Abstract
Long-term potentiation (LTP) of synaptic transmission in the CA1 region of the hippocampus depends on activation of N-methyl-D-aspartate receptors (NMDARs), which can be regulated by Ca2+-dependent release of D-serine from astrocytes. The detailed mechanism underlying astrocytic D-serine release is still unknown. In this study, we found that clamping astrocytic [Ca2+] at 100-150 nM or puffing artificial cerebrospinal fluid (ACSF) into the extracellular space (weak mechanical stimulation) enhanced synaptic activation of NMDARs. The enhancement was blocked by the NMDAR glycine site antagonist DCKA, glycine saturation, and infusion of astrocytes with D-Amino Acid Oxidase (DAAO) and the serine racemase inhibitor L-erythro-3-hydroxyaspartate (HoAsp), suggesting the involvement of astrocytic D-serine release. Intracellular 100-150 nM [Ca2+] or puffing ACSF stimulated astrocytes to generate D-serine-containing large vesicles (1-3 μm), exocytotic fusion of which released D-serine. The formation of astrocytic large vesicles involved intracellular fusion of small vesicles and/or other organelles. Spontaneous fusion of large vesicles occurred occasionally in astrocytes at rest, contributing to baseline D-serine levels, which increased the rising slope of NMDAR post-burst potentiation (PBP) without altering the PBP peak amplitude. Thus, under physiological conditions, astrocytic D-serine release by large vesicles facilitated weak theta-burst (TBS consisting of 5 bursts), but not strong TBS (TBS consisting of 10 bursts) stimulation-induced LTP.
Keywords: long-term potentiation, astrocyte, Ca2+ signaling, D-serine release, large vesicle
INTRODUCTION
Long-term potentiation (LTP), a persistent and activity-dependent increase in synaptic strength, is widely regarded as a cellular mechanism underlying learning and memory (Bliss and Collingridge, 1993, Milner et al., 1998, Whitlock et al., 2006). Activation of N-methyl-D-aspartate receptors (NMDARs) is one well-studied pathway for induction of LTP (Bliss and Collingridge, 1993, Milner et al., 1998, Martin et al., 2000, Muller et al., 2002, Whitlock et al., 2006). D-serine, an endogenous co-agonist for NMDARs in the brain, binds the NMDAR glycine site (Hashimoto et al., 1992, Wolosker et al., 2008) to facilitate glutamate activation of the receptor. Recent studies have suggested that astrocytes, a major type of glial cells, may participate in synaptic plasticity by releasing D-serine (Schell et al., 1995, Yang et al., 2003, Panatier et al., 2006, Henneberger et al., 2010). Mothet et al., (2005) reported that cultured astrocytes release D-serine through Ca2+- and SNARE-dependent exocytosis. However, the detailed mechanism for astrocytic D-serine release is still unknown. On the other hand, neuronal origin of D-serine was recently reported (Kartvelishvily et al., 2006, Balan et al., 2009), and the exact role of astrocytic D-serine release in synaptic plasticity needs to be further studied.
Recent studies have also suggested that astrocytes actively participate in synaptic plasticity by releasing other gliotransmitters, such as glutamate and ATP (Deng et al., , Kang et al., 1998, Araque et al., 2001, Parri and Crunelli, 2001, Fellin et al., 2004, Liu et al., 2004, Pascual et al., 2005, Jourdain et al., 2007, Agulhon et al., 2008, Navarrete and Araque, 2008, Shigetomi et al., 2008, Di Castro et al., 2011, Panatier et al., 2011, Min and Nevian, 2012). However, in transgenic mice with either astrocyte-specific knock-in of the mas-related gene A1 receptor (MrgA1R), an orphan G-protein coupled receptor that increases intracellular [Ca2+], or knock-out of IP3R2, experiments failed to demonstrate functions of astrocytic Ca2+ in synaptic plasticity (Fiacco et al., 2007, Petravicz et al., 2008, Agulhon et al., 2010). As a result, there is a heated debate about whether astrocytic Ca2+ signaling and Ca2+-dependent release of gliotransmitters play any roles in synaptic plasticity (Smith, 2010, Wenker, 2010). The study on the mechanism and function of astrocytic D-serine release will be helpful for clarifying the debate.
In this study, we used two-photon microscopy to image astrocytic vesicles and found that astrocytes release D-serine by a large vesicle that was generated from intracellular fusion of small vesicles. Under physiological conditions, astrocytic D-serine release facilitates weak theta-burst stimulation (TBS consisting of 5 bursts), but not strong TBS (10 bursts)-induced LTP.
EXPERIMENTAL PROCEDURES
Slice preparation
Brain slices were prepared as described previously (Kang et al., 1998). Briefly, 18- to 30-day-old (P18-P30) of either sex Sprague-Dawley rats were deeply anaesthetized with sodium pentobarbital (55 mg/kg) and then decapitated. Transverse brain slices of 300 or 400 µm thickness were cut with a vibratome (TPI, St Louis, MO, USA) in a cutting solution containing (mM): 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, 0.5 CaCl2, 10 glucose, 26 NaHCO3 and 230 sucrose. Slices containing the hippocampus were incubated in artificial cerebrospinal fluid (ACSF) gassed with 5% CO2/95% O2 for 1-4 hours and then transferred to a recording chamber (1.5 ml) perfused continually (3 ml/min) with ACSF gassed with 5% CO2/95% O2 at room temperature (23-25°C) for recording and imaging. ACSF contained (mM): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 MgCl2, 2 CaCl2, 10 glucose and 26 NaHCO3 (pH at 7.4 when gassed with 5% CO2/95% O2).
Patch-clamp recording
Cells were visualized with a 60[.dotmath]/0.90 nA water immersion lens on an Olympus BX51 upright microscope (Olympus Optical Co., NY, USA) equipped with infra-red differential inference contrast (IR-DIC) optics. Patch-clamp recording: Patch pipette electrodes (5–10 MΩ) were pulled from KG-33 glass capillaries (inner diameter 1.0 mm, outer diameter 1.5 mm, Garner Glass Co., Claremont, CA, USA) using a P-97 electrode puller (Sutter Instrument Co., Novato, CA, USA). Pyramidal neurons in the CA1 pyramidal layer and astrocytes in stratum radiatum were identified by their distinct DIC morphology and electrophysiological properties as described previously (Kang et al., 1998). Passive astrocytes (Steinhauser et al., 1994, Chvatal et al., 1995) with low input resistance (<20 MΩ), no voltage-dependent currents, and no AMPAR (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor) currents (Fig. 1A-C) were selected for all experiments. Complex astrocytes with high input resistance (>20 MΩ), voltage-gated K+ channels, and AMPAR currents were rejected. Astrocytes were voltage-clamped at -80 mV. Cells with a seal resistance < 5 GΩ, a holding current negative than -500 pA, or changes in series resistance >10% of control were rejected from further analysis. The control patch pipette solution for astrocytes contained (in mM): 123 K-gluconate, 10 KCl, 1 MgCl2, 10 HEPES, 1 ATP, 0.2 GTP, and 4 glucose (pH adjusted to 7.2 with KOH). Minimal air pressure was applied before patching and the time of patching-induced mechanical stimulation was limited in 5-10 s. Recorded signals were filtered through an 8-pole Bessel low-pass filter with 2 kHz cut-off frequency and sampled using the PCLAMP 10.2 acquisition program (Axon Instruments Inc.) with an analog-digital point sample interval of 50 or 100 μs.
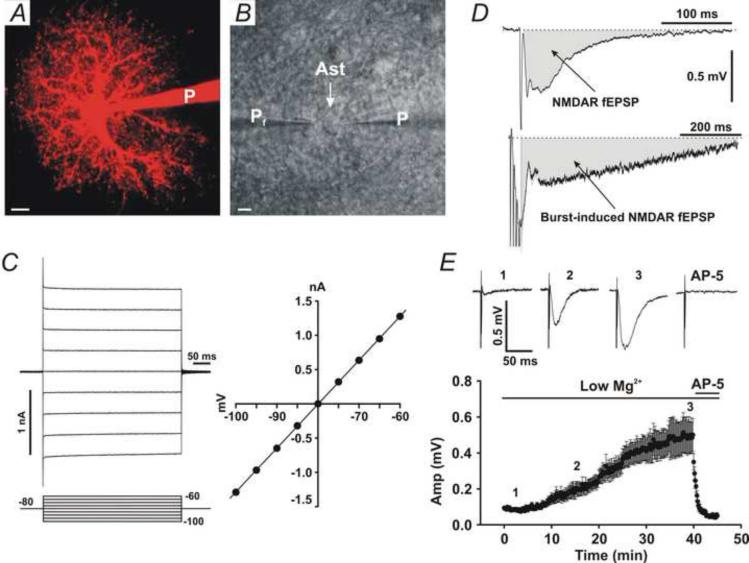
Fig. 1. Passive astrocytes and measurement of NMDAR fEPSPs.
