suggested that chronic exposure to elevated levels of Mn may alter cognitive domains mediated by the frontal cortex and subcortical structures (Josephs et al. 2005). However, there is relatively little or no information on Mn-induced effects on brain structures outside of the basal ganglia.
The present study is part of an on-going multidisciplinary effort to characterize the neurological consequences of chronic exposure to Mn in non-human primates. The monkeys described in this communication have been previously shown to have subtle deficits in cognitive function and fine motor control (Schneider et al. 2006). They exhibit decreased levels of amphetamine-induced dopamine release in the striatum measured by positron emission tomography (Guilarte et al. 2006a) and decreased cerebral cortex N-acetylaspartate/creatine ratio measured by magnetic resonance spectroscopy indicative of neuronal loss or dysfunction (Guilarte et al. 2006b). We now present new evidence that in the frontal cortex of these same animals, Mn exposure produces gene expression changes indicative of a cellular stress response that produces extensive degeneration and diffuse amyloid-β (Aβ) plaques. These findings show for the first time that the frontal cortex, a brain region that accumulates significantly lower levels of Mn that basal ganglia structures, is susceptible to Mn-induced neurodegeneration.
Materials and methods
Manganese administration and animal care
Young adult male research Cynomologous macaques, 5–6 years of age at the start of the study, were used. All animal studies were reviewed and approved by the Johns Hopkins and the Thomas Jefferson University Animal Care and Use Committee. These animals are part of an on-going multidisciplinary study assessing the behavioral, neuroimaging, and neuropathological consequences of chronic Mn exposure. Four animals were used for the gene array studies that received intravenous injections of manganese sulfate (3.3–5.0 mg Mn/kg) into the saphenous vein under 1–3% isoflurane anesthesia approximately once per week for approximately 40 weeks. These animals, and three naïve animals of the same age, were killed by ketamine injection (20–30 mg/kg) followed by an overdose of pentobarbital (100 mg/kg) and the brains harvested.
Brains were collected and embedded in warm 3% low-melting point agarose. After the agarose hardened, the brains were sliced in a series of 0.4-cm coronal slabs using a commercial meat slicer. For each brain slab, one of the hemispheres was immediately frozen on dry ice and the other hemisphere was post-fixed for immunohistochemistry. Brain slabs were stored at −80°C until use. Alternating brain tissue slabs from the right hemisphere were processed for histochemistry in two different ways. The right hemisphere of the first and every other slab thereafter was post-fixed, paraffin embedded, and 5-µm thick sections were cut using a microtome and stained with hematoxylin and eosin. The remaining slabs from the right hemisphere were immersed in 4% p-formaldehyde in 0.1 M phosphate buffer for 28 h, cryoprotected with 20% glycerol, 0.5% dimethylsulfoxide in phosphate buffer, and frozen at −80°C. Fifty microns thick sections were cut using a freezing microtome and processed (free-floating) for immunohistochemistry.
Microarray analysis
Brains were stored at −80°C until use for microarray analysis. Frontal cortex samples (areas 8 and 9 from the superior frontal lobe) of the four 3.3–5.0 mg Mn/kg injected animals and three naϊve control animals were dissected, weighed, and RNA isolated using the Qiagen RNAEasy kit (Qiagen, Valencia, CA, USA). RNA from each animal was processed independently so that a total of seven arrays were performed (n = 3, control; n = 4, Mn-exposed). National Institute on Aging Human MGC Nylon cDNA arrays (Baltimore, MD, USA), consisting of 9600 features that correspond to 6766 unique genes (Nadon et al. 2005), were used to analyze the differences in gene expression between Mn-treated and naïve non-human primates. Log transformed hybridization intensities were used for Z-score transformation, yielding a comparison of microarray data independent of the original hybridization intensity Z-ratios represent a unit of standard deviation from the normalized mean of zero for a particular gene (Cheadle et al. 2003). Z-ratios ±1.5 are statistically significantly different from control (p < 0.05), and represent a measure of fold change between treatments. Gene identities and functions were established using the National Center of Biotechnology Information (NCBI) Entrez Gene search engine (http://www.ncbi.nlm.nih.gov).
Immunohistochemistry
Tissue sections were pre-treated with 3% H2O2 and 10% methanol in Tris-buffered saline for 20 min followed by treatment with 20% formic acid for 10 min (for 6E10 only) and incubation with 5% normal horse serum and 0.2% Triton X-100 in Tris-buffered saline for 1 h. Further, sections were incubated with mouse 6E10 antibodies reactive to amino acid residue 1–17 of human A4 peptide (1 : 1000, 48 h at 4°C; Signet, Dedham, MA, USA), rabbit antibodies against amyloid-β precursor-like protein 1 (APLP1) (1 : 10 000; Calbiochem, Gibbstown, NJ, USA), p53 (1 : 500; Cell signaling, Danvers, MA, USA), hydroxynonenol (1 : 5000; Alpha Diagnostic, San Antonio, TX, USA), and glial fibrillary acidic protein (GFAP) (1 : 5000; Dako, Carpinteria, CA, USA) followed by incubation with the corresponding secondary biotinylated IgG (1 : 250, Vector, Burlingame, CA, USA; 1.5 h, at 25°C) and the avidin-biotin peroxidase complex solution (1 : 170, 1 h at 25°C). The reaction product was visualized with 0.25 mg/mL 3,3′-diaminobenzidine and 0.03% H2O2. Additional sets of sections were stained using FD Neurosilver Kit I (Ellicott City, MD, USA). Sections were mounted on slides, stained with cresyl violet, dehydrated in graded ethanols, and coverslipped using dibutyl phthalate xylene (DPX, Sigma, St. Louis, MO, USA) mounting media.
Metals analysis In brain tissue
Concentrations of Mn, copper, zinc, and iron were measured in frontal cortex tissue from control and Mn-exposed animals as previously described (Guilarte et al. 2006a).
Statistics
Microarray data were analyzed by conversion to Z-ratios. For a detailed discussion of methodology, see Cheadle et al. (2003). Metal concentrations for control and Mn-exposed tissue were compared using a Student’s t-test, with p < 0.05 used as a threshold for statistical significance.
Results
Animal husbandry, dosing regimen, and Mn tissue concentrations
The dosing regimen, cumulative Mn dose, time of exposure, blood and brain Mn concentrations, and general characteristics of the animals used in this study have been described (Guilarte et al. 2006a,b). These animals have also been shown to express subtle deficits in cognitive function, primarily spatial working memory (Schneider et al. 2006).
Gene expression changes in Mn-exposed frontal cortex
Table 1 shows that from a total of 6766 unique genes represented on the array, 61 genes were significantly up-regulated and four genes were significantly down-regulated by Mn. Microarray data were normalized by Z-score transformation in order to calculate significant changes in gene expression between control and Mn-exposed animals (Cheadle et al. 2003). Only those genes with a Z-ratio ± 1.5 (statistically significantly different from control,p < 0.05) are reported. Positive Z-ratios indicate that gene expression was significantly higher in Mn-treated animals relative to controls while negative Z-ratios indicates genes whose expression was significantly lower in Mn-treated animals relative to controls. Gene expression changes in Mn-exposed animals were categorized according to known biological functions and are listed in order from highest to lowest Z-ratio (Table 1). Genes related to cell cycle regulation and DNA repair, apoptosis, cholesterol homeostasis, proteasome/ubiquitin function, protein folding, axonal/vesicular transport, and inflammation were modulated by Mn exposure. The most highly up-regulated gene in the frontal cortex of Mn-treated animals with a Z-ratio of 2.90 was APLP1. Because of the limited amount of tissue, as the frontal cortex has been used for several other biological measurements, and because the RNA extraction procedure produced a low yield, there was only sufficient RNA for the gene array studies and we could not confirm the gene array findings using PCR. Therefore, as an alternative approach we used immunohistochemistry in frontal cortex tissue from the opposite hemisphere to confirm the increase in APLP1 gene expression.
Table 1.
