Abstract
Objective
Our goal was to engineer cartilage in vivo using auricular chondrocytes that underwent clinically relevant expansion and using methodologies that could be easily translated into health care practice.
Design
Sheep and human chondrocytes were isolated from auricular cartilage biopsies and expanded in vitro. To reverse dedifferentiation, expanded cells were either mixed with cryopreserved P0 chondrocytes at the time of seeding onto porous collagen scaffolds or proliferated with basic fibroblast growth factor (bFGF). After 2-week in vitro incubation, seeded scaffolds were implanted subcutaneously in nude mice for 6 weeks. The neocartilage quality was evaluated histologically; DNA and glycosaminoglycans were quantified. Cell proliferation rates and collagen gene expression profiles were assessed.
Results
Clinically sufficient over 500-fold chondrocyte expansion was achieved at passage 3 (P3); cell dedifferentiation was confirmed by the simultaneous COL1A1/3A1 gene upregulation and COL2A1 downregulation. The chondrogenic phenotype of sheep but not human P3 cells was rescued by addition of cryopreserved P0 chondrocytes. With bFGF supplementation, chondrocytes achieved clinically sufficient expansion at P2; COL2A1 expression was not rescued but COL1A1/3A1genes were downregulated. Although bFGF failed to rescue COL2A1 expression during chondrocyte expansion in vitro, elastic neocartilage with obvious collagen II expression was observed on porous collagen scaffolds after implantation in mice for 6 weeks.
Conclusions
Both animal and human auricular chondrocytes expanded with low-concentration bFGF supplementation formed high-quality elastic neocartilage on porous collagen scaffolds in vivo.
Keywords: engineered cartilage, chondrocytes, clinical-scale cell expansion, dedifferentiation
Introduction
The limited ability of cartilage to regenerate promotes a search for new repair strategies. Engineered cartilaginous tissue is being developed as a viable substitute for diseased or missing cartilage, including auricular.1
One of the major obstacles to wider clinical use of engineered cartilage is the availability and sufficient quantity of autologous cartilage-producing cells. Stem cells derived from various sources hold promise; however, at present, autologous chondrocytes remain the most reliable and the only near-term clinical source of engineered cartilage. Auricular chondrocyte yield averages 9.7 million cells/g human ear cartilage2 and is low because of low cellularity of cartilage tissue.3 Therefore, approximately 0.3 million chondrocytes can be harvested from a 5 mm diameter (30 mg) biopsy. Using current methodologies, a 300- to 500-fold increase will be required to engineer a replacement adult-sized human ear.4
Generating the desired cell number can be achieved by extensive chondrocyte proliferation in vitro in conventional monolayer culture. However, with repeated passaging, chondrocytes rapidly dedifferentiate, acquire a more fibroblastic phenotype, and lose their potential to form high-quality neocartilage.5 Many experimental methodologies have been developed to address the loss of chondrocyte cartilage-forming ability during expansion; most would be challenging to scale-up for clinical applications and to overcome the regulatory hurdles.