(A) Two-photon microscopy imaging of a passive astrocytes patched with Alexa Fluor-594 (100 μM). (B) DIC imaging of a passive astrocyte (Ast) patched by a patch pipette (P). A field recording pipette (Pf) was placed in the immediate vicinity of the patched astrocyte. Scale bars in A and B, 5 μm. (C) Left traces, whole-cell voltage-clamp recording from a passive astrocyte showing membrane currents in response to voltage steps (Bottom lines). The number indicates voltage in mV. Right panel, the I-V curve shows a linear I-V relationship. (D) Upper trace, measurement of a testing stimulus-induced NMDAR fEPSP (Grey area). Bottom trace, measurement of a burst stimulation-induced NMDAR fEPSP (Grey area). Data are from a representative experiment. (E) Changes in NMDAR fEPSPs in low Mg2+ solution during recording. Upper traces, representative NMDAR fEPSP traces from the time indicated in the bottom panel (1, 2, and 3). Bottom panel, time-dependent changes in NMDAR fEPSP amplitude (Amp).
Field potential recording
Glass pipettes with 4–7 MΩ resistance were filled with the standard slice solution (ACSF) and placed at the position 2-3 μm from the soma of patched astrocytes (Fig. 1B, Pf). A bipolar Cluster Electrode (FHC, Bowdoin, ME, USA) was placed in CA1 stratum radiatum to stimulate CA3-CA1 Shaffer collateral fibers. Test stimulus was at an intensity of 1.0 – 1.5 μA (an half of the maximal intensity), 0.1 ms duration, and 10 s intervals. High-frequency stimulation (HFS) for inducing LTP consisted of two trains of 1 s, 100 Hz stimuli at intervals of 5 min. Theta-burst stimulation (TBS) for inducing LTP consisted of two trains of 10 bursts (strong TBS) or four trains of 5 bursts (weak TBS), each having four 100 Hz stimuli with an inter-burst duration of 200 ms. The inter-train interval was 5 min. In all experiments, Bicuculline (1(S),9(R)-(−)-Bicuculline methchloride, 10 μM) was added to the superfusate to inhibit GABAergic activity. NMDAR-mediated field excitatory postsynaptic potentials (NMDAR fEPSPs) were isolated pharmacologically by relieving the Mg2+-blockade of NMDARs with the superfusate containing 0 mM MgCl2 and 4 mM CaCl2, plus adding the AMPA receptor antagonist, 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX, 10 μM). To quantify activation of NMDARs, we measured the area under the curve of NMDAR fEPSPs evoked by the single test stimulus (Fig. 1D, Left trace) or by the burst stimulation (Fig. 1D, Right trace). When starting perfusion of slices with the low Mg2+/high Ca2+ solution, NMDAR fEPSPs were often very small (Fig. 1E, 1). The amplitude of NMDAR fEPSPs increased along with recording time (Fig. 1E, 2), and reached the maximal value after 40-50 min (Fig. 1E, 3). The maximal NMDAR fEPSP was fully blocked by the NMDAR antagonist, AP-5 (Fig. 1E, AP-5), suggesting that it is a NMDAR-mediated fEPSP. To avoid this time-dependent change in NMDAR fEPSPs, we pre-incubated slices in the low Mg2+/high Ca2+/NBQX solution for 50-60 min before recording. In such a way, we obtained constant large baseline NMDAR fEPSPs.
Fluorescence imaging
A customized two-photon Olympus BX61WI microscope with a 60×/0.90 nA water immersion lens was used to detect fluorescence signals. A Mai-Tai™ laser (Solid-State Laser Co., Mountain View, CA) tuned to 830 or 890 nm was used for excitation, and imaging acquisition was controlled by Olympus Fluoview FV300 software (Olympus America INC, Melville, NY). In the transfluorescence pathway, a 565 nm dichroic mirror was used to separate green and red fluorescence, and HQ525/50 and HQ605/50 high pass filters were placed in the “green” and “red” pathways, respectively, to eliminate transmitted or reflected excitation light (Chroma Technology Corp., Rockingham, VT, U.S.A.). Fluorescence images were scanned in X-Y-T or X-Y-Z modes at intervals of 2-5 s. Alexa Fluor-594 was detected via the red pathway with the HQ605/50 filter. Fluo-4 fluorescence or anti-D-serine antibody fluorescence was detected using the green pathway. Baseline fluorescence (F0) was the average of four images during pre-stimulus baseline imaging, and ΔF/F was calculated as (ΔF/F)(t) = (F(t)-F0)/F0. The position shift in the X-Y section during 5 min scanning was 0.5 ± 0.3 μm (mean ± SD, n = 30 slices). The identification criteria for large vesicles were a round Alexa Fluor-594 and Fluo-4 positive structure that rapidly disappeared. Disappearance of large vesicles was confirmed by X-Y-Z scanning.
Immunofluorescence for D-serine
After patching astrocytes with pipettes containing tonic 100 nM [Ca2+] and Alexa Fluor-594, slices (200 μm) were fixed with 2% paraformaldehyde and 2% glutaraldehyde for 1 hour on ice (Stevens et al., 2003). After washing 3 times with cold PBS, slices were treated with 5% donkey serum/0.3% TritonX-100 PBS solution for 30 min in room temperature. Slices were then treated with the anti-D-serine antibody (Rabbit, 1:2000 by 1% donkey serum/0.1% TritonX-100/PBS, Millipore, Billerica, MA USA) for 24 hours, followed by a fluorescencein (FITC)-conjugated second antibody (Goat anti-Rabbit IgG, 1:250 by 1% donkey serum/0.1% TritonX-100/PBS, Millipore, Billerica, MA USA) for 2 hours at room temperature. Finally, antibody fluorescence in Alexa Fluor-594-labeled astrocytes was examined under two-photon microscopy.
Weak mechanical stimulation
Puffing ACSF was used to produce weak mechanical stimulation. Glass pipettes with resistances of 7–10 MΩ were used to puff ACSF. Six puffs were delivered at intervals of 1 min with 3–5 psi air pressure pulses of 200 ms duration. Puffing pressure and duration were controlled by a Picospritzer III (Parker Hannifin Co. General Valve Operation, Cleveland, OH), and intervals were controlled by a Master-8 stimulator (A.M.P.I., Israel). Puffing pipettes were placed in stratum radiatum of CA1 50–100 μm below the slice surface.
Data analysis and Statistics
Patch-clamp data were analyzed using Clampfit 10.2 (Axon Instruments Inc., CA, USA), Origin 6.0 (OriginLab Co., Northampton, MA, USA), and CorelDraw 12 (Corel Co. Ontario, Canada) programs. The slope of fEPSPs, and area under the curve of NMDAR fEPSPs were analyzed offline using Clampfit 10.2. The mean slope of fEPSPs after LTP was measured 30–35 min after the second HFS and relative to baseline before LTP induction. Statistic analysis was performed by using Sigmaplot 8.0 (SSI, San Jose, CA, USA) or Origin 6.0 software. Statistical data are presented as mean ± SEM unless otherwise indicated.
Drugs
Fluo-4 potassium and Alexa Fluor-594 were purchased from Molecular Probes (Carlsbad, California, USA). AP-5 and MRS2179 was purchased from Tocris Bioscience Inc. (Ellisville, MO, USA). LC-TT was purchased from List Biological Laboratories, Inc. (Campbell, CA, USA). HoAsp was purchased from Wako Chemicals USA, Inc. (Richmond, VA, USA). Glycine, DAAO, NBQX, Bicuculline, and other chemicals were purchased from Sigma-Aldrich Co (St. Louis, MO, USA).