Genes identified in the microarray analysis as being statistically significantly different in Mn-exposed animals from control animals in frontal cortex tissue
| GeneID | Gene symbol | Function | Z-ratio |
|---|---|---|---|
| Amyloid precursor protein regulation | |||
| 333 | APLP1 | Metal and protein binding | 2.90 |
| Apoptosis | |||
| 10670 | RRAGA | Metalloprotease; participates in TNFα-mediated cell death |
2.72 |
| 5074 | PAWR | Tumor suppressor gene | 1.98 |
| 54205 | CYCS | e– transport chain; apoptosis | 1.76 |
| 725 | C4BPB | C4b binding on apoptotic cells | 1.58 |
| Cholesterol metabolism/transport | |||
| 3422 | IDI1 | Cholesterol biosynthesis | 2.34 |
| 335 | APOA1 | Cholesterol efflux | 1.89 |
| 10654 | PMVK | Cholesterol biosynthesis | 1.76 |
| Axonal/vesicular transport | |||
| 27067 | STAU2 | mRNA transport; spine formation | 2.48 |
| 51626 | DYNC2LI1 | Dynein subunit | 2.37 |
| 51429 | SNX9 | EGFR degradation; vesicle recycling | 2.31 |
| 79814 | AGMAT | Polyamine biosynthesis; neurotransmission |
2.18 |
| 400 | ARL1 | Vesicle regulation | 2.02 |
| 89953 | KLC4 | Kinesin light chain | 1.96 |
| 9022 | CLIC3 | Voltage-gated ion transport | 1.85 |
| 10490 | VTI1B | Vesicle transport | 1.68 |
| Inflammatory/immune response | |||
| 6374 | CXCL5 | Neutrophil activation | 2.57 |
| 7462 | LAT2 | T and B cell activation | 2.32 |
| 7551 | ZNF3 | Immune cell activation | 2.06 |
| 3459 | IFNGR1 | Immune response | 1.84 |
| 7039 | TGFA | Mitogenic response | 1.75 |
| 6696 | SPP1 | Immune response | 1.73 |
| Cell cycle/transcription/DNA repair/biosynthesis | |||
| 9204 | ZMYM6 | DNA binding | 2.72 |
| 2068 | ERCC2 | Nucleotide excision repair | 2.55 |
| 11055 | ZPBP | Transcription | 2.36 |
| 51204 | CCDC44 | DNA integrase | 2.31 |
| 4801 | NFYB | p53-dependent transcription factor | 2.25 |
| 84307 | ZNF397 | DNA binding | 2.18 |
| 991 | CDC20 | Cell cycle regulation | 2.11 |
| 29922 | NME7 | Nucleotide biosynthesis | 2.11 |
| 3615 | IMPDH2 | Guanine biosynthesis | 2.06 |
| 5326 | PLAGL2 | Recognizes DNA | 1.96 |
| 22803 | XRN2 | RNA metabolism | 1.95 |
| 65989 | DLK2 | EGF homology | 1.93 |
| 1022 | CDK7 | Cell cycle | 1.89 |
| 3487 | IGFBP4 | Prolongs insulin growth factors | 1.74 |
| 4830 | NME1 | Nucleoside kinase | −1.53 |
| 7334 | UBE2N | Non-canonical ubiquitin conjugation | −1.69 |
| Proteasome/protein folding/protein turnover | |||
| 64224 | HERPUD2 | ER stress; protein folding | 2.40 |
| 9810 | RNF40 | E3 ubiquitin ligase | 2.37 |
| 5685 | PSMA4 | Proteosome 20S subunit | 1.96 |
| 55033 | FKBP14 | Protein folding; peptidyl isomerase | 1.83 |
| 5684 | PSMA3 | Proteosome 20S subunit | 1.52 |
| 10450 | PPIE | Protein folding; peptidyl isomerase |
−1.59 |
| Others | |||
| 57655 | GRAMD1A | Glucosyltransferase | 2.86 |
| 302 | ANXA2 | Cytoskeletal stability | 2.58 |
| 25974 | MMACHC | Vitamin B12 metabolism | 2.43 |
| 7957 | EPM2A | Glycogen metabolism | 2.33 |
| 65083 | NOL6 | Actin binding | 2.13 |
| 8813 | DPMI | GPI anchoring | 2.08 |
| 81929 | SEH1L | Nuclear pore | 2.05 |
| 2052 | EPHX1 | Detoxication of epoxides | 2.03 |
| 2993 | GYPA | Glycophorin A | 1.98 |
| 3423 | IDS | Lysosomal degradation | 1.62 |
| 84773 | CYHR1 | Cysteine rich, binds zinc and galectin-3 |
1.56 |
| 84992 | PIGY | GPI biosynthesis | −1.59 |
| Mn-responsive genes with unknown function | |||
| 84830 | C6ORF105 | 2.73 | |
| 85016 | C11ORF70 | 2.54 | |
| 54951 | COMMD8 | 2.33 | |
| 84304 | NUDT22 | 2.13 | |
| 83732 | RIOK1 | 2.10 | |
| 84832 | MGC12538 | 2.05 | |
| 80346 | REEP4 | 2.04 | |
| 55127 | HEATR1 | 1.91 | |
| 84303 | CHCDH6 | 1.85 | |
Gene expression changes were categorized according to known biological functions and are listed in order from highest to lowest Z-ratio. Genes related to cell cycle regulation and DNA repair, apoptosis, cholesterol homeostasis, proteasome/ubiquitin function, protein folding, axonal/vesicular transport, and inflammation were modulated by Mn exposure. APLP1, amyloid-β precursor-like protein 1.
Amyloid-β precursor-like protein 1 and amyloid-β immunohistochemistry
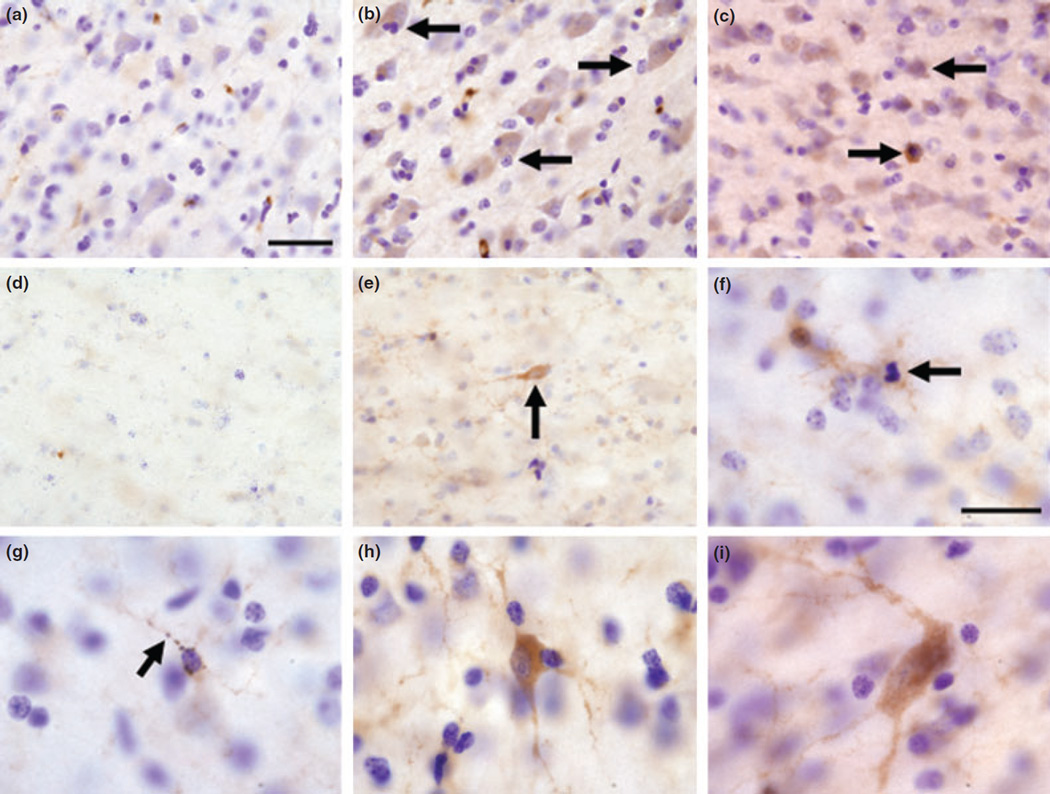
Figure 1 shows that in the frontal cortex of control animals, APLP1 labeling in cortical neurons was very faint (Fig. 1a) with little or no expression in white matter (Fig. 1d). However, Mn-exposed animals expressed darkly stained APLP1-positive neurons in the gray matter morphologically similar to pyramidal cells and cortical interneurons (Fig. 1b, c, and h). APLP1 immunostaining was also increased in subcortical white matter cells from Mn-exposed animals. Some of the APLP1-positive cells detected in the white matter (Fig. 1e) and in the border of the gray and white matter (Fig. 1i) were morphologically similar to interstitial neurons. In deep cortical layers adjacent to the white matter and in deeper regions of the white matter, glial cells expressed increased APLP1 staining in their processes (Fig. 1e–g). In Fig. 1f, there is an APLP1-positive glial cell with a condensed nucleus, resembling apoptosis. Together, these findings confirm that the protein encoded by the APLP1 gene is increased in the frontal cortex of Mn-exposed monkeys.
Fig. 1.
Representative images of APLP1 immunohistochemistry in the frontal cortex of control (a and d) and Mn-exposed animals (b, c, and e–i). Control animals exhibited faint APLP1 staining in cortical neurons (a) and in the white matter (d). In the frontal cortex of Mn exposed animals, there were darkly stained cells whose morphology resembles pyramidal cells (arrows in b) and cortical interneurons (arrow in c and cell in panel h). White matter interstitial neurons with fusiform morphology (arrow in e) and polymorphous morphology (i) also expressed increased APLP1 labeling. An overall increase in APLP1 was detected in glial cell processes in the white matter (e–g). The increase is apparent by comparing panel (d) (control) and panel (e) (Mn-exposed) white matter. In panel (f), there are two glial cells that expressed increased APLP1 immunoreactivity and one of the glial cells appears to have a condensed nucleus resembling apoptosis (arrow in f). In panel (g), there is a glial cell whose processes are APLP1 positive with a beaded appearance as if it was undergoing degeneration (arrow in g). Scale bar: (a–e) 40 and (f–i) 20 µm.