Few methodologies are promising for immediate translation into health care practice. Redifferentiation of bovine articular passage 2 (P2) chondrocytes that were expanded beyond 200-fold was achieved in vitro in a mixed or in a side-by-side culture with either freshly isolated or cryopreserved primary P0 chondrocytes.6-10 Although this approach is promising, in vitro experiments published to date employed only articular chondrocytes and these results have not been validated in vivo. However, different types of cartilage vary significantly in their anatomy and composition, cell yield, chondrocyte proliferative capacity, and, most important, in the quality and stability of engineered cartilage.11,12
A variety of exogenous cytokines and growth factors has been evaluated to prevent chondrocyte dedifferentiation during experimental in vitro expansion13; however, scale-up logistics and regulatory challenges complicate their advancement into clinical practice. In one of the approaches, dedifferentiation of human auricular and nasal chondrocytes during in vitro expansion was prevented by supplementing culture medium with basic fibroblast growth factor (bFGF); the resulting engineered cartilage was successfully used for the clinical reconstruction of nasal and external ear defects in Japan14-18 and nasal defects in Switzerland.19 Earlier studies demonstrated that bFGF supplementation increased both cell yield during monolayer expansion and the ability of expanded chondrocytes to re-express cartilage-specific molecules during subsequent 3D culture in vitro.20,21
The present study is part of our ongoing effort to expedite the development of an engineered living replacement ear for human auricular reconstruction. We have successfully engineered auricular cartilage in immunocompromised rodents22,23 and in a large immunocompetent animal model24 using moderately expanded P1 chondrocytes. The goal of the present study was to engineer cartilage in vivo using chondrocytes that underwent clinically relevant expansion and methodologies that could be easily translated into health care practice. Sheep and human chondrocytes were isolated from small auricular cartilage biopsies and expanded in vitro beyond 500-fold. To reverse dedifferentiation, expanded cells were mixed with cryopreserved P0 chondrocytes at the time of seeding onto porous collagen scaffolds. Alternatively, chondrocytes were proliferated in the presence of bFGF to preserve their cartilage-forming ability. Seeded scaffolds were implanted subcutaneously on the backs of immunocompromised mice after 2 weeks in vitro incubation, and the quality of the resulting neocartilage was evaluated 6 weeks after implantation.
Materials and Methods
Chondrocyte Isolation and Culture
Chondrocytes were isolated from sterilely harvested sheep (n = 3, mean age 12.0 ± 0.9 months) and human (n = 3, mean age 39.3 ± 19.6, age range = 21-60 years) auricular cartilage as previously described.24 A biopsy of native cartilage from the middle third of the ear lobe was reserved for histology and processed as described below. Cartilage was minced into 1 mm3 pieces and digested with 0.1% collagenase type II (Worthington Biochemical Corp., Lakewood, NJ) at 37°C for 16 hours with agitation. Digests were filtered through 100 µm cell strainers (BD, Franklin Lakes, NJ) and washed with phosphate-buffered saline. Freshly isolated chondrocytes were plated at 3 × 103 cells/cm2 into vented 25 cm2 cell culture flasks (BD Falcon, Franklin Lakes, NJ) and the rest were cryopreserved. Culture medium was Ham’s F12 (Invitrogen Co., Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich Co., St. Louis, MO), 100 U/mL penicillin, 100 µg/mL streptomycin, 292 µg/mL l-glutamine, and 0.1 mM nonessential amino acids (all from Invitrogen). The chondrocytes were passaged twice and plated at the same density into vented 150 cm2 cell culture flasks (BD Falcon); they were detached with 0.05% trypsin-EDTA (Mediatech Inc., Manassas, VA) and used for the study. At each passage, a cell aliquot was frozen for RNA extraction. Population doublings were calculated using the equation PD = Log10(N/N0) × 3.33, where N is the number of harvested and N0 the number of plated cells.
Scaffold Preparation and Cell Seeding
Porous bovine dermis–derived type I collagen 2 mm thick sheets were provided by DSM Biomedical (Exton, PA). Five millimeter diameter disks were made using dermal biopsy punches (Acuderm Inc., Ft. Lauderdale, FL). Scaffolds were sterilized with cold ethylene oxide gas prior to seeding. Chondrocytes were suspended in the culture medium at 50 × 106 cells/mL and 50 µL of cell suspension was pipetted onto each side of a scaffold in a 20 minute interval; the scaffold was then flipped every 20 minutes for 3 hours to achieve a more uniform distribution of cells. Constructs were cultured in ultra-low attachment well plates (Corning) in culture medium supplemented with 50 µg/mL ascorbic acid (Wako Pure Chemical Industries Ltd., Osaka, Japan) on a Talboys standard orbital shaker (Henry Troemner LLC, Thorofare, NJ) at 40 rpm in standard incubator conditions for 2 weeks. The culture medium was changed twice a week.