RESULTS
High astrocytic [Ca2+] enhances NMDAR activation by triggering D-serine release
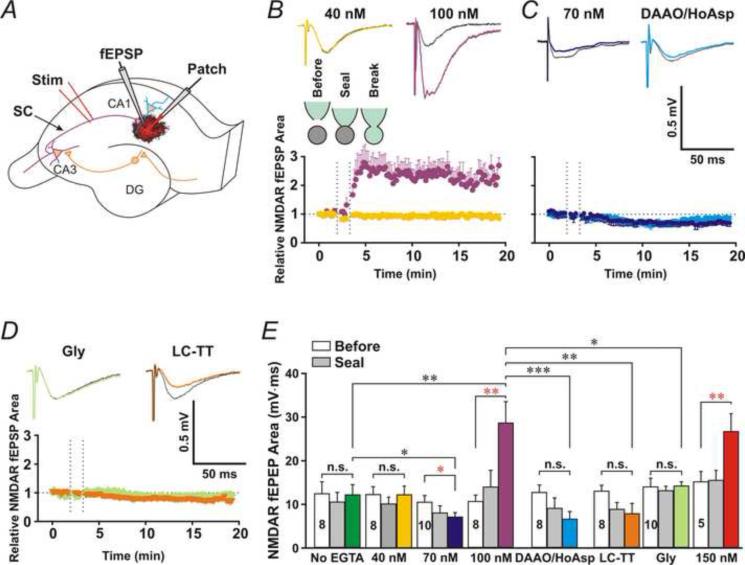
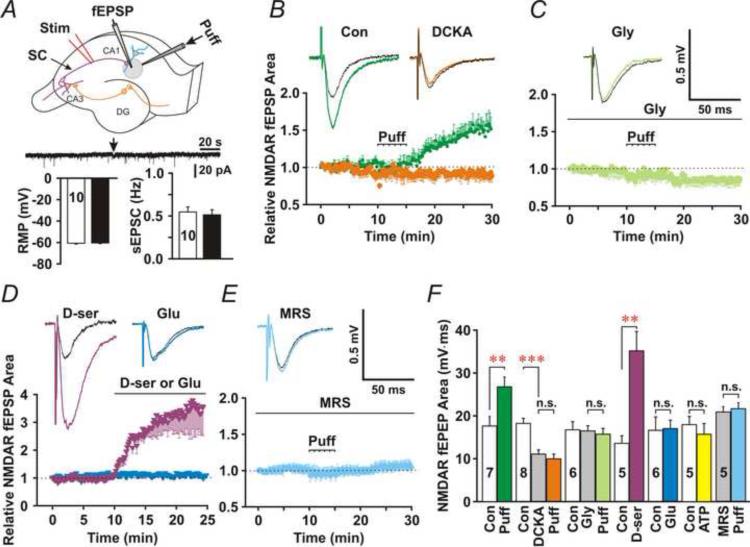
To investigate astrocytic Ca2+-induced D-serine release, we patched passive astrocytes (Fig. 1A-C) in stratum radiatum of the CA1 area and monitored Shaffer collateral (SC)-evoked field NMDAR-mediated EPSPs (NMDAR fEPSPs) in their immediate vicinity (Fig. 2A). We isolated NMDAR EPSPs pharmacologically by relieving the Mg2+-blockade of NMDARs with the superfusate containing 0 mM MgCl2, enhancing Ca2+ driven force with 4 mM CaCl2, and blocking AMPA receptors with the AMPA receptor antagonist, 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX, 10 μM). The area under the curve of NMDAR fEPSPs (Fig. 1D) was measured before and after patching astrocytes in the whole-cell configuration. We added 0.5 mM EGTA/0.1 mM CaCl2, 0.5 mM EGTA/0.15 mM CaCl2, 0.5 mM EGTA/0.2 mM CaCl2 or 0.5 mM EGTA/0.245 mM CaCl2 to the patch pipette solution for astrocytes, which clamped astrocytic [Ca2+] at 40 nM, 70nM, 100 nM or 150 nM (free [Ca2+] calculated using the website Ca-EGTA calculator, Maxchelator, pH = 7.2; Ionic = 1.4 N; Temperature = 24°C). Patching astrocytes with the pipette solution containing 100 nM [Ca2+] or 150 nM [Ca2+] significantly increased the area under the curve of NMDAR fEPSPs (Fig. 2B and E, 100 nM and 150 nM), compared to before patching (Fig. 2E, 100 nM, Open bar, P < 0.01, paired t-test, n = 8). Patching astrocytes with the control no EGTA pipette solution or the pipette solution containing 40 nM free [Ca2+] did not significantly alter NMDAR fEPSPs (Fig. 2B and E, No EGTA and 40 nM). Clamping astrocytic [Ca2+] at 70 nM decreased the area under the curve of NMDAR fEPSPs (Fig. 2D and E, 70 nM), in consistent with the previous study reported by Henneberger et al. (Henneberger et al., 2010). To test whether astrocytic D-serine release is necessary for the enhancement of NMDAR fEPSPs, we added the D-Amino Acid Oxidase (DAAO, 50 μg/ml) and the serine racemase inhibitor, L-erythro-3-hydroxyaspartate (HoAsp, 400 μM) to the 100 nM [Ca2+] astrocyte patch solution. In the presence of DAAO and HoAsp, NMDAR fEPSPs (Fig. 2C and E, DAAO/HoAsp) were significantly smaller compared to 100 nM [Ca2+] alone (Fig. 2B and E, 100 nM/Purple, P < 0.001, Student's unpaired t-test, n = 8 for both groups), which suggests that the enhancement of NMDAR activation results from astrocytic D-serine release. To confirm this hypothesis, we applied glycine (0.1 mM) in the superfusate to saturate NMDAR glycine-binding sites before patching astrocytes. Glycine itself did not significantly increase the area under the curve of NMDAR fEPSPs (Con: 14.0±2.1, Gly: 14.7±2.6, P = 0.38, paired t-test, n = 5). However, after saturating NMDAR glycine-binding sites, infusion of astrocytes with 100 nM [Ca2+] no longer increased NMDAR fEPSPs (Fig. 2D and E, Gly/Moon green, P = 0.86 compared to baseline before patching, paired t-test, n = 8), supporting that the enhancement of NMDAR activation did result from astrocytic release of D-serine.
Fig. 2. High astrocytic [Ca2+] enhances synaptic activation of NMDARs.
(A) Experimental arrangement: NMDAR fEPSP (fEPSP) was recorded from the immediate vicinity of a patched passive astrocyte (Patch). (B) Upper traces, representative NMDAR fEPSPs before (Black traces) and after (Colored traces) patching astrocytes with the 40 or 100 nM [Ca2+] patch pipette solution. Bottom panel, the time course of the relative area under the curve of NMDAR fEPSPs for control (Green) or 100 nM [Ca2+] (Purple) pipette solution. The dashed lines indicate the start time of the cell-attached (Seal) and whole-cell configurations (Break). (C) Patching astrocytes with the 70 nM [Ca2+] patch pipette solution (Blue) or the 100 nM [Ca2+] patch pipette solution containing DAAO (50 μg/ml) and HoAsp (400 μM) (Cyan) decreased NMDAR fEPSPs. (D) Saturation of NMDAR glycine sites with bath application of glycine (Gly, 0.1 mM, Moon green) or intracellular application of vesicular fusion toxin LC-TT (0.3 μM, Orange) blocked the astrocytic [Ca2+]-induced increase. (E) Statistic data for the mean area under the curve of NMDAR fEPSPs before (Open bars) and after patching astrocytes in the cell-attached (Grey bars) and whole-cell (Colored bars) configuration. Data are presented as average SEM. *, **, and *** denote P < 0.05, 0.01 and 0.001, respectively, paired t-test (Red) or Student's unpaired t-test (Black). n.s., no statistical significance.
To test whether D-serine release from astrocytes involves the activity of soluble N-ethylmaleimide factor-attached protein receptors (SNAREs), we added light-chain tetanus toxin (LC-TT, 0.3 μM), which enzymatically cleaves the SNARE protein synaptobrevin, to the 100 nM [Ca2+] pipette solution for astrocytes. In the presence of LC-TT, the area under the curve of NMDAR fEPSPs (Fig. 2D and E, LC-TT/Orange) was significantly smaller than 100 nM [Ca2+] alone (P < 0.01, Student's unpaired t-test, n = 8 for each group). These data suggest that SNAREs-dependent fusion is involved in astrocytic D-serine release that enhances NMDAR activation.