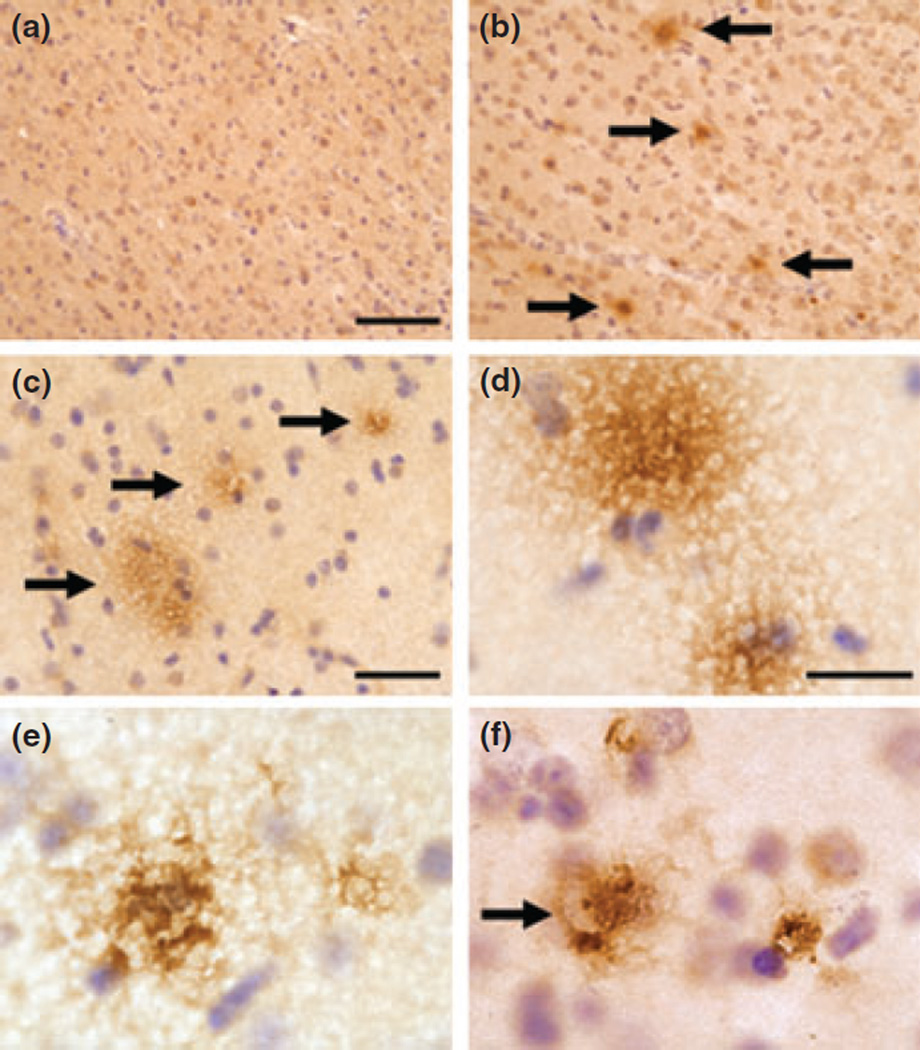
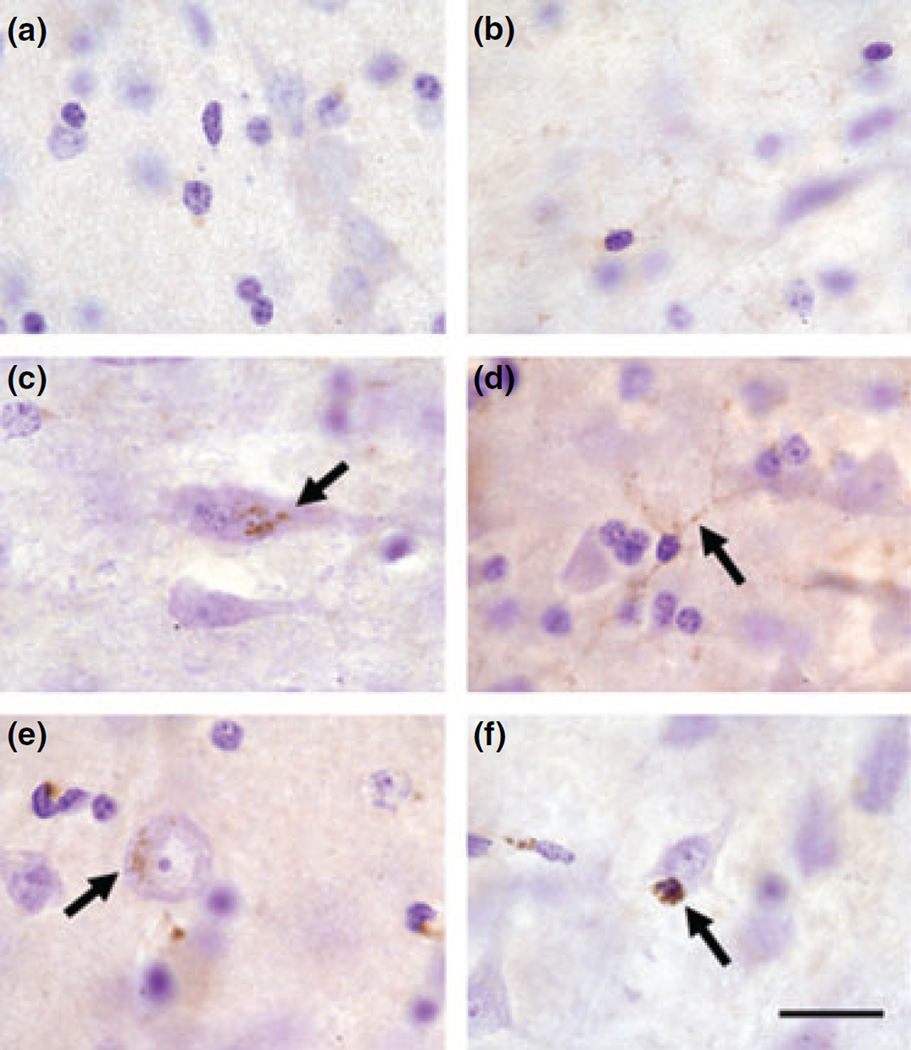
Based on these observations, we also performed Aβ immunohistochemistry (6E10 antibody) to assess the presence of Aβ plaques in Mn-exposed animals. As expected, immunostaining for Aβ in control animals (all animals less than 10 years of age) did not show extracellular aggregation (Fig. 2a). Panels in Fig. 2b – f depicts immunostaining in Mn-exposed monkeys. Extracellular Aβ aggregates resemble diffuse plaques that have been described in the aging canine and monkey brain (Kimura et al. 2005; Czasch et al. 2006) or at the early stages of Alzheimer’s disease (AD) (Alafuzoff et al. 2006). Further, Aβ aggregation sometimes surrounded Nissl-negative soma of large cortical neurons that appear to have been lost (Fig. 2f). These findings suggest the early stages of Aβ plaques, but further studies are needed to provide a quantitative assessment and confirmation in additional animals.
Fig. 2.
Representative images of amyloid-β immunohistochemistry (6E10 antibody) in the frontal cortex of control (a) and Mn-exposed animals (b–f). No extracellular amyloid-β-positive aggregates were observed in the frontal cortex of control animals (a). However, diffuse amyloid-β plaques were present in the gray matter of Mn-exposed animals (b–d; arrows in b and c). In panel (f) (arrow) there is the appearance of extracellular amyloid-β aggregation in a Nissl-negative cell body resembling the soma of a neuron that has been lost. Scale bar: (a and b) 80, (c) 40, and (d–f) 20 µm.