Construct Implantation
All procedures were carried out in accordance with the Massachusetts General Hospital (Boston, MA) guidelines and approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital (2012N000144). The disks were implanted subcutaneously on the backs of female athymic nude (nu/nu) mice, 6 weeks old (Cox-7 Laboratories, Massachusetts General Hospital, Boston, MA). Anesthesia was achieved with an intraperitoneal injection of 75 mg/kg ketamine and 5 mg/kg xylazine. Under aseptic conditions, three midline incisions were made and a total of six subcutaneous pockets were created with blunt dissection. One construct was placed into each pocket and the skin was closed with absorbable sutures. All experiments were performed in triplicate.
Experimental Conditions
To evaluate the ability of extensively expanded animal and human auricular chondrocytes to form quality neocartilage, the following experiments were performed:
Experiment 1: Extensively expanded chondrocytes mixed with freshly harvested chondrocytes. Sheep and human auricular chondrocytes were expanded in monolayer culture to P3 and mixed with cryopreserved P0 chondrocytes of the same origin at a 80:20 ratio before seeding.8
Experiment 2: Extensively expanded chondrocytes cultured in medium conditioned with freshly harvested chondrocytes. Sheep and human auricular chondrocytes were expanded in monolayer culture to P3 and seeded onto the scaffold. Seeded constructs were incubated in medium conditioned with sheep P0 chondrocytes for 2 weeks in vitro prior to implantation. To make conditioned medium, freshly isolated P0 sheep auricular chondrocytes were plated in standard growth medium at high density (20 × 103/cm2), allowed to adhere for 2 days, and then incubated in serum-free medium.25 Conditioned medium was collected, centrifuged to pellet debris, and supplemented with 10% FBS prior to use on constructs seeded with P3 chondrocytes.
Experiment 3: Chondrocytes extensively expanded in medium containing bFGF. Sheep and human auricular chondrocytes were expanded to P3 in monolayer using culture medium supplemented with 5 ng/mL bFGF (R&D Systems, Minneapolis, MN) and seeded onto scaffolds.
P3 chondrocytes expanded in standard growth medium served as controls in all experiments.
Histological and Immunochemical Analyses
All implants were harvested at 6 weeks postimplantation. Half of each explant was fixed in 10% buffered formalin for histology and the other half snap-frozen and stored at −80°C for biochemical analysis. Paraffin-embedded specimens were sectioned at 8 µm. Sections were stained with hematoxylin and eosin, safranin O, toluidine blue, and elastin (Verhoeff’s elastin staining kit, American MasterTech Scientific, Lodi, CA) using standard protocols. Collagen type II and I were detected by immunohistochemistry. Tissue sections were pretreated with 1 mg/mL pepsin in Tris–HCl (pH 2.0) for 15 minutes at room temperature followed by peroxidase block and serum block (MOM kit, Vector Laboratories, Burlingame, CA). Mouse anti-human collagen type I antibody (1:100, clone I-8H5, EMD Millipore, Temecula, CA) or mouse anti-human collagen type II antibody (1:100, clone 6B3, Millipore) were applied for 30 minutes at room temperature. The EnVision+ System kit (Dako, Carpinteria, CA) was used and sections were counterstained with hematoxylin.
Biochemical Assays
For the DNA content, frozen samples were weighed and DNA extracted and purified with the DNeasy kit (Qiagen Inc., Valencia, CA). Total DNA content was determined using the PicoGreen dsDNA assay (Invitrogen). For GAG quantification, minced samples were lyophilized for 24 hours and digested overnight at 60°C in 125 µg/mL papain type III (Sigma-Aldrich) in phosphate buffer with EDTA pH 6.5 (100 mM disodium phosphate, 10 mM ethylenediaminetetraacetic acid, and 10 mM l-cysteine). GAG content was determined spectrophotometrically using the Blyscan Glycosaminoglycan Assay Kit (Biocolor Ltd., Carrickfergus, UK). The GAG and DNA content in engineered cartilage was expressed as % of native GAG and % of native GAG/DNA ratio. The GAG content averaged 118.4 ± 40.5 and 141.2 ± 25.3 µg/mg dry tissue for sheep and human native auricular cartilage, respectively. GAG/DNA ratio was 1.0 ± 0.2 for both species.