High astrocytic [Ca2+] induces large D-serine-containing vesicles
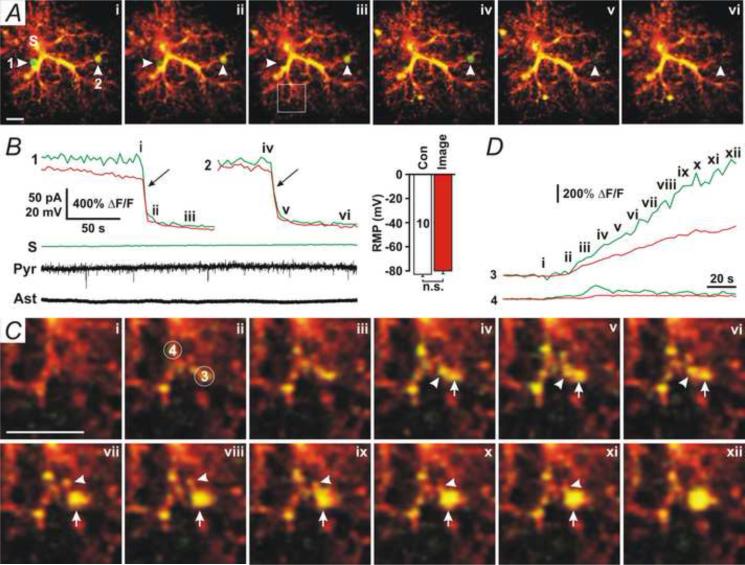
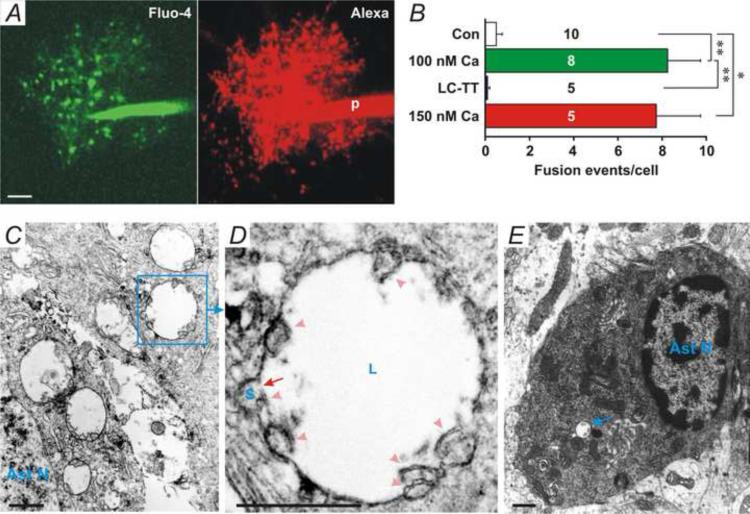
To further investigate the mechanism underlying astrocytic D-serine release, we used two-photon microscopy to image astrocytes that were patched with the 100 nM [Ca2+] pipette solution added with the fluorescent dye, Alexa Fluor-594 (100 μM), and membrane impermeable Ca2+ dye, Fluo-4 potassium (100 μM). When the whole-cell configuration was formed in astrocytes, Alexa Fluor-594- and Fluo-4-positive large vesicles appeared in the processes of patched astrocytes and disappeared suddenly, indicating that vesicular fusion had occurred (Fig. 3A, arrowheads, and Movie 1). Fusion of large vesicles was not associated with slow inward currents (SICs) (Angulo et al., 2004, Fellin et al., 2004) in nearby pyramidal neurons (Fig. 3B, Pyr), suggesting that these large vesicles contain [glutamate] too low to evoke SICs. Fluo-4 fluorescence in the soma (Fig. 3A and B, S) and the resting membrane potential (RMP) of patched astrocytes (Fig. 3B, Ast and Right insert) did not significantly alter during two-photon imaging, suggesting that large vesicles were not due to laser light-induced photo-damage of cells. The Alexa Fluor-594 and Fluo-4 fluorescence intensity in large vesicles (Fig. 3A and C, 1-3) was significantly higher than in nearby processes, suggesting that the large vesicle is a compartment isolated from the cytoplasm. Sustained increases in Fluo-4 fluorescence in the local processes of astrocytes were first observed before formation of large vesicles (Fig. 3C, ii–iii), and then large vesicles were generated from enlargement of Alexa Fluor-594- and Fluo-4-positive compartments (Fig. 3C, ii-xii, and Movie 2), implying that Ca2+ increases are involved in the generation of large vesicles. Nearby small Alexa Fluor-594- and Fluo-4-positive compartments (Fig. 3C, iv-xii, arrowheads) merged into large vesicles during their enlargement (Fig. 3C, iv-xii, arrows), suggesting that the enlargement is probably due to intracellular fusion of small compartments. The mean diameter of large vesicles before fusion measured by Alexa Fluor-594 was 2.33 ± 0.04 μm (mean ± SEM, range: 1-3 μm, n =143), significantly smaller than high [glutamate]-induced large vesicles (3.8 ± 0.1 μm, n = 68, P < 0.001, Student's unpaired t-test) (Xu et al., 2007). Fusion of Alexa Fluor-594/Fluo-4-positive vesicles smaller than 1 μm was not observed, suggesting that large vesicles (>1 μm) are the major type of readily releasable vesicles in astrocytes. When astrocytes were patched with the control pipette solution, formation and fusion of large vesicles were occasionally observed (3 of 10 cells, Movie 3), suggesting that large vesicles can occur at rest. The number of fusion events per cell in the control group (Fig. 4B, Con) was significantly lower than in astrocytes with 100 nM [Ca2+] (Fig. 4B, 100 nM, P < 0.01, Student's unpaired t-test, n = 10 and 8 cells, respectively).
Fig. 3. High astrocytic [Ca2+] induces large vesicles.
(A) Two-photon microscopy images of an astrocyte that was patched with the pipette solution added with 100 nM [Ca2+], Fluo-4 potassium (100 μM), and Alexa Fluor-594 (100 μM). Two large vesicles (1 and 2) were undergoing fusion (i-ii and iv-v). (B) The time course of Fluo-4 (Green) and Alexa Fluor-594 (Red) fluorescence from the two large vesicles (1 and 2) and the soma (S) indicated in A, showing the occurrence of fusion events (arrows). Whole-cell voltage-clamp recording in a nearby pyramidal neuron (Pyr) with apical dendrites passing through the domain of the patched astrocyte showed no fusion-associated SICs. Whole-cell current-clamp recording in the patched astrocyte (Ast) showed no changes in resting membrane potential (RMP). Representative data were chosen from 8 experiments. Right insert, the mean RMP of patched astrocytes during DIC imaging (Con) or fluorescence imaging (Image). (C) The enlarged image in the square area in A-iii. Alexa Fluor-594 and Fluo-4 fluorescence first increased (ii – iii, 3 and 4) and then a large vesicle (3) formed. Arrows indicate the large vesicle, and arrowheads indicate that two small Alexa Fluor 594- and Fluo4-positive compartments were merging into the large vesicle. (D) The time course for Fluo-4 (Green) and Alexa Fluor-594 (Red) fluorescence from the circles (3 and 4) indicated in C. Scale Bars in A and C, 5 μm.
Fig. 4. Formation and fusion of large vesicles.
(A) X-Y-Z overlaid Fluo-4 (Green) and Alexa Fluor-594 (Red) fluorescence images of an astrocyte patched with the 100 nM [Ca2+] pipette solution added with 0.2 μM LC-TT. Many Fluo-4- and Alexa Fluor-594-positive large vesicle-like structures appeared in the processes. (B) The number of vesicles per cells with different pipette solutions. Fusion events in 60 min were counted. * and ** denotes P < 0.05 and 0.01, respectively, Student's unpaired t-test. The sample number is presented in each bar. (C) An EM image of large vesicles in an astrocyte. Ast N, astrocytic nucleus. (D) Enlarged picture from the indicated area in C, showing intracellular fusion between a large vesicle (L) and a small vesicle (S). There are multiple double bilayer membrane fragments (arrowheads) near the fusion pore, indicating that multiple intracellular fusion events had occurred. (E) An EM image of an astrocyte in a fresh non-treatment slice. Only few large vesicles (arrow) were found in control astrocytes (3 vesicles in 9 astrocytes).
Since vesicular fusion in astrocytes is SNAREs-dependent (Liu et al., 2011) and D-serine release is SNAREs-dependent (Fig. 2D, LC-TT), we further tested whether fusion of large vesicles is blocked by LC-TT. When astrocytes were patched with the 100 nM [Ca2+] pipette solution added with 0.2 μM LC-TT, Alexa Fluor-594- and Fluo-4-positive large vesicle-like structures were accumulated in their processes (Fig. 4A); however, few fusion events occurred (Fig. 4B, LC-TT, and Movie 4). These results further support that fusion of large vesicles depends on SNAREs. The accumulation of large vesicles suggests that 0.2 μM LC-TT blocks fusion but not generation of large vesicles.
To further investigate formation of large vesicles in astrocytes, we used electron microscopy. Slices were post-experimentally fixed, and astrocytes were labeled with the anti-GFAP antibody. Multiple large vesicles with plain content were found in GFAP-labeled astrocytes (Fig. 4C). Enlarged image of a large vesicle from Fig. 4C (Squared area) showed that a small vesicle (Fig. 4D, S) was fusing into the large vesicle (Fig. 4D, L). A double bilayer membrane fragment (Fig. 4D, arrowhead) was found near the fusion pore (Fig. 4D, arrow), indicating that fusion had occurred at a “vertex” ring around the disc of apposed boundary (Wang et al., 2002, Fratti et al., 2004, Jun et al., 2006). In this large vesicle, there were 7 double bilayer membrane fragments (Fig. 4D, arrowheads), indicating 7 small vesicles or other organelles had fused into this large vesicle. These results support that large vesicles are generated from intracellular fusion of small vesicles and/or other organelles such as lysosomes (Alvarez de Toledo and Fernandez, 1990, Hafez et al., 2003). EM images of astrocytes in a fresh non-treatment slice (Fig. 4E) showed that only few large vesicles (3 vesicles in 9 astrocytes) were observed, indicating large vesicles are rare under control conditions.
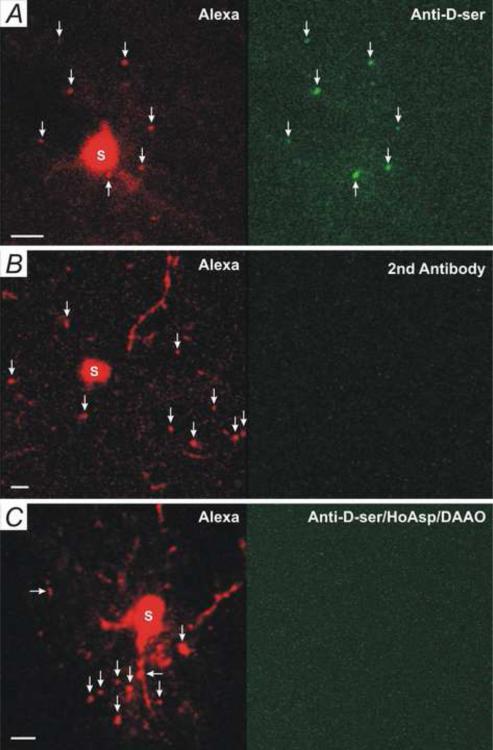
To further demonstrate that these large vesicles contain D-serine, we examined immunofluorescence of an anti-D-serine antibody in astrocytic large vesicles. Large vesicles were induced by patching astrocytes with the 100 nM [Ca2+] pipette solution added with Alexa Fluor-594, by which patched astrocytes (Fig. 5A, Red) and large vesicles (Fig. 5A, Red, arrows) could be identified after antibody-staining. In contrast to the low level of anti-D-serine fluorescence in astrocytic soma (Fig. 5A, S) and processes, a high level of anti-D-serine fluorescence was found in large vesicles (Fig. 5A, Green, arrows). As a control, slices were treated with the second antibody only, no high fluorescence intensity (Fig. 5B, Green, 2nd Antibody) was found in Alexa Fluor-594-labeled large vesicles (Fig. 5B, Red, arrows). When adding HoAsp (400 μM) and DAAO (50 μg/ml) to the 100 nM [Ca2+] patch pipette solution for astrocytes, a low level of anti-D-serine fluorescence in large vesicles was observed (Fig. 5C, arrows). These results suggest that astrocytic large vesicles contain high levels of D-serine.