Neurodegenerative changes in Mn-exposed frontal cortex
Silver staining
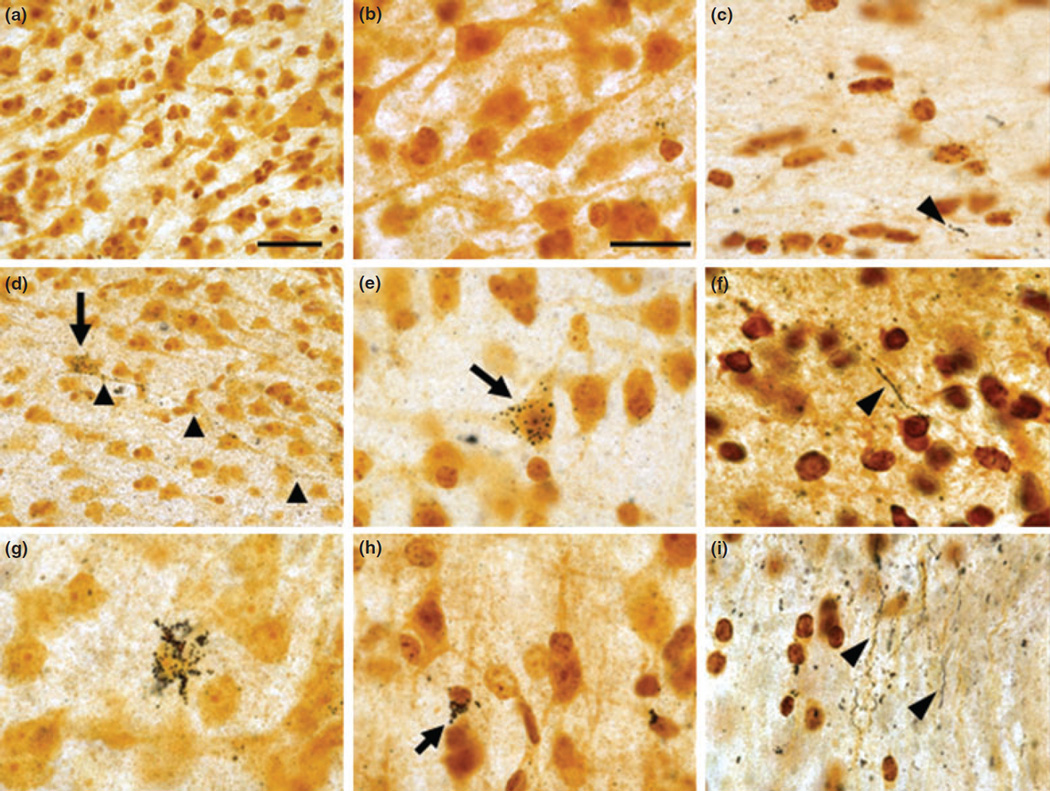
Silver staining was used to assess cellular degeneration in the frontal cortex of Mn-exposed animals. In control animals there was little evidence of silver grain accumulation in gray matter (Fig. 3 a and b) with occasional short segments of white matter axonal fibers accumulating silver grains (Fig. 3c). In Mn-exposed animals, there were pyramidal cells with marked silver grain accumulation in the soma (Fig. 3d) and along the entire length of the axonal projection (Fig. 3d). Large pyramidal cells and cortical interneurons expressed various degrees of silver grain accumulation or argyrophilia (Fig. 3e, g, and h). Multiple argyrophilic axonal fibers indicative of degenerative changes were also present in the white matter of Mn-exposed animals (Fig. 3f and i).
Fig. 3.
Representative images of silver staining in control animals (a–c) and Mn-exposed animals (d–i). In control animals, there was no significant accumulation of silver grains in any cell type in the gray matter (a and b). However, there were small occasionally silver-positive fiber segments in the white matter (see arrowhead in c). In Mn-exposed frontal cortex, there were pyramidal cells with marked silver grain accumulation in the soma (arrow in d) and along the axonal projection (arrowheads in d). Silver grain accumulation in a large pyramidal neuron is depicted in panel (e) (see arrow). Argyrophilic cortical cells resembling cortical interneurons are shown in panels (g and h) (see arrow in h). In the white matter of Mn-exposed animals, there were long axonal processes with silver accumulation indicative of degenerative changes (see arrowheads in f and i). Scale bar: (a and d) 40 and in the remaining panels 20 µm.
Histological examination
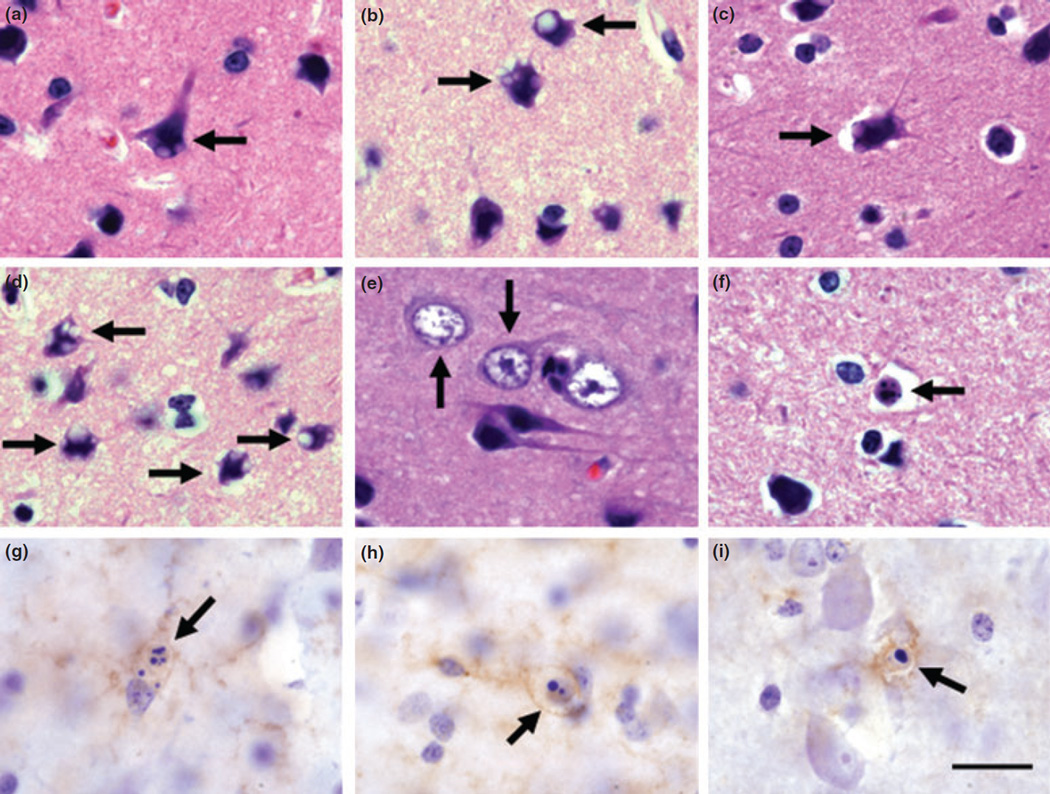
A common morphological feature observed in the frontal cortex of Mn-exposed monkeys was the presence of single or multiple intracytoplasmic vacuoles in the neuronal soma and dendrites (Fig. 4a – d). Intracytoplasmic vacuoles were present in large pyramidal cells and cortical interneurons (Fig. 4a – d). We also observed cortical neurons with hypertrophic nuclei in which the nucleus almost completely filled the entire volume of the cell soma with little or no visible cytoplasm (Fig. 4e). This type of morphological alteration has been recently described in mild cognitive impairment and in the AD brain (Riudavets et al. 2007).
Fig. 4.
Morphological changes in the frontal cortex of Mn-exposed animals. Cortical cells of various morphological types displayed single or multiple intracytoplasmic vacuoles (arrows in a–d). We also observed cells in the frontal cortex of Mn-exposed animals with hyper-trophic nuclei and reduced cytoplasm (arrows in e). Cortical cells with apoptotic stigmata were present in Mn-exposed frontal cortex (arrows in f–i). Apoptotic cells could be seen with two or more spherical bodies (arrows in f–h) or with a single, dense, and spherical nuclear body (arrow in i). Cellular remnants of cells with apoptotic stigmata expressed APLP1 (g), hydroxynonenol (h), or p53 (i) immunolabeling. Panels (a–f) are hematoxylin and eosin staining and (g–i) are count-erstained with Nissl. Scale bar: (for all panels) 20 µm.
Histology also showed cortical cells at various stages of apoptosis in the frontal cortex of Mn-exposed animals (Fig. 4f – i). Apoptotic stigmata consisted of two or more dense spheroid nuclear inclusions (Fig. 4f – h) or a single large and dense spheroid nuclear inclusion (Fig. 4i) possibly representing different stages of apoptosis. Cellular remnants in apoptotic cells stained with APLP1, p53, or hydroxynonenol (lipid peroxidation marker) suggesting an association of these markers with apoptotic cell death (Fig. 4g – i).
Glial fibrillary acidic protein immunohistochemistry
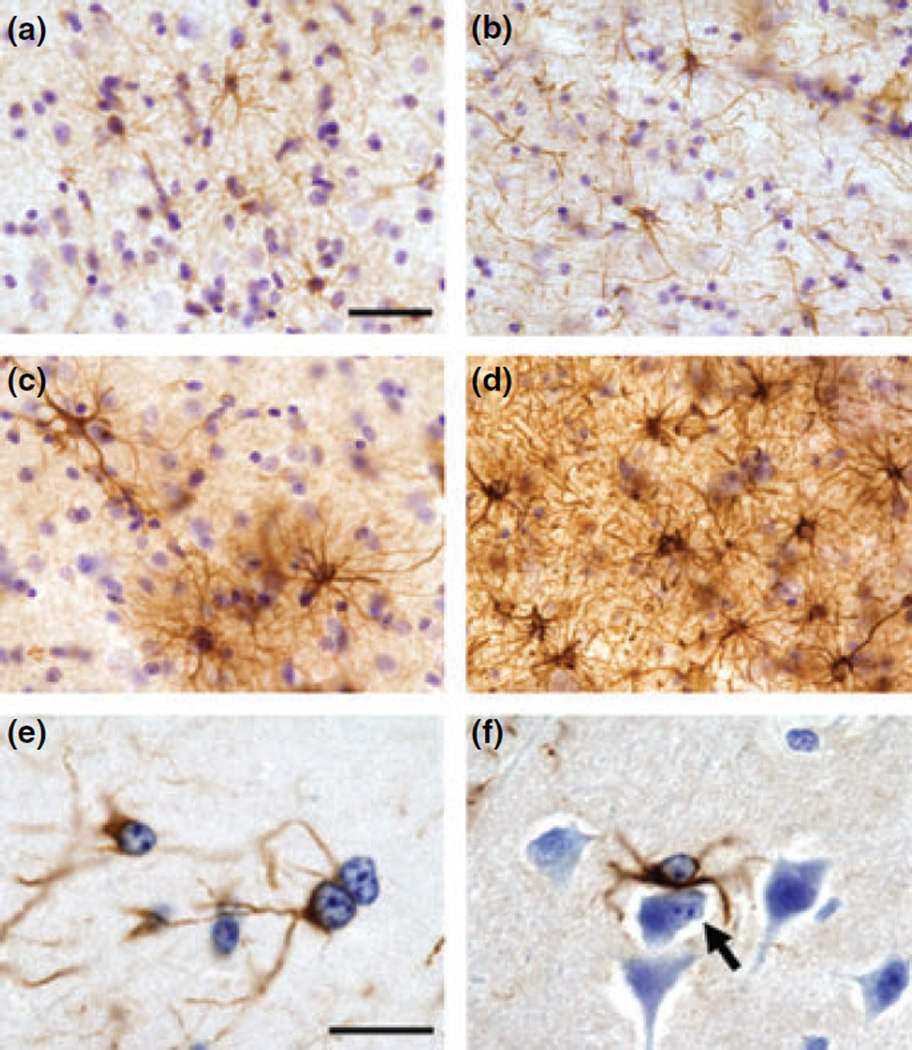
Glial fibrillary acidic protein was used to assess glial cell activation in the frontal cortex. In the gray matter and white matter of Mn-exposed animals, there were fibrous and protoplasmic astrocytes that expressed increased GFAP levels and hypertrophic processes consistent with astrocytosis (Fig. 5). We also observed numerous Alzheimer’s type II astrocytes consistent with what has been described in the Mn-exposed brain (Hazell et al. 2006) (Fig. 5).