Gene Expression Analysis
Total RNA was extracted with the RNeasy Mini kit (Qiagen) and concentration determined by absorbance. First-strand cDNA was synthesized using SuperScript III (Life Technologies). Relative gene expression profiles were determined using end point PCR. Primers were designed that span introns. For each reaction, template cDNA derived from 10 ng of input RNA was used. Thermal cycling parameters used were: 95°C for 30 seconds (one cycle); 95°C for 30 seconds, †°C for 30 seconds, 68°C for 30 seconds (30 cycles); † denotes optimal annealing temperatures listed in Table 1. Reaction products were then electrophoresed on a 2% agarose gel. Specificity of the product was confirmed by fragment length of the observed band. Relative expression levels were determined in ImageJ and normalized using GAPDH.
Table 1.
Primers Used for RT-PCR, Including Annealing Temperatures and Size of the Amplified Products.
| Gene | Primer | Annealing Temperature (°C) | Product Size (bp) |
|---|---|---|---|
| Human COL1A1 | Forward: TAA AGG GTC ACC GTG GCT | 52 | 355 |
| Reverse: CGA ACC ACA TTG GCA TCA | |||
| Human COL2A1 | Forward: AAG ATG GTC CCA AAG GTG CTC G | 57 | 500 |
| Reverse: AGC TTC TCC TCT GTC TCC TTG C | |||
| Human COL3A1 | Forward: GGA AAT GTA AAG AAG GCC CTG AAG CT | 57 | 151 |
| Reverse: GTG TTC GAT ATT CAA AGA CTG TTT TGCT | |||
| Sheep COL1A1 | Forward: AGG GAC CCA AAG GAG ACA CT | 55 | 198 |
| Reverse: GCA CGG AAA TTC CTG TTG AT | |||
| Sheep COL2A1 | Forward: CGT CAC CTA CCA CTG CAA GA | 52 | 219 |
| Reverse: GGG AGA CGT GAG GTC TTC TG | |||
| Sheep COL3A1 | Forward: CTT TTC GCT CTG CTT CAT CC | 58 | 216 |
| Reverse: TTC TCC AAA CGG GAT TTC AG | |||
| GAPDH | Forward: CTC ACT GGC ATG GCC TTC CG | 52 | 293 |
| Reverse: ACC ACC CTG TTG CTG TAG CC |
COL1A1 = collagen type I; COL2A1 = collagen type II; COL3A1 = collagen type III; GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analysis
Values were expressed as mean ± standard deviation. Statistical analyses were performed using Student’s t-test; P < 0.05 was considered significant.