Fig. 5. Large vesicle contains D-serine.
(A) Anti-D-serine antibody immunofluorescence (Green) of an astrocyte that had been patched with the 100 nM [Ca2+] Alexa Fluor-594 pipette solution. Alexa Fluor-594-stained large vesicles (Red, arrows) are Anti-D-serine-positive (Green, arrows). Data are representative chosen from 8 experiments. (B) A control slice treated with the second antibody only (2nd Antibody). Data are chosen from three experiments. (C) An astrocyte were patched with the 100 nM [Ca2+] Alexa Fluor-594 pipette solution added with HoAsp (400 μM) and DAAO (50 μg.ml). No high levels of anti-D-serine (Green) were found in large vesicles (Red, arrows). Data are representative chosen from 5 experiments. Scale bars in A, B, and C: 5 μm.
Mechanical stimulation induces D-serine release from astrocytic large vesicles
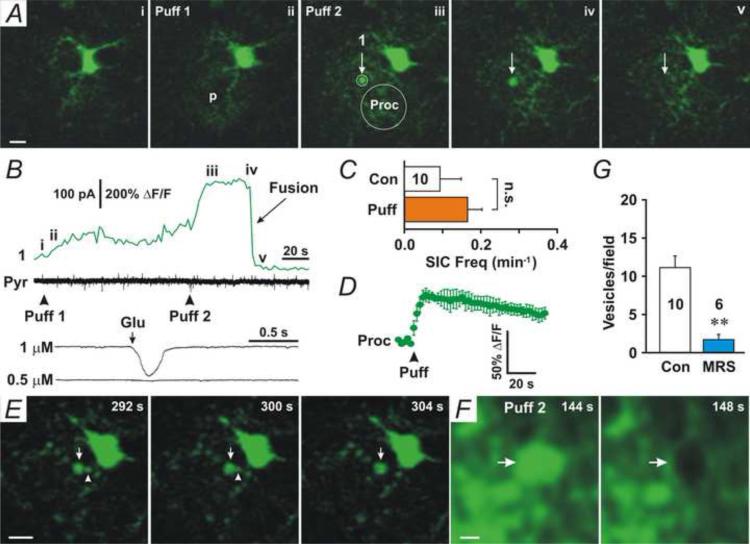
Mechanical stimulation has been used for stimulating astrocytic Ca2+ signaling and glutamate release (Charles et al., 1991, Angulo et al., 2004, Liu et al., 2011). To confirm that large vesicles are not due to patching of astrocytes, we used a weak mechanical stimulus produced by puffing ACSF into the extracellular space. We repetitively puffed ACSF (six puffs, intervals of 60 s) into CA1 stratum radiatum 50–100 μm beneath the slice surface via a puffer pipette (R = 7–10 MΩ). During puffs, the frequency of spontaneous EPSCs (sEPSCs) or the resting membrane potential (RMP) of pyramidal neurons were not altered (Fig. 7A, Bottom panel), suggesting no direct effects of puffs on neuronal activity. Since large vesicles are Fluo-4-positive (Fig. 3A), we extracellularly loaded astrocytes with Fluo-4 by pre-incubating slices with Fluo-4 AM (5 μM) and monitored Fluo-4-positive large vesicles during puffs. Puffing ACSF induced many Fluo-4-positive large vesicles in astrocytes (Movie 5). Fluo-4-positive round structures started to appear after 1–2 puffs (Fig. 6A, V, and Movie 5) and disappeared suddenly (Fig. 6A and B, iv-v and Fusion). Puffing ACSF into the surface layer of slices (<30 μm from the surface) could not, however, induce such Fluo-4-positive large vesicles. Rapid disappearance of vesicular Fluo-4 fluorescence represented diffusion of Ca2+ and Fluo-4 into the extracellular space via the fusion pore. Fusion of the first Fluo-4-positive large vesicle occurred at 48–156 s (mean: 101.6±14.0 s, n = 10 slices) after the first puff. The mean diameter of puffing ACSF-induced large vesicles was 2.5 ± 0.1 μm (range: 1–4 μm, n = 80), similar to the large vesicles induced by patching astrocytes with 100-150 nM Ca2+. When fusion of large vesicles occurred, no associated SICs were observed in whole-cell recording from pyramidal neurons whose apical dendrites passed through the puffed area (Fig. 6B, Black traces, and 6C, Puff), confirming the low [glutamate] level in these large vesicles. Puffing 1 μM glutamate to dendrites of pyramidal neurons induced inward currents (Fig. 6B, Glu/1 μM) similar to SICs, but puffing 0.5 μM glutamate (Fig. 6B, Glu/0.5 μM) did not, indicating that [glutamate] in large vesicles is probably lower than 1 μM. Puffing ACSF induced a long-lasting increase in Fluo-4 fluorescence in local astrocytic processes surrounding the puffer pipette (Fig. 6A and D, Proc), implying that sustained astrocytic Ca2+ increases may be related to the generation of large vesicles. Large vesicles were developed from smaller Fluo-4-positive compartments (Fig. 6E, arrowheads, and Movie 6) and fusion events occurred as their size enlarged to more than 1 μm. To confirm that the round Fluo-4-positive structure is a large vesicle, we monitored the morphology of large vesicles after Fluo-4 fluorescence disappeared. The FM lipophilic styryl dye, FM 1-43 (20 μM), was added to the ACSF puffer pipette solution and puffed into Fluo-4-loaded slices. Immediately following disappearance of vesicular Fluo-4 fluorescence (Fig. 6F, 144 s, arrow), FM 1-43-negative large vesicles with collapsed volume were observed (Fig. 6F, 148 s, arrow, and Movie 7), confirming that the disappearance of fluorescence was due to diffusion of Ca2+ and dye into the extracellular space through the fusion pore. The results also suggest that diffusion of vesicular Ca2+ is faster than vesicular collapse.
Fig. 7. Weak mechanical stimulation enhances activation of NMDARs.
(A) Experimental arrangement: fEPSP, extracellular field recording pipette; Puff, puffer pipette; SC, Schaffer collateral; Stim, stimulation electrode. Grey circle indicates puffed area. (B) Puffing ACSF induced an enhancement of NMDAR fEPSPs, which was blocked by the NMDAR glycine site antagonist DCKA (750 nM). Upper traces, sample traces (average of 30 traces) before (Black lines) and after puffs from control (Purple line) or DCKA group (Orange line). Bottom panel, relative mean area under the curve of NMDAR fEPSPs was plotted against time. (C) In the presence of glycine (0.1 mM), the puffing ACSF-induced increase in NMDAR fEPSPs was inhibited. (D) Bath application of D-serine (D-ser, 10 μM) increased NMDAR fEPSPs similarly as puffing ACSF did, but glutamate (Glu, 10 μM) did not. (E) The P2Y1 receptor antagonist MRS2179 (30 μM) inhibited the increase in NMDAR fEPSPs. (F) Summary for NMDAR fEPSP amplitude in B-E. Data are presented as mean±SEM. Red ** and *** denote significance of P < 0.01 and 0.001, respectively, compared to baseline (paired t-test). The sample number is present in each bar.
Fig. 6. Weak mechanical stimulation induces large vesicles in astrocytes.
(A) Two-photon microscopy images of a representative astrocyte that was preloaded with Fluo-4 AM at the time indicated in B. The position of the puffer pipette is indicated (p). The first puff induced an increase in Fluo-4 fluorescence in the astrocytic processes (i–ii). A high Fluo-4 fluorescence vesicle (1) appeared after the second puff (iii) and suddenly disappeared (iv–v). (B) Fluo-4 fluorescence in the circled area (1) indicated in A. Middle trace (Pyr), whole-cell voltage-clamp recording from a nearby pyramidal neuron with apical dendrites passing through the puffed area. Bottom traces, whole-cell recording from a representative pyramidal neuron, showing that local application (Glu and arrow) of 1 μM glutamate induced inward currents (1 μM), but 0.5 μM glutamate did not (0.5 μM). (C) The frequency of SICs during the control period (Con) and after puffs (Puff). (D) The mean relative Fluo-4 fluorescence intensity from puffed processes (Proc) before and after puffing (Puff). n = 10 slices. (E) A large vesicle was developed from small vesicles. A small vesicle (arrowhead) was being merged into a large vesicle (arrow). Images were from the same astrocyte in A. (F) Fluo-4 and FM 1-43 fluorescence showed a large vesicle with Fluo-4 fluorescence (144 s, arrow). After its Fluo-4 fluorescence disappeared (fusion occurred), the large vesicle was FM 1-43-negative with a reduced size (148 s, arrow). FM 1-43 (20 μM) was added to the puffer pipette solution (ACSF). (G) The bath application of the P2Y1 receptor antagonist MRS2179 (MRS) inhibited puffing ACSF-induced generation of large vesicles.