Fig. 5.
Representative images of glial fibrillary acidic protein (GFAP) immunohistochemistry in control (a and b) and Mn-exposed (c–f) frontal cortex. In control animals, astrocytes expressed a normal ramified morphology in the gray (a) and white matter (b). In Mn-exposed frontal cortex, there were areas in which GFAP-labeled astrocytes were increased in number and expressed hypertrophic processes in both the gray (c) and white matter (d). Alzheimer’s type II astrocytes were frequently observed in Mn-exposed animals (e and f). Alzheimer’s type II astrocytes are often paired (see adjacent cells in e) and express enlarged nuclei with prominent nucleoli. In panel (f), there is an Alzheimer’s type II astrocyte adjacent to a cell undergoing apoptosis (arrow in f). Scale bar: (a–d) 40 and (e and f) 20 µm.
p53 immunohistochemistry
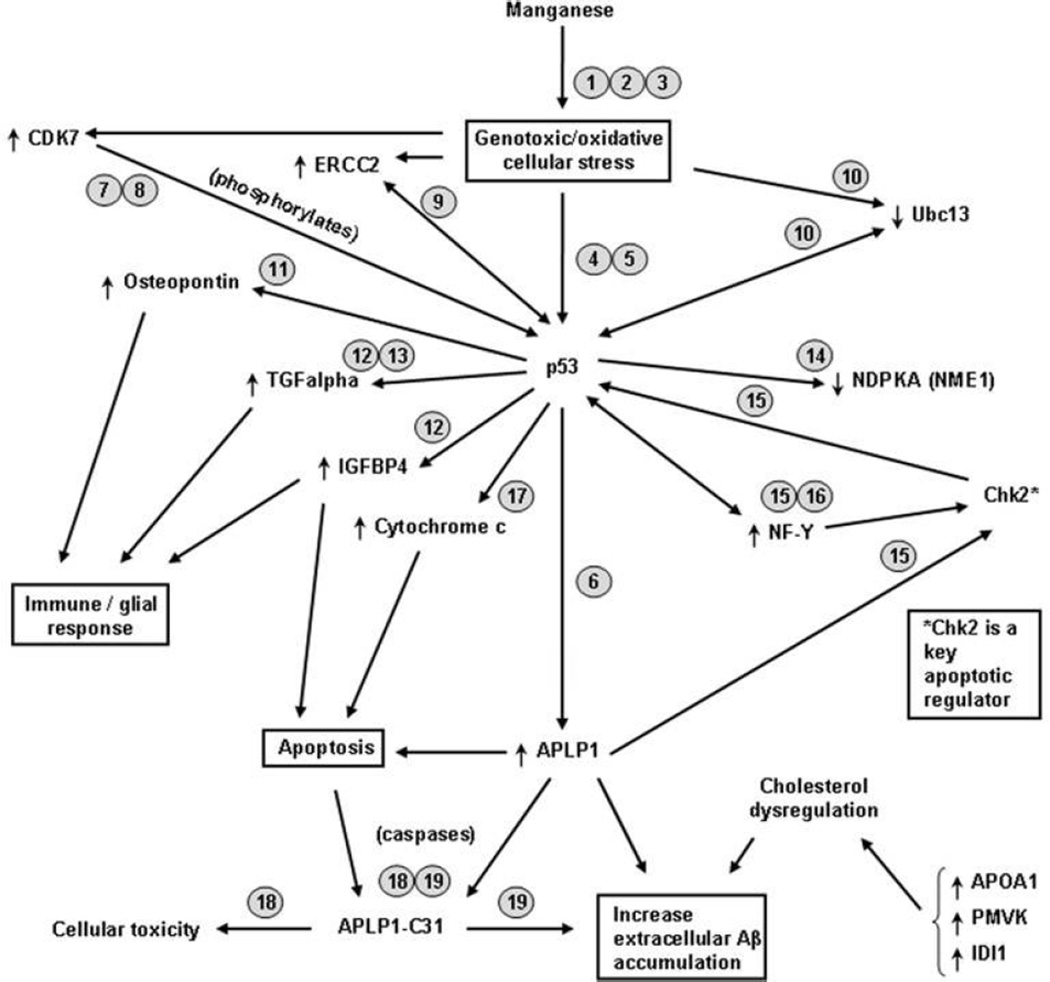
Several of the genes altered by chronic Mn-exposure are known to be activated by p53 or their gene product interacts with p53 to modify its function (see Scheme 1). Therefore, we performed p53 immunohistochemistry to determine if p53 protein levels were increased in the frontal cortex of Mn-exposed animals. Figure 6a and b show that there was little or no p53 immunoreactivity in control tissue except in blood vessels (not shown). However, neurons in Mn-exposed animals expressed increased levels of p53 label in the cytoplasm with a punctate appearance (Fig. 6c). p53 staining in the cytoplasm was also present in neurons with pale hypertrophic nuclei (Fig. 6e). Increased p53 immunoreactivity was also observed in glial processes and nuclei (Fig. 6d and f). These findings indicate that chronic Mn exposure produces a cellular stress response that increases p53 levels in cortical neurons and glial cells.
Scheme 1.
Putative mechanisms of Mn-induced neurodegeneration involving p53 related genes. Arrows next to gene name indicate if the gene is increased or decreased in Mn-exposed frontal cortex. Number next to gene designation represents the citation documenting the effect described: 1 (HaMai and Bondy 2004); 2 (Hirata 2002); 3 (Latchoumycandane et at. 2005); 4 (Culmsee and Mattson 2005); 5 (Miller et a1. 2000); 6 (Tang et al. 2007); 7 (Lu et al. 1997); 8 (Ko et al. 1997); 9 (Wang et al. 1995); 10 (Laine et al. 2006); 11 (Morimoto et al. 2002); 12 (Wang et al. 2001); 13 (Shin et al. 1995); 14 (Cervoni et al. 2006); 15 (Matsui et al. 2004); 16 (Romier et al. 2003); 17 (Jin et al. 2006); 18 (Galvan et al. 2002); 19 (Dumanchin-Njock et al. 2001).
Fig. 6.
Representative p53 immunohistochemistry in the frontal cortex of control (a and b) and Mn-exposed animals (c–f). There was little or no p53 labeling in cortical neurons (a) or white matter glial cells (b) in control animals. In the frontal cortex of Mn-exposed animals there were cortical neurons with increased punctate p53 immunoreactivity in the cytoplasm (arrow in c). In some cases, cortical neurons with p53 cytoplasmic labeling exhibited hypertrophic nuclei with pale staining (arrow in e). Increased p53 immunostaining was present in glial processes (arrow in d) and in their nucleus (arrow in f). Scale bar: (for all panels) 20 µm.
Metal concentrations in brain tissue
Concentrations of Mn, copper, zinc, and iron were measured in the frontal cortex by high-resolution inductively coupled plasma mass spectroscopy and presented in Table 2. As expected, Mn-exposure produced a significant increase (72.4%; p < 0.05; one-tail Student’s t-test) in Mn concentrations. The mean ± SEM of frontal cortex Mn concentrations were: controls (n = 3) 0.207 ± 0.03 µg Mn/g tissue and Mn-exposed (n = 4) 0.357 ± 0.06 µg Mn/g tissue. Copper concentrations were also significantly increased (47.3%; p < 0.05; one-tail Student’s t-test) in Mn-exposed frontal cortex relative to control tissue. The mean ± SEM of frontal cortex copper concentrations were: controls (n = 3) 3.19 ± 0.29 µg copper/g tissue and Mn-exposed (n = 4) 4.70 ± 0.56 µg copper/g tissue. Chronic Mn exposure did not alter iron or zinc concentrations in the frontal cortex (Table 2).