Results
Combination of Sheep but Not Human P3 and P0 Auricular Chondrocytes Produced Neocartilage In Vivo
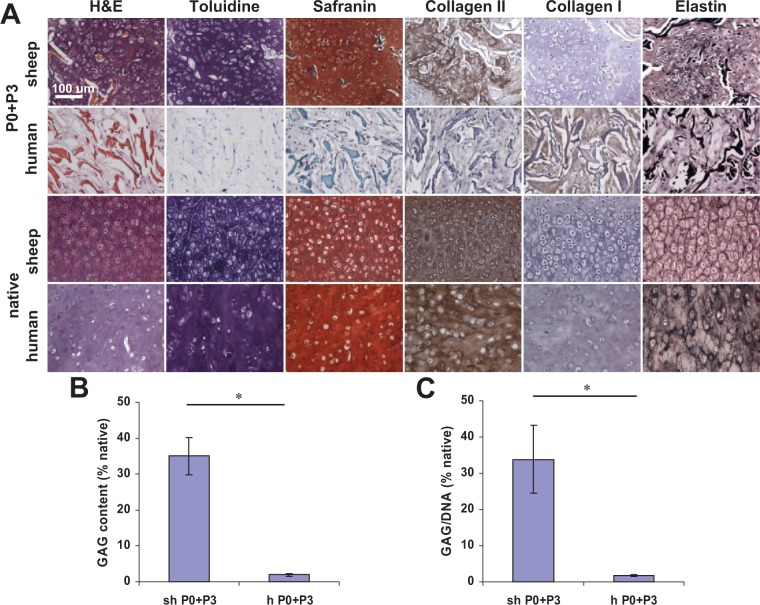
Based on histological evaluation, neocartilage formed from sheep P3 auricular chondrocytes mixed with cryopreserved P0 chondrocytes of the same origin at 6 weeks implantation in nude mice (Fig. 1A). Cells were located in lacunae structures and surrounded by cartilaginous matrix. Furthermore, matrix stained intensely for glycosaminoglycan (GAG) as evident from toluidine blue and safranin O staining and for type II collagen based in the immunohistochemical staining. Staining for collagen type I was negative in the neocartilage. Elastin fibers were sparse and stained faintly. No neocartilage formed in samples seeded with a mixture of human P3 and P0 chondrocytes; matrix stained positively only for collagen type I and not for collagen type II or other cartilage-specific stains. No neocartilage formed from sheep or human P3 chondrocytes without P0 supplementation (not shown). Quantitative extracellular matrix analysis (Fig. 1B, C) confirmed histological findings; GAG content in neocartilage made from P3+P0 sheep chondrocytes reached 35.0 ± 5.2% that of native sheep auricular cartilage and negligible amount of GAG was determined in human P3+P0 samples. The GAG to DNA ratio in P3+P0 sheep samples reached 33.8 ± 9.4% of native sheep auricular cartilage and was negligible in P3+P0 human samples. GAG content and GAG/DNA ratio were negligible in P3 sheep and human samples (not shown).
Figure 1.
Histological and biochemical assessment of elastic cartilage engineered from a mixture of passaged P3 auricular chondrocytes and cryopreserved P0 cells after 6 weeks in nude mice as compared to native sheep and human auricular cartilage. (A) Neocartilage formed from sheep cells as evidenced by positive toluidine blue, safranin, collagen type II, and elastin stains. No neocartilage formed from human cells as confirmed by the absence of cartilage-specific glycosaminoglycans (GAG) staining and positive collagen type I staining. Collagen scaffold fibers stained red on hematoxylin–eosin (H&E) stained sections. Scale bar: 100 µm. (B) GAG and (C) GAG/DNA ratio quantification confirmed histological results. *P < 0.01.
Sheep but Not Human P3 Auricular Chondrocytes Partially Redifferentiated after Incubation in Medium Conditioned with Sheep P0 Chondrocytes
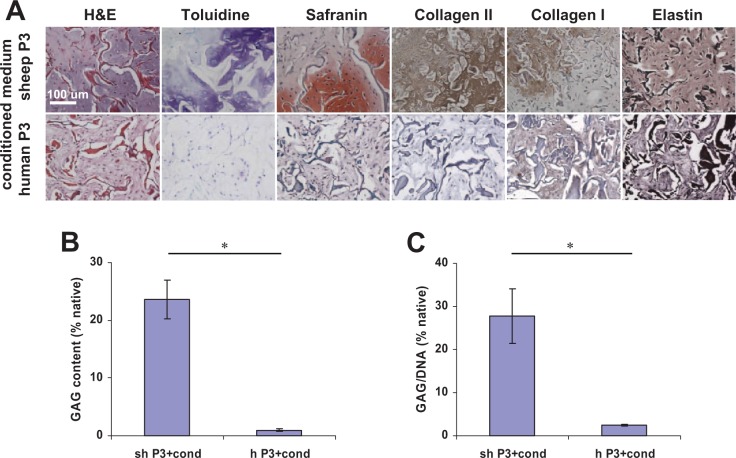
Sheep P3 auricular chondrocytes seeded onto collagen scaffolds and incubated in medium conditioned with P0 chondrocytes partially redifferentiated after 6 weeks implantation in mice. Histologically, neocartilage was of poor quality and not contiguous as evidenced by cartilage-specific stains (Fig. 2A). No elastin was observed histologically. Human chondrocytes failed to redifferentiate and form neocartilage. Histological data were confirmed by GAG quantification (Fig. 2B, C); GAG content was low in sheep samples and was negligible in human samples.