To test whether purinergic receptors are involved in weak mechanical stimulation-induced large vesicles, we applied the P2Y1 receptor antagonist MRS2179 (MRS, 30 μM) in the superfusate. In the presence of MRS2179, puffing ACSF-induced generation of large vesicles was inhibited (Fig. 6G, MRS). The results suggest that P2Y1 receptors are involved in weak mechanical stimulation-induced generation of large vesicles.
To test whether fusion of large vesicles leads to D-serine release, we recorded NMDAR fEPSPs before, during and after puffs. The recording pipette for NMDAR fEPSPs (Fig. 7A, fEPSP) was placed in the puffed area (Fig. 7A, Grey circle). After 6 puffs, the area under the curve of NMDAR fEPSPs slowly increased and reached a plateau after 15 min (Fig. 7B, Green). To test whether the increase in NMDAR EPSPs is due to D-serine release, we first used the NMDAR glycine site antagonist, 5,7-dichlorokynurenic acid (DCKA, 750 nM). DCKA itself only partially blocked NMDAR EPSPs (Fig. 7F, DCKA). However, in the presence of DCKA, puffing ACSF no longer induced increases in NMDAR EPSPs (Fig. 7B and F, Orange). Then we used glycine (0.1 mM) to saturate the glycine binding site of NMDARs. Glycine blocked puffing ACSF-induced enhancement of NMDAR EPSPs (Fig. 7C and F, Moon Green). These results suggest that weak mechanical stimulation induces large D-serine-containing vesicles in astrocytes, which enhance NMDAR activation. To further test the involvement of D-serine and glutamate in NMDAR activation, we perfused slices with D-serine (10 μM) or glutamate (10 μM). Similar to puffs, D-serine significantly increased the amplitude of NMDAR EPSPs (Fig. 7D and F, D-ser), while glutamate (10 μM, Fig. 7D and F, Glu) or ATP (10 μM, Fig. 7F, ATP) did not, suggesting that D-serine rather than glutamate and ATP is involved in the enhancement of NMDAR activation. Last, we used the P2Y1 receptor antagonist, MRS2179 that blocked puffing ACSF-induced generation of large vesicles (Fig. 6G), to confirm that fusion of large vesicles releases D-serine. In the presence of MRS2179, puffing ACSF did not induce significant changes in NMDAR fEPSPs (Fig. 7E and F, MRS), further supporting that astrocytes release D-serine by large vesicles.
Functions of astrocytic D-serine release
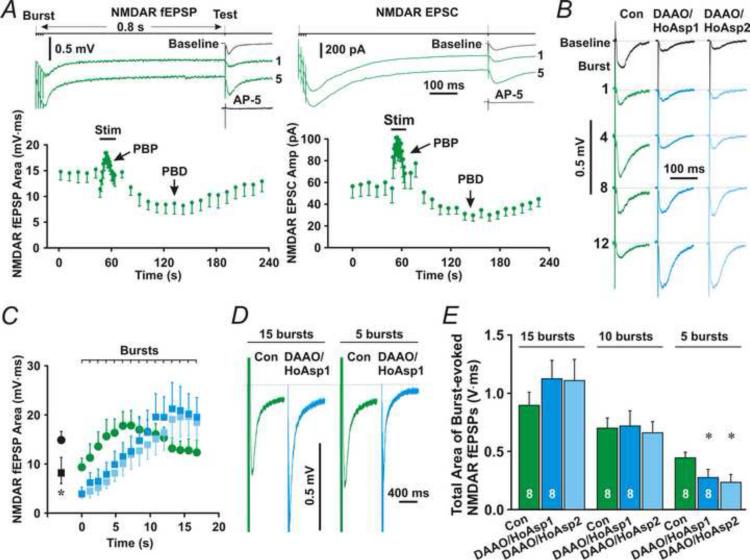
To investigate functions of astrocytic D-serine release in long-term synaptic plasticity, we first examine effects of astrocytic D-serine release on theta burst-induced activation of NMDARs. We monitored NMDAR activation during application of a theta-burst stimulation (TBS) that consists of 15 bursts (each burst contained four, 100 Hz stimuli). In order to be able to measure NMDAR fEPSPs between two bursts, an inter-burst interval of 1.2 s was used. A test stimulus was delivered 0.8 s after each burst (Fig. 8A, top line, Test) to evoke NMDAR fEPSPs. After several bursts, a post-burst potentiation (PBP or PTP) of NMDAR fEPSPs (Fig. 8A, left upper traces, 1 and 5; left bottom panel, PBP) appeared. Following PBP, a post-burst depression (PBD) was observed (Fig. 8A, left panel, PBD) (Andersson and Hanse, , Xie et al., 1996). Whole-cell recording of evoked NMDAR-mediated EPSCs (NMDAR EPSC) from pyramidal neurons exhibited similar PBP and PBD (Fig. 8A, right panel). Consistent with previous reports (Xie et al., 1996), PBP, with a decay constant of 14.9 ± 1.2 s (mean ± SEM), did not persist longer than 1 min after the burst stimulation ended. PBD lasted longer than PBP and was recovered to the baseline with a half recovery time of 240.9 ± 9.4 s (mean ± SEM).
Fig. 8. Effects of astrocytic D-serine on TBS-induced NMDAR activation.
(A) Left upper traces are NMDAR fEPSPs evoked by the first (1) or fifth (5) burst (Burst) followed by test stimulus-induced NMDAR fEPSPs (Test). A nearby astrocyte was patched with the control pipette solution. Left bottom panel, the time course of test stimulus-induced NMDAR fEPSPs before, during, and after the burst stimulation (Stim), showing post-burst potentiation (PBP) and post-burst depression (PBD). Right upper traces, whole-cell recording from a representative pyramidal neuron showing NMDAR EPSCs evoked by the first or fifth burst followed by test stimulation. Right bottom panel, the time course of NMDAR EPSCs. (B) Representative field potential recording traces showing test stimulus-evoked NMDAR fEPSPs following the first (1), fourth (4), eighth (8), or twelfth burst (12) in the absence (Con) or presence of DAAO (50 μg/ml) and HoAsp (400 μM) in astrocytes (DAAO/HoAsp). DAAO/HoAsp1 and DAAO/HoAsp2 denote NMDAR fEPSPs during the first and second trains of the burst stimulation, respectively. The black traces (Baseline) are baseline NMDAR or AMPAR fEPSPs before the burst stimulation. (C) The mean area under the curve of NMDAR fEPSPs evoked by test stimuli after each burst (Burst). (D) Averaged burst-evoked NMDAR fEPSPs for total 15 (15 bursts) or first 5 (5 bursts) bursts from a representative experiment. (E) The mean total area under the curve of burst-evoked NMDAR fEPSPs for total 15 (15 bursts), first 10 (10 bursts), or first 5 (5 bursts) bursts. * denotes P < 0.05 compared to control, Student's unpaired t-test. The sample size is given in left two bars.
To evaluate the role of astrocytic D-serine release in LTP under physiological conditions, we added DAAO and HoAsp to the control astrocyte patch solution. The role of astrocytic D-serine release on synaptic activation of NMDARs was examined. Astrocytes were patched for 20 min before application of TBS to clear out D-serine in stored vesicles. In the presence of DAAO and HoAsp in astrocytes, baseline NMDAR fEPSPs (Fig. 8B and C, DAAO/HoAsp1, Baseline) were significantly smaller than the control group (Fig. 8B and C, Con, Baseline, P < 0.05, Student's unpaired t-test, n = 8 for each group). In agreement with the spontaneous fusion of large vesicles at rest, these results suggest that D-serine is spontaneously released from astrocytes at rest and contributes to baseline activation of NMDARs. Additionally, compared with control NMDAR fEPSPs (Fig. 8B and C, Green), DAAO and HoAsp in astrocytes delayed the PBP peak of NMDAR fEPSPs (Fig. 8B and C, Cyan). However, DAAO and HoAsp did not alter the PBP peak amplitude (Fig. 8B and C, Cyan vs. Green).
To test whether there is existing stored D-serine that is not blocked by DAAO and HoAsp during the first stimulation protocol (Henneberger et al., 2010), we applied the burst stimulation twice with an interval of 5 min. The slope and peak of NMDAR PBPs induced by the first and second trains of the burst stimulation were similar (Fig. 8B and C, Cyan and Ice blue). The total area under the curve of NMDAR fEPSPs induced by the first train of the burst stimulation (Fig. 8E, 15 bursts, Cyan) was not significantly different from that induced by the second train (Fig. 8E, 15 bursts, Ice blue, P = 0.78, paired t-test, n = 8). These results are consistent with DAAO and HoAsp eliminating release of D-serine from almost all vesicles. Less existing stored D-serine was probably due to spontaneous and continuing fusion of large vesicles.