Table 2.
Average tissue metal concentrations (± SEM) measured by inductively coupled plasma mass spectroscopy, expressed as µg metal/g tissue, in the frontal cortex of Mn-exposed and control animals
| Mn | Cu | Fe | Zn | |
|---|---|---|---|---|
| Control | 0.20 ± 0.02 | 3.40 ± 0.47 | 30.65 ± 4.80 | 11.97 ± 1.10 |
| Mn-Treated | 0.36 ± 0.05 | 4.70 ± 0.56 | 31.23 ±3.72 | 13.16 ± 1.46 |
| p- value | 0.04* | 0.04* | 0.93** | 0.54** |
p-values for the Mn and Cu measurements were derived using a one-tailed Student’s t-test;
p-values for Fe and Zn measurements were derived using a two-tailed Student’s t-test.
Discussion
The present study provides new evidence that chronic exposure to Mn, a heavy metal that is ubiquitously present in the environment, produces a cellular stress response and neurodegeneration in the frontal cortex of non-human primates. To our knowledge, this is the first report describing Mn-induced neuropathology in the frontal cortex of non-human primates. We also show that chronic Mn exposure altered the expression of genes associated with the amyloid precursor protein (APP) family, cell cycle regulation and DNA repair, apoptosis, cholesterol homeostasis, proteasome/ubiquitin function, protein folding, axonal/ vesicular transport, and inflammation. The finding that several cell cycle regulation genes were altered by Mn exposure suggests an attempt of cell cycle re-entry. A central hypothesis that explains many of the pathological changes in neurodegenerative disease such as AD is the activation of the cell cycle machinery in post-mitotic differentiated neurons (Raina et al. 2000; Yang et al. 2003). We should note, however, that one of the limitations of this study is the inability to confirm the Mn-induced changes of multiple genes identified in the array, with the exception of APLP1 which was confirmed by immunohistochemistry.
Several of the genes altered in the frontal cortex of Mn-exposed animals are documented to be p53 target genes or their protein product interacts with p53 to alter its function (see Scheme 1). p53 is a transcription factor known to regulate several major cellular functions including gene transcription, DNA synthesis and repair, cell cycle regulation, senescence, and apoptosis (Culmsee and Mattson 2005). Activation of p53 is known to occur following insults that produce genotoxic or oxidative stress (Morrison and Kinoshita 2000; Culmsee and Mattson 2005). Manganese produces oxidative stress in cell culture systems (Hirata 2002; HaMai and Bondy 2004; Latchoumycandane et al. 2005) and our present results suggest that p53 activation may play a central role in the gene expression changes documented in the frontal cortex of Mn-exposed animals (Scheme 1).
Involvement of p53 activation in Mn-induced neurotoxicity is suggested by increased p53 reactivity in the cytoplasm of cortical neurons and in the nucleus and processes of glial cells in the frontal cortex of Mn-exposed animals (Fig. 6). This pattern of p53 subcellular localization has been described in the brain of patients with neurodegenerative diseases including AD (de la Monte et al. 1997). In Mn-exposed frontal cortex, p53 immunoreactivity was also observed in cells with apoptosis stigmata (Fig. 4). Non-transcriptional p53-induced apoptosis mediated by p53 localization in the cytoplasm or in the mitochondria has been documented (Moll et al. 2005).
Amyloid-β precursor-like protein 1 was the most highly up-regulated gene in the frontal cortex of Mn-exposed animals and increased protein expression was confirmed by immunohistochemistry (Table 1 and Fig. 1). APLP1 is a member of the APP family that consists of APP, APLP1, and APLP2 and is the only gene in this family that is expressed exclusively in the brain (Kim et al. 1995). The human APLP1 gene has been mapped to the long arm of chromosome 19 in the same region containing a late-onset familial AD locus (Kim et al. 1995). APLP1 is enriched at the post-synaptic density in the cerebral cortex and in the hippocampus (Kim et al. 1995) and it has been found in dystrophic neurites and senile plaques in the AD brain (Bayer et al. 1997; McNamara et al. 1998). APLP1 can also selectively increase APP shedding. Ectodomain shedding is a key regulatory step in the generation of Aβ peptide (Neumann et al. 2006). The promoter region of the APLP1 gene has specificity protein 1 (SP-1) and activator protein 1 (AP-1) regulatory sites as well as a heat-shock element consensus sequence that may confer sensitivity to a variety of stressful and pathologic conditions (Zhong et al. 1996). Therefore, increased APLP1 expression may be an indicator of cellular stress. Consistent with this notion, a recent study has shown that APLP1 is a novel p53 transcriptional target gene that is induced by genotoxic stress (Tang et al. 2007). Further, among the APP family members, only APLP1 is increased specifically by p53 during genotoxic stress (Tang et al. 2007). This same study showed that increased APLP1 expression enhances neuronal apoptosis and neurodegeneration (Tang et al. 2007). This finding is consistent with our observation that in Mn-exposed frontal cortex, cell remnants with apoptosis stigmata exhibited increased APLP1 and p53 expression suggesting involvement of these two factors in Mn-induced apoptotic cell death (Fig. 4). Our finding of increased p53 immunoreactivity and extracellular Aβ deposition has important mechanistic implications. LaFerla et al. (1996) have shown that in Aβ transgenic mice, brain regions expressing p53 activation, DNA fragmentation and marked neuronal cell death, extracellular deposits of Aβ become evident with local activation of astrocytes. All of these conditions are observed in the frontal cortex of Mn-exposed animals suggestive of Mn-induced dysregulation of APP processing and Aβ extracellular accumulation (see Scheme 1).
A question raised by our present findings relates to potential mechanism(s) by which a Mn-induced increase in APLP1 expression promotes neurodegenerative changes in the frontal cortex. Although increased APLP1 expression has been detected in dystrophic neurites and in Aβ plaques in AD (McNamara et al. 1998), its precise role in plaque formation or neurodegeneration is not known. It is thought that the loss of synapses and neuronal cell death characteristic of AD is due to the neurotoxic effects of amyloid-β1–42, a toxic peptide resulting from the proteolysis of APP by β- and γ-secretases. However, unlike APP, APLP1 lacks the Aβ domain (Li and Sudhof 2004). On the other hand, APP is also cleaved in the intracellular carboxy-terminus by caspases to generate a C31 cytotoxic peptide (Galvan et al. 2002). This peptide is present in the brains of AD patients (Lu et al. 2000) and triggers a selective increase in amyloid-β1–42 in mammalian cells (Dumanchin-Njock et al. 2001). Galvan et al. (2002) reported that the caspase cleavage sites in APP are conserved in APLP1 and it generates a carboxy-terminus-C31 peptide (APLP1-C31) with a degree of cytotoxicity similar to the APP-C31 peptide. Relevant to our present findings of increased APLP1 protein expression in apoptotic neurons and glial cells in the Mn-exposed frontal cortex, the APLP1-C31 peptide induces apoptotic cell death in both neuronal and glial cells (Galvan et al. 2002). Taken together, these observations suggest that increased levels of APLP1-C31 peptide may produce neurodegenerative changes and diffuse Aβ plaques in Mn-exposed frontal cortex. These observations provide a potential link by which chronic exposure to toxic concentrations of Mn may mediate changes in the processing of APLP1 to promote neurodegeneration and diffuse Aβ plaques in the brain. Studies using primary cortical cultures are needed to directly test this hypothesis.
A novel observation in the present study was the marked expression of APLP1 in interstitial neurons of the white matter in the Mn-exposed frontal cortex (Fig. 1). White matter interstitial neurons are remnant neurons of the cortical subplate and are the oldest cells in the neocortex. They are NADPH-diaphorase and nitric oxide synthase positive and they receive projections from the thalamus, brainstem, and the nucleus basalis of Meynert (Smiley et al. 1998). They have been described as being cholinoniceptic (Smiley et al. 1998) and alterations in the number of interstitial neurons have been noted in AD (Kowall and Beal 1988). Their possible involvement in Mn-induced neurodegeneration needs further exploration.
Environmental exposure to metals has been implicated as a risk factor for neurodegenerative disease, including AD (Marx 2003; Liu et al. 2006). In particular, accumulation or dysregulation of brain copper homeostasis has been associated with Aβ plaques in the AD brain (Bush 2003). We have previously shown that in the basal ganglia of monkeys used in the current study, Mn exposure not only increased brain Mn concentrations but it also increased copper concentrations with no change in zinc or iron levels (Guilarte et al. 2006a). In the current study, we confirm that Mn exposure produces a similar effect in the frontal cortex as Mn-exposed animals expressed an increase in Mn and copper concentrations with no effect on zinc or iron. Therefore, it appears that Mn, in and of itself, is neurotoxic but increasing brain Mn concentrations to toxic levels also dysregulates copper homeostasis in the brain. An increase in brain copper concentrations by Mn may contribute to the neurodegenerative changes and diffuse Aβ plaques documented in the present study as copper has been implicated in AD pathology (Waggoner et al. 1999; Bush 2003; Marx 2003). The increase in copper concentration measured in the brain of Mn-exposed monkeys is consistent with two recent reports showing increased copper concentration in saliva (Wang et al. 2008) and in serum (Squitti et al. 2007) of workers occupationally exposed to Mn. Therefore, our findings of increased brain copper concentrations in non-human primates by chronic Mn exposure are consistent with human studies.