Figure 2.
Histological and biochemical assessment of neocartilage engineered from passaged P3 chondrocytes after in vitro incubation in medium conditioned by P0 chondrocytes for 2 weeks and implantation in nude mice for 6 weeks. (A) The quality of neocartilage formed from sheep cells was poor as evidenced by toluidine blue, safranin, collagen type II, and elastin stains. No neocartilage formed from human cells as confirmed by the absence of cartilage-specific glycosaminoglycans (GAG) staining and positive collagen type I staining. Collagen scaffold fibers stained red on hematoxylin–eosin (H&E) stained sections. Scale bar: 100 µm. (B) GAG and (C) GAG/DNA ratio quantification confirmed histological results. *P < 0.01.
Both Sheep and Human P3 Auricular Chondrocytes Expanded in the Presence of bFGF Formed High-Quality Neocartilage In Vivo
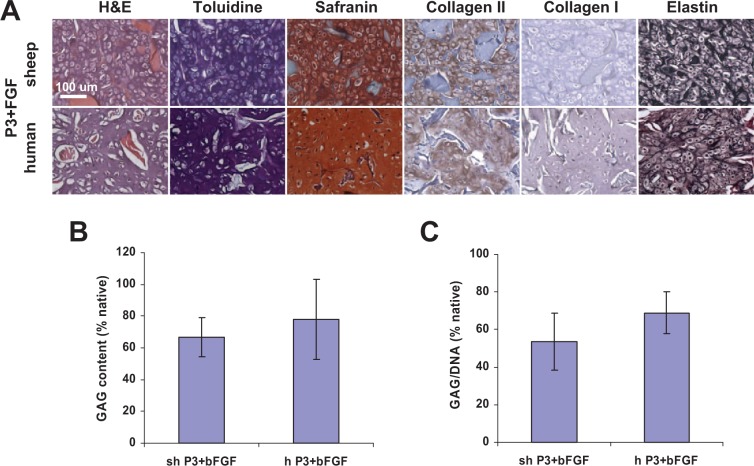
Based on the results of histological evaluation, neocartilage was produced from both sheep and human P3 auricular chondrocytes expanded in the presence of bFGF (Fig. 3A). Chondrocytes were residing in lacunae and intense toluidine blue and safranin O staining was observed for the engineered cartilage matrix and positive staining was seen for type II but not type I collagen. Moreover, elastin fibers in both sheep and human engineered cartilage were mature and stained intensely after 6-week implantation in nude mice. GAG content in sheep and human neocartilage reached 66.6 ± 12.3% and 78.0 ± 25.4% of corresponding native cartilage, respectively (Fig. 3B). GAG to DNA ratio reached 53.6 ± 15.1% and 68.9 ± 11.16% of native sheep and human cartilage, respectively (Fig. 3C). The GAG content and GAG/DNA ratio of cartilage engineered from sheep and human P3 auricular chondrocytes expanded with bFGF supplementation was significantly higher than that of neocartilage engineered from P3 and P0 mixture (P < 0.01).
Figure 3.