Because astrocytic D-serine release shifts the PBP peak to the left without changing the peak amplitude, it may only influence the activation of NMDARs induced by a few of bursts. Indeed, the total area under the curve of NMDAR fEPSPs evoked by the total 15 bursts or the first 10 bursts in the DAAO/HoAsp1 or DAAO/HoAsp2 group (Fig. 8D and E, 15 and 10 bursts, Cyan and Ice blue) was not significantly different from controls (Fig. 8D and E, 15 and 10 bursts, Green, P = 0.2, 0.15, 0.91, and 0.76, respectively, Student's unpaired t-test, n = 8 for each group), which suggests that astrocytic D-serine release plays a minor role in the total activation of NMDARs stimulated by 10 or 15 bursts. However, the total area under the curve of NMDAR fEPSPs evoked by the first five bursts in either the DAAO/HoAsp1 or DAAO/HoAsp2 group (Fig. 8D and E, 5 bursts, Cyan and Ice blue) was significantly smaller than controls (Fig. 8D and E, 5 bursts, Green, P < 0.05 for both DAAO/HoAsp1 and DAAO/HoAsp2, Student's unpaired t-test, n = 8 for each group). These results suggest that astrocytic D-serine release only contributes significantly to NMDAR activation evoked by 5 or fewer bursts.
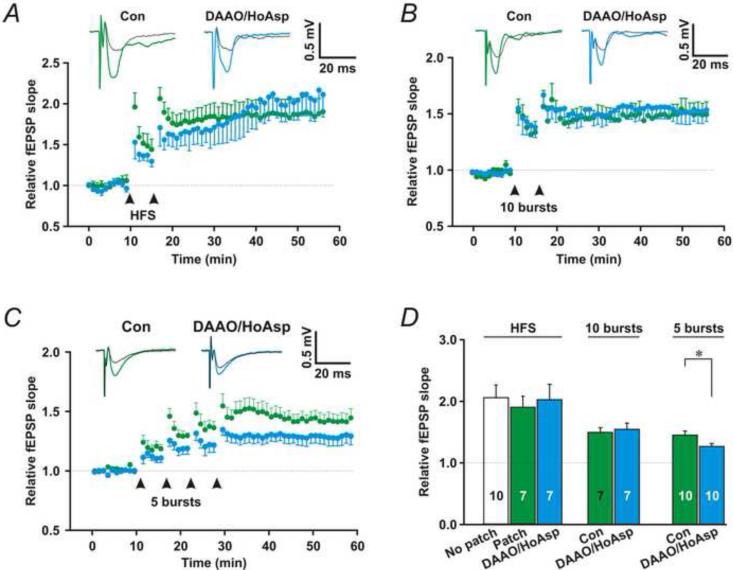
Next, we tested the role of astrocytic D-serine release in promoting induction of LTP. We patched astrocytes and monitored Shaffer collateral (SC)-evoked field excitatory postsynaptic potentials (fEPSPs) in their immediate vicinity (Fig. 2A). To compare LTP induced by strong and weak stimulation, we used high-frequency SC stimulation (HFS, 100 Hz for 1 s, strong stimulation), theta-burst stimulation (TBS) consisting of 10 bursts (strong stimulation), and TBS consisting of 5 bursts (weak stimulation). Patching nearby astrocytes with the control pipette solution did not influence the induction of LTP (Fig. 9D, No patch vs. patch, P = 0.63, Student's unpaired t-test, n = 10 and 7, respectively). Patching nearby astrocytes with the control pipette solution added with DAAO and HoAsp did not significantly reduce strong stimulation (HFS and 10 burst TBS)-induced LTP (Fig. 9A, B and D, HFS/ DAAO/HoAsp and 10 burst/DAAO/HoAsp, P = 0.79 and 0.64, respectively, compared with control, Student's unpaired t-test, n = 7 for each group). However, DAAO and HoAsp in astrocytes did partially block LTP induced by the weak stimulation (five burst TBS) repeated four times at 5 min intervals (Fig. 9C and D, 5 bursts/DAAO/HoAsp, P < 0.05 compared with control, Student's unpaired t-test, n = 10 for each group). These results suggest that, under physiological conditions, astrocytic D-serine release is most important in favoring LTP elicited by synaptic activity right at the threshold for inducing LTP.
Fig. 9. Astrocytic D-serine release facilitates LTP induction.
(A)-(C) Upper traces, representative fEPSPs before (Black traces) and after LTP induction (Colored traces) in the absence (Green) or presence of DAAO and HoAsp (Cyan) in nearby astrocytes. Bottom panels, the relative slopes of fEPSPs. LTP was induced by HFS delivered twice (A, arrows), TBS consisting of 10 bursts delivered twice (B, arrows), or TBS consisting of 5 bursts delivered four times (C, arrows). (D) Summary for LTP induction in A-C. Red * and ** denote significance of P < 0.05 and 0.01, respectively, compared to baseline (paired t-test); Black *, **, and *** indicate P < 0.05, 0.01, and 0.001, respectively, compared to the control group or 100 nM [Ca2+] alone. The sample number is present in each bar.
DISCUSSION
Our results suggest that exocytotic fusion of large vesicles is responsible for astrocytic D-serine release. Multiple observations support this hypothesis. The first, clamping astrocytic [Ca2+] at 100 or 150 nM induced both generation/fusion of large vesicles and astrocytic D-serine release. The second, tetanus toxin blocked both fusion of large vesicles and astrocytic D-serine release. The third, astrocytic large vesicles contained high levels of D-serine. The fourth, weak mechanical stimulation induced both generation/fusion of large vesicles and astrocytic D-serine release. The last, the P2Y1 receptor antagonist, MRS2179, inhibited both puffing ACSF-induced generation of large vesicles and activation of NMDARs. The enhancement of NMDAR fEPSPs by fusion of large vesicles was not due to astrocytic release of other gliotransmitters, such as glutamate or ATP, because application of glutamate (Fig. 7F, Con vs. Glu) or ATP (Fig. 7F, Con vs. ATP) did not induce significant changes in NMDAR fEPSPs. The Fluo-4-and Alexa Fluor-594-positive round structures are not membrane swellings or blubbing because the Fluo-4 and Alexa Fluor-594 fluorescence intensity in large vesicles (Fig. 3B and D, 3) is much higher than in nearby astrocyte processes (Fig. 3B and D, 4), suggesting that large vesicles are the compartment isolated from the intracellular space of processes. Higher levels of D-serine in large vesicles than in soma and processes and the ultrastructure of large vesicles confirm this hypothesis. Large vesicles could not be due to laser-induced photo-damage of cells because: 1) RMP of patched astrocytes was similar to RMP of control astrocytes (Fig. 3B, Insert); 2) somatic [Ca2+] did not remarkably altered (Fig. 3B, S); 3) puffing-induced large vesicles only appeared in and near the puffed area (Movie 5); 4) there were very few large vesicles in control slices and no increases in number of large vesicles as continuous exposure to laser; 5) puffing-induced large vesicles could be blocked by the P2Y1 receptor antagonist (Fig. 6G).
Astrocytic large vesicles are different from synaptic-like microvesicles previously reported by other groups (Zhang et al., 2004, Montana et al., 2006, Jourdain et al., 2007). The difference is that: 1) the size of large vesicles is 1-3 μm, whereas the size of synaptic-like microvesicles is < 0.1 μm; 2) large vesicles contain high D-serine but low [glutamate]v under physiological conditions, while synaptic-like microvesicles contain high [glutamate]. Astrocytic large vesicles with high D-serine but low [glutamate] could have advantages for modulating synaptic plasticity. Astrocytes by fusing these large vesicles do not induce frank depolarizing currents (SICs) in postsynaptic neurons, but do synchronously modulate synapses in their domain by binding to the NMDAR glycine site. Under some pathological conditions where extracellular [glutamate] is elevated (>5 mM), astrocytic large vesicles may contain both glutamate and D-serine, which are co-released and evoke NMDAR-mediated depolarizing currents in surrounding pyramidal neurons (Angulo et al., 2004, Fellin et al., 2004).
Fusion events of large vesicles that were smaller than 1 μm were rare, which suggests that large vesicles rather than small vesicles make up the majority of vesicles in the readily releasable pool in astrocytes. Mergence of small compartments into large vesicles during their formation suggests that large vesicles are developed from small compartments (Williams et al., 2006). The results from EM experiments (Fig. 4C and D) confirmed this hypothesis. These large vesicles may be generated by intracellular fusion of similar small vesicles and/or other organelles such as lysosomes (Alvarez de Toledo and Fernandez, 1990, Hafez et al., 2003). The blockade of fusion by LC-TT indicates that fusion of large vesicles is driven by SNAREs, which is characteristic of vesicular fusion in astrocytes (Liu et al., 2011). One advantage for astrocytes to use such large vesicles rather than small vesicles to release D-serine is that the greater volume allows fusion of a single vesicle to influence a larger number of synapses, sufficient to produce significant effects on synaptic activation of NMDARs. Thus, an astrocyte can modify all the synapses in its domain synchronously.