In summary, our present studies provide new evidence on Mn-induced effects in the frontal cortex, a brain region that has not been previously associated with Mn neurotoxicity and which accumulates much lower concentrations of Mn than basal ganglia structures. Our data suggest that Mn-induced neurodegeneration is not solely defined by the degree of Mn accumulation in brain structures but also by the intrinsic vulnerability of a particular brain region to Mn-induced neurotoxicity.
Acknowledgements
This work was supported by National Institute of Environmental Health Sciences Grant number ES010975 to TRG and in part by the intramural Research Program of the NIH, National Institute on Aging. NCB was supported by NIEHS training Grant #T32 ES07141.
Abbreviations used
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- APLP1
amyloid-β precursor-like protein 1
- APP
amyloid precursor protein
- GFAP
glial fibrillary acidic protein
- Mn
manganese
References
- Alafuzoff I, Pikkarainen M, Al Sarraj S, et al. Interlaboratory comparison of assessments of Alzheimer disease-related lesions: a study of the Brain Net Europe Consortium. J. Neuropathol. Exp. Neurol. 2006;65:740–757. doi: 10.1097/01.jnen.0000229986.17548.27. [DOI] [PubMed] [Google Scholar]
- Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast-Pettersen R, Ellingsen DG, Hetland SM, Thomassen Y. Neuropsychological function in manganese alloy plant workers. Int. Arch. Occup. Environ. Health. 2004;11:277–287. doi: 10.1007/s00420-003-0491-0. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Paliga K, Weggen S, Wiestler OD, Beyreuther K, Multhaup G. Amyloid precursor-like protein 1 accumulates in neuritic plaques in Alzheimer’s disease. Acta Neuropathol (Berl.) 1997;94:519–524. doi: 10.1007/s004010050745. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Mergler D, Baldwin M, Panisset M, Bowler R, Roels HA. Neurobehavioral functioning after cessation of manganese exposure: a follow-up after 14 years. Am. J. Ind. Med. 2007;50:831–840. doi: 10.1002/ajim.20407. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–326. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, et al. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup. Environ. Med. 2007;64:167–177. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush AI. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- Cervoni L, Egistelli L, Eufemi M, d’Abusco AS, Altieri F, Lascu I, Turano C, Giartosio A. DNA sequences acting as binding sites for NM23/NDPK proteins in melanoma M14 cells. J. Cell. Biochem. 2006;98:421–428. doi: 10.1002/jcb.20808. [DOI] [PubMed] [Google Scholar]
- Cheadle C, Vawter MP, Freed WJ, Becker KG. Analysis of microarray data using Z score transformation. J. Mol. Diagn. 2003;5:73–81. doi: 10.1016/S1525-1578(10)60455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem. Biophys. Res. Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- Czasch S, Paul S, Baumgartner W. A comparison of immunohistochemical and silver staining methods for the detection of diffuse plaques in the aged canine brain. Neurobiol. Aging. 2006;27:293–305. doi: 10.1016/j.neurobiolaging.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Dumanchin-Njock C, Alves da Costa CA, Mercken L, Pradier L, Checler F. The caspase-derived C-terminal fragment of betaAPP induces caspase-independent toxicity and triggers selective increase of Abeta42 in mammalian cells. J. Neurochem. 2001;78:1153–1161. doi: 10.1046/j.1471-4159.2001.00513.x. [DOI] [PubMed] [Google Scholar]
- Galvan V, Chen S, Lu D, Logvinova A, Goldsmith P, Koo EH, Bredesen DE. Caspase cleavage of members of the amyloid precursor family of proteins. J. Neurochem. 2002;82:283–294. doi: 10.1046/j.1471-4159.2002.00970.x. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, et al. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp. Neurol. 2006a;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL, Degaonkar M, Chen MK, Barker PB, Syversen T, Schneider JS. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: a 1H–MRS and MRI study. Toxicol. Sci. 2006b;94:351–358. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- HaMai D, Bondy SC. Oxidative basis of manganese neurotoxicity. Ann. NY Acad. Sci. 2004;1012:129–141. doi: 10.1196/annals.1306.010. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Normandin L, Norenberg MD, Kennedy G, Yi JH. Alzheimer type II astrocytic changes following sub-acute exposure to manganese and its prevention by antioxidant treatment. Neurosci. Lett. 2006;396:167–171. doi: 10.1016/j.neulet.2005.11.064. [DOI] [PubMed] [Google Scholar]
- Hirata Y. Manganese-induced apoptosis in PC12 cells. Neurotoxicol. Teratol. 2002;24:639–653. doi: 10.1016/s0892-0362(02)00215-5. [DOI] [PubMed] [Google Scholar]
- Iinuma Y, Kubota M, Uchiyama M, Yagi M, Kanada S, Yamazaki S, Murata PL, Okamoto K, Suzuki M, Nitta K. Whole-blood manganese levels and brain manganese accumulation in children receiving long-term home parenteral nutrition. Pediatr. Surg Int. 2003;19:268–272. doi: 10.1007/s00383-002-0929-6. [DOI] [PubMed] [Google Scholar]
- Jin YJ, Wang J, Qiao C, Hei TK, Brandt-Rauf PW, Yin Y. A novel mechanism for p53 to regulate its target gene ECK in signaling apoptosis. Mol. Cancer Res. 2006;4:769–778. doi: 10.1158/1541-7786.MCR-06-0178. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Manganese: a high-octane dispute. Science. 2003;300:926–928. doi: 10.1126/science.300.5621.926. [DOI] [PubMed] [Google Scholar]
- Kim TW, Wu K, Xu JL, McAuliffe G, Tanzi RE, Wasco W, Black IB. Selective localization of amyloid precursor-like protein 1 in the cerebral cortex postsynaptic density. Brain Res. Mol. Brain Res. 1995;32:36–44. doi: 10.1016/0169-328x(95)00328-p. [DOI] [PubMed] [Google Scholar]
- Kim EA, Cheong HK, Choi DS, Sakong J, Ryoo JW, Park I, Kang DM. Effect of occupational manganese exposure on the central nervous system of welders: (1)H magnetic resonance spectroscopy and MRI findings. Neurotoxicology. 2007;28:276–283. doi: 10.1016/j.neuro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Kimura N, Yanagisawa K, Terao K, Ono F, Sakakibara I, Ishii Y, Kyuwa S, Yoshikawa Y. Age-related changes of intracellular Abeta in Cynomolgus monkey brains. Neuropathol Appl. Neurobiol. 2005;31:170–180. doi: 10.1111/j.1365-2990.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- Klos KJ, Ahlskog JE, Kumar N, Cambern S, Butz J, Burritt M, Fealey RD, Cowl CT, Parisi JE, Josephs KA. Brain metal concentrations in chronic liver failure patients with pallidal Tl MRI hyperintensity. Neurology. 2006a;67:1984–1989. doi: 10.1212/01.wnl.0000247037.37807.76. [DOI] [PubMed] [Google Scholar]
- Klos KJ, Chandler M, Kumar N, Ahlskog JE, Josephs KA. Neuropsychological profiles of manganese neurotoxicity. Eur J. Neurol. 2006b;13:1139–1141. doi: 10.1111/j.1468-1331.2006.01407.x. [DOI] [PubMed] [Google Scholar]
- Ko LJ, Shieh SY, Chen X, Jayaraman L, Tamai K, Taya Y, Prives C, Pan ZQ. p53 is phosphorylated by CDK7-cyclin H in a p36MAT1-dependent manner. Mol. Cell. Biol. 1997;17:7220–7229. doi: 10.1128/mcb.17.12.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaki H, Maisawa S, Sugai K, Kobayashi Y, Hashimoto T. Tremor and seizures associated with chronic manganese intoxication. Brain Dev. 1999;21:122–124. doi: 10.1016/s0387-7604(98)00074-6. [DOI] [PubMed] [Google Scholar]
- Kowall NW, Beal MF. Cortical somatostatin, neuropeptide Y, and NADPH diaphorase neurons: normal anatomy and alterations in Alzheimer’s disease. Ann. Neurol. 1988;23:105–114. doi: 10.1002/ana.410230202. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Hall CK, Ngo L, Jay G. Extracellular deposition of beta-amyloid upon p53-dependent neuronal cell death in transgenic mice. J. Clin. Invest. 1996;98:1626–1632. doi: 10.1172/JCI118957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine A, Topisirovic I, Zhai D, Reed JC, Borden KL, Ronai Z. Regulation of p53 localization and activity by Ubcl3. Mol Cell. Biol. 2006;26:8901–8913. doi: 10.1128/MCB.01156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J. Pharmacol. Exp. Ther. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Levy BS, Nassetta WJ. Neurologic effects of manganese in humans: a review. Int. J. Occup. Environ. Health. 2003;9:153–163. doi: 10.1179/oeh.2003.9.2.153. [DOI] [PubMed] [Google Scholar]
- Li Q, Sudhof TC. Cleavage of amyloid-beta precursor protein and amyloid-beta precursor-like protein by BACE 1. J. Biol. Chem. 2004;279:10542–10550. doi: 10.1074/jbc.M310001200. [DOI] [PubMed] [Google Scholar]
- Liu G, Huang W, Moir RD, Vanderburg CR, Lai B, Peng Z, Tanzi RE, Rogers JT, Huang X. Metal exposure and Alzheimer’s pathogenesis. J. Struct. Biol. 2006;155:45–51. doi: 10.1016/j.jsb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Lu H, Fisher RP, Bailey P, Levine AJ. The CDK7-cycH-p36 complex of transcription factor IIH phosphorylates p53, enhancing its sequence-specific DNA binding activity in vitro. Mol. Cell. Biol. 1997;17:5923–5934. doi: 10.1128/mcb.17.10.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DC, Rabizadeh S, Chandra S, Shayya RF, Ellerby LM, Ye X, Salvesen GS, Koo EH, Bredesen DE. A second cytotoxic proteolytic peptide derived from amyloid beta-protein precursor. Nat. Med. 2000;6:397–404. doi: 10.1038/74656. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Albini E, Benedetti L, Borghesi S, Coccaglio R, Malara EC, Parrinello G, Garattini S, Resola S, Alessio L. High prevalence of Parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am. J. Ind. Med. 2007;50:788–800. doi: 10.1002/ajim.20494. [DOI] [PubMed] [Google Scholar]
- Marx J. Neuroscience. Possible role for environmental copper in Alzheimer’s disease. Science. 2003;301:905. doi: 10.1126/science.301.5635.905a. [DOI] [PubMed] [Google Scholar]
- Matsui X, Katsuno Y, Inoue T, Fujita F, Joh X, Niida H, Murakami H, Itoh M, Nakanishi M. Negative regulation of Chk2 expression by p53 is dependent on the CCAAT-binding transcription factor NF-Y. J. Biol. Chem. 2004;279:25093–25100. doi: 10.1074/jbc.M403232200. [DOI] [PubMed] [Google Scholar]
- McNamara MJ, Ruff CX, Wasco W, Tanzi RE, Thinakaran G, Hyman BX. Immunohistochemical and in situ analysis of amyloid precursor-like protein-1 and amyloid precursor-like protein-2 expression in Alzheimer disease and aged control brains. Brain Res. 1998;804:45–51. doi: 10.1016/s0006-8993(98)00653-2. [DOI] [PubMed] [Google Scholar]
- Meral H, Kutukcu Y, Atmaca B, Ozer F, Hamamcioglu K. Parkinsonism caused by chronic usage of intravenous potassium permanganate. Neurologist. 2007;13:92–94. doi: 10.1097/01.nrl.0000253089.20746.a8. [DOI] [PubMed] [Google Scholar]
- Miller FD, Pozniak CD, Walsh GS. Neuronal life and death: an essential role for the p53 family. Cell Death Differ. 2000;7:880–888. doi: 10.1038/sj.cdd.4400736. [DOI] [PubMed] [Google Scholar]
- Moll UM, Wolff S, Speidel D, Deppert W. Transcription-independent pro-apoptotic functions of p53. Curr Opin. Cell Biol. 2005;17:631–636. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Sohn YK, Wands JR. Correlates of p53-and Fas (CD95)-mediated apoptosis in Alzheimer’s disease. J. Neurol. Sci. 1997;152:73–83. doi: 10.1016/s0022-510x(97)00131-7. [DOI] [PubMed] [Google Scholar]
- Morimoto I, Sasaki Y, Ishida S, Imai K, Tokino X. Identification of the osteopontin gene as a direct target of TP53. Genes Chromosomes Cancer. 2002;33:270–278. doi: 10.1002/gcc.10020. [DOI] [PubMed] [Google Scholar]
- Morrison RS, Kinoshita Y. The role of p53 in neuronal cell death. Cell Death Differ. 2000;7:868–879. doi: 10.1038/sj.cdd.4400741. [DOI] [PubMed] [Google Scholar]
- Nadon NL, Mohr D, Becker KG. National Institute on Aging microarray facility - resources for gerontology research. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:413–415. doi: 10.1093/gerona/60.4.413. [DOI] [PubMed] [Google Scholar]
- Neumann S, Schobel S, Jager S, Trautwein A, Haass C, Pietrzik CU, Lichtenthaler SF. Amyloid precursor-like protein 1 influences endocytosis and proteolytic processing of the amyloid precursor protein. J. Biol. Chem. 2006;281:7583–7594. doi: 10.1074/jbc.M508340200. [DOI] [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J. Neuropathol. Exp. Neurol. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Raina AK, Zhu X, Rottkamp CA, Monteiro M, Takeda A, Smith MA. Cyclin’ toward dementia: cell cycle abnormalities and abortive oncogenesis in Alzheimer disease. J. Neu-rosci. Res. 2000;61:128–133. doi: 10.1002/1097-4547(20000715)61:2<128::AID-JNR2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Riudavets MA, Iacono D, Resnick SM, O’brien R, Zonderman AB, Martin LJ, Rudow G, Pletnikova O, Troncoso JC. Resistance to Alzheimer’s pathology is associated with nuclear hypertrophy in neurons. Neurobiol. Aging. 2007;28:1484–1492. doi: 10.1016/j.neurobiolaging.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Agudelo Y, Riojas-Rodriguez H, Rios C, Rosas I, Sabido PE, Miranda J, Siebe C, Texcalac JL, Santos-Burgoa C. Motor alterations associated with exposure to manganese in the environment in Mexico. Sci. Total Environ. 2006;368:542–556. doi: 10.1016/j.scitotenv.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Rollin H, Mathee A, Levin J, Theodorou P, Wewers F. Blood manganese concentrations among first-grade schoolchildren in two South African cities. Environ. Res. 2005;97:93–99. doi: 10.1016/j.envres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Romier C, Cocchiarella F, Mantovani R, Moras D. The NF-YB/NF-YC structure gives insight into DNA binding and transcription regulation by CCAAT factor NF-Y. J. Biol. Chem. 2003;278:1336–1345. doi: 10.1074/jbc.M209635200. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118:222–231. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin TH, Paterson AJ, Kudlow JE. p53 stimulates transcription from the human transforming growth factor alpha promoter: a potential growth-stimulatory role for p53. Mol. Cell Biol. 1995;15:4694–4701. doi: 10.1128/mcb.15.9.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikk K, Taba P, Haldre S, Bergquist J, Nyholm D, Zjablov G, Asser T, Aquilonius SM. Irreversible motor impairment in young addicts - ephedrone, manganism or both? Acta Neurol Scand. 2007;115:385–389. doi: 10.1111/j.1600-0404.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Mesulam MM. Infracortical interstitial cells concurrently expressing m2-muscarinic receptors, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate-diaphorase in the human and monkey cerebral cortex. Neuroscience. 1998;84:755–769. doi: 10.1016/s0306-4522(97)00524-1. [DOI] [PubMed] [Google Scholar]
- Squitti R, Gorgone G, Binetti G, Ghidoni R, Pasqualetti P, Draicchio F, Albini E, Benedetti L, Lucchini R, Rossigni PM. Metals and oxidative stress in Parkinson’s Disease from industrial areas with exposure to environmental toxins or metal pollution. G Ital. Med. Lav. Ergon. 2007;29:294–296. (In Italian) [PubMed] [Google Scholar]
- Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24:667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Tang X, Milyavsky M, Goldfinger N, Rotter V. Amyloid-beta precursor-like protein APLP1 is a novel p53 transcriptional target gene that augments neuroblastoma cell death upon genotoxic stress. Oncogene. 2007;26:7302–7312. doi: 10.1038/sj.onc.1210542. [DOI] [PubMed] [Google Scholar]
- Waggoner DJ, Bartnikas TB, Gitlin JD. The role of copper in neurodegenerative disease. Neurobiol. Dis. 1999;6:221–230. doi: 10.1006/nbdi.1999.0250. [DOI] [PubMed] [Google Scholar]
- Walsh MP. The global experience with lead in gasoline and the lessons we should apply to the use of MMT. Am. J. Ind. Med. 2007;50:853–860. doi: 10.1002/ajim.20483. [DOI] [PubMed] [Google Scholar]
- Wang XW, Yeh H, Schaeffer L, Roy R, Moncollin V, Egly JM, Wang Z, Freidberg EC, Evans MK, Taffe BG. p53 modulation of TFIIH-associated nucleotide excision repair activity. Nat. Genet. 1995;10:188–195. doi: 10.1038/ng0695-188. [DOI] [PubMed] [Google Scholar]
- Wang L, Wu Q, Qiu P, Mirza A, McGuirk M, Kirschmeier P, Greene JR, Wang Y, Pickett CB, Liu S. Analyses of p53 target genes in the human genome by bioinformatic and microarray approaches. J. Biol. Chem. 2001;276:43604–43610. doi: 10.1074/jbc.M106570200. [DOI] [PubMed] [Google Scholar]
- Wang D, Du X, Zheng W. Alteration of saliva and serum concentrations of manganese, copper, zinc, cadmium and lead among career welders. Toxicol. Lett. 2008;176:40–47. doi: 10.1016/j.toxlet.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, et al. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J. Neurosci. 2003;23:2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Wu K, Black IB, Schaar DG. Characterization of the genomic structure of the mouse APLP1 gene. Genomics. 1996;32:159–162. doi: 10.1006/geno.1996.0096. [DOI] [PubMed] [Google Scholar]