Histological and biochemical assessment of elastic cartilage engineered from passaged P3 auricular chondrocytes expanded in the presence of bFGF after 6 weeks in nude mice. (A) Neocartilage formed from both sheep and human cells as evidenced by positive toluidine blue, safranin, collagen type II, and elastin stains. Elastin fibers were dense and strongly stained. Collagen scaffold fibers stained red on hematoxylin–eosin (H&E) stained sections. Scale bar: 100 µm. (B) GAG and (C) GAG/DNA ratio quantification confirmed histological results.
bFGF Supplementation Affected Chondrocyte Morphology, Proliferation Rates, and Collagen Gene Expression in Monolayer Culture
Morphology
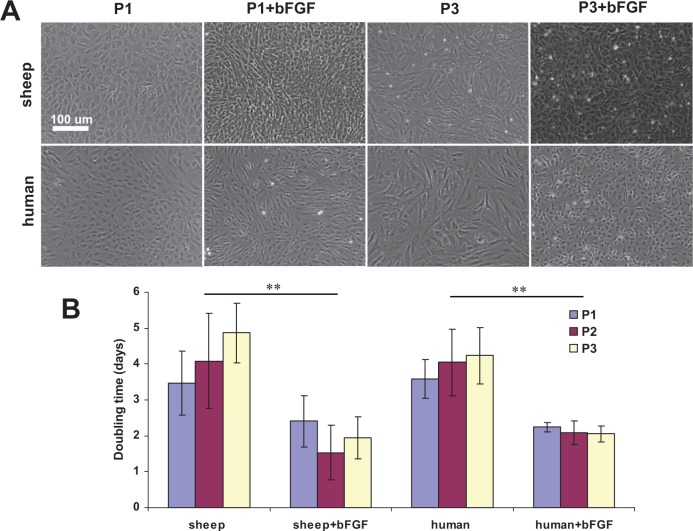
Morphology of both sheep and human auricular chondrocytes changed within the first several days after culture medium supplementation with bFGF (Fig. 4A). Cells from both species became smaller and more elongated whereas chondrocytes cultured in standard medium preserved their size and rounded shape. Interestingly, by passage 3, chondrocytes cultured with bFGF supplementation regained a more rounded shape and their size remained unchanged. At the same time, sheep and human auricular chondrocytes cultured in standard medium became larger and acquired fibroblast-like morphology.
Figure 4.
Effect of bFGF on sheep and human auricular chondrocyte morphology and doubling time. (A) With bFGF supplementation, cells from both species became smaller and more elongated whereas chondrocytes cultured in standard medium preserved their size and rounded shape. By passage 3, chondrocytes cultured with bFGF supplementation regained more rounded shape and their size remained unchanged; sheep and human auricular chondrocytes cultured in standard medium became larger and acquired fibroblast-like morphology. (B) bFGF supplementation significantly reduced population doubling times (**P < 0.02); these findings remained constant regardless of passage number.
Expansion
Both sheep and human auricular chondrocytes proliferated well in 2D culture. bFGF supplementation significantly reduced population doubling times (P < 0.02); these findings remained constant regardless of passage number (Fig. 4B). Cumulative cell expansion fold was significantly greater (P < 0.02) in all cell cultures supplemented with bFGF as compared to those without supplementation (Table 2). Sheep chondrocyte yield was always significantly greater than that of human (P < 0.05 and P < 0.02 for passages 2 and 3, respectively) with the exception of passage 1 (P > 0.1). Target clinically relevant cumulative expansion exceeding 500-fold was achieved at P2 for both sheep and human auricular chondrocytes (2030.9 ± 279.2 and 1599.9 ± 77.7, respectively) expanded with bFGF supplementation. Chondrocytes expanded in standard medium needed to undergo an additional passage to exceed the target 500-fold expansion.
Table 2.
Cumulative Expansion Fold of Human and Sheep Chondrocytes.
| Expansion Fold (