Functions of astrocytic release of gliotransmitters including D-serine are now hotly debated. In this study, we found that under physiological conditions, a limited amount of D-serine is released from astrocytic large vesicles and modulates baseline activation of NMDARs. Astrocytic D-serine only affects activation of NMDARs by the first few bursts and facilitates the induction threshold of LTP. The peak of NMDAR activation induced by late bursts may depend on either D-serine release from other sources such as neurons (Rosenberg et al., 2010) or high-frequency stimulation-induced glutamate release, which alone activates NMDARs because the NMDAR glycine site blocker DCKA only partially blocked activation of NMDAR fEPSPs (Fig. 7B and F, DCKA). The role of astrocytic D-serine release in LTP induction from this study is inconsistent with the previous study by Rusakov's group (Henneberger et al., 2010). Their conclusion is based on the hypothesis that D-serine is stored in vesicles and can't be cleared by metabolic blockers. However, our results showed that fusion of large vesicles spontaneously and continuously occurred and therefore patching astrocytes with DAAO and HoAsp for 20 min could block almost all D-serine in patched astrocytes (Fig. 8B and C, DAAO/HoAsp1 vs. DAAO/HoAsp2). Because sustained high astrocytic [Ca2+] and weak mechanical stimulation of astrocytes may only occur in pathological conditions, such as epileptic seizures, hypoxic ischemia, concussion, and traumatic brain injury, high astrocytic [Ca2+]- or weak mechanical stimulation-induced large vesicles may be involved in such pathological functions.
Supplementary Material
Highlights.
High astrocytic [Ca2+] or weak mechanical stimulation induces astrocytic Dserine release.
Astrocytes release D-serine by fusion of a large vesicle.
Formation of large vesicles involves intracellular fusion of small vesicles.
Astrocytic D-serine release facilitates threshold stimulation-induced LTP.
ACKNOWLEDGMENTS
We thank Dr. Maiken Nedergaard for providing advice. This work was supported by grant C020925 from the NY State Spinal Cord Injury Research Board, grant W81XWH-07-2-0126 from Citizens United for Research in Epilepsy, US Army Medical Research and Material Command, and NIH grant ES006189.
Comprehensive list of abbreviations
- ACSF
artificial cerebrospinal fluid
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- AP5
D-(-)-2-Amino-5-phosphonopentanoic acid
- DAAO
D-Amino Acid Oxidase
- DCKA
5,7-dichlorokynurenic acid
- fEPSP
field recording of excitatory post-synaptic potential
- HFS
High-frequency stimulation
- HoAsp
L-erythro-3-hydroxyaspartate
- LC-TT
light-chain tetanus toxin
- LTP
long-term potentiation
- MRS2179
2'-Deoxy-N6-methyladenosine 3',5'-bisphosphate tetrasodium salt
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione
- NMDAR
N-methyl-D-aspartate receptor
- NMDAR fEPSP
NMDAR-mediated fEPSP
- NMDAR EPSC
NMDAR-mediated excitatory post-synaptic current
- PBP
post-burst potentiation
- PBD
post-burst depression
- SIC
slow inward current
- TBS
theta-burst stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agulhon C, Fiacco TA, McCarthy KD. (Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–1254. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. (What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de Toledo G, Fernandez JM. (Compound versus multigranular exocytosis in peritoneal mast cells. J Gen Physiol. 1990;95:397–409. doi: 10.1085/jgp.95.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Hanse E. (Astrocytes impose postburst depression of release probability at hippocampal glutamate synapses. J Neurosci. 30:5776–5780. doi: 10.1523/JNEUROSCI.3957-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. (Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG. (Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- Balan L, Foltyn VN, Zehl M, Dumin E, Dikopoltsev E, Knoh D, Ohno Y, Kihara A, Jensen ON, Radzishevsky IS, Wolosker H. (Feedback inactivation of D-serine synthesis by NMDA receptor-elicited translocation of serine racemase to the membrane. Proc Natl Acad Sci U S A. 2009;106:7589–7594. doi: 10.1073/pnas.0809442106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. (A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. (Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Chvatal A, Pastor A, Mauch M, Sykova E, Kettenmann H. (Distinct populations of identified glial cells in the developing rat spinal cord slice: ion channel properties and cell morphology. Eur J Neurosci. 1995;7:129–142. doi: 10.1111/j.1460-9568.1995.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Deng Q, Terunuma M, Fellin T, Moss SJ, Haydon PG. (Astrocytic activation of A1 receptors regulates the surface expression of NMDA receptors through a Src kinase dependent pathway. Glia. 59:1084–1093. doi: 10.1002/glia.21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. (Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;14:1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. (Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD. (Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54:611–626. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Fratti RA, Jun Y, Merz AJ, Margolis N, Wickner W. (Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J Cell Biol. 2004;167:1087–1098. doi: 10.1083/jcb.200409068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez I, Stolpe A, Lindau M. (Compound exocytosis and cumulative fusion in eosinophils. J Biol Chem. 2003;278:44921–44928. doi: 10.1074/jbc.M306013200. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K. (The presence of free D-serine in rat brain. FEBS Lett. 1992;296:33–36. doi: 10.1016/0014-5793(92)80397-y. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. (Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. (Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Jun Y, Thorngren N, Starai VJ, Fratti RA, Collins K, Wickner W. (Reversible, cooperative reactions of yeast vacuole docking. Embo J. 2006;25:5260–5269. doi: 10.1038/sj.emboj.7601413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. (Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. (Neuron-derived D-serine release provides a novel means to activate N-methyl-D-aspartate receptors. J Biol Chem. 2006;281:14151–14162. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M. (Astrocyte-mediated activation of neuronal kainate receptors. Proc Natl Acad Sci U S A. 2004;101:3172–3177. doi: 10.1073/pnas.0306731101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Sun L, Xiong Y, Shang S, Guo N, Teng S, Wang Y, Liu B, Wang C, Wang L, Zheng L, Zhang CX, Han W, Zhou Z. (Calcium triggers exocytosis from two types of organelles in a single astrocyte. J Neurosci. 2011;31:10593–10601. doi: 10.1523/JNEUROSCI.6401-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. (Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. (Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Min R, Nevian T. (Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci. 2012;15:746–753. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. (Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- Muller D, Nikonenko I, Jourdain P, Alberi S. (LTP, memory and structural plasticity. Curr Mol Med. 2002;2:605–611. doi: 10.2174/1566524023362041. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A. (Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. (Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Panatier A, Vallee J, Haber M, Murai KK, Lacaille JC, Robitaille R. (Astrocytes are endogenous regulators of basal transmission at central synapses. Cell. 2011;146:785–798. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V. (Pacemaker calcium oscillations in thalamic astrocytes in situ. Neuroreport. 2001;12:3897–3900. doi: 10.1097/00001756-200112210-00008. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. (Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Petravicz J, Fiacco TA, McCarthy KD. (Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28:4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D, Kartvelishvily E, Shleper M, Klinker CM, Bowser MT, Wolosker H. (Neuronal release of D-serine: a physiological pathway controlling extracellular D-serine concentration. FASEB J. 2010;24:2951–2961. doi: 10.1096/fj.09-147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. (D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. (Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci. 2008;28:6659–6663. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. (Neuroscience: Settling the great glia debate. Nature. 2010;468:160–162. doi: 10.1038/468160a. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, Jabs R, Kettenmann H. (Properties of GABA and glutamate responses in identified glial cells of the mouse hippocampal slice. Hippocampus. 1994;4:19–35. doi: 10.1002/hipo.450040105. [DOI] [PubMed] [Google Scholar]
- Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF. (D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci U S A. 2003;100:6789–6794. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Seeley ES, Wickner W, Merz AJ. (Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell. 2002;108:357–369. doi: 10.1016/s0092-8674(02)00632-3. [DOI] [PubMed] [Google Scholar]
- Wenker I. (An active role for astrocytes in synaptic plasticity? J Neurophysiol. 2010;104:1216–1218. doi: 10.1152/jn.00429.2010. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. (Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Williams SM, Diaz CM, Macnab LT, Sullivan RK, Pow DV. (Immunocytochemical analysis of D-serine distribution in the mammalian brain reveals novel anatomical compartmentalizations in glia and neurons. Glia. 2006;53:401–411. doi: 10.1002/glia.20300. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Dumin E, Balan L, Foltyn VN. (D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J. 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- Xie X, Barrionuevo G, Berger TW. (Differential expression of short-term potentiation by AMPA and NMDA receptors in dentate gyrus. Learn Mem. 1996;3:115–123. doi: 10.1101/lm.3.2-3.115. [DOI] [PubMed] [Google Scholar]
- Xu J, Peng H, Kang N, Zhao Z, Lin JH, Stanton PK, Kang J. (Glutamate-induced exocytosis of glutamate from astrocytes. J Biol Chem. 2007;282:24185–24197. doi: 10.1074/jbc.M700452200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. (Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci U S A. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zorec R, Haydon PG. (Fusion-related release of glutamate from astrocytes. J Biol Chem. 2004;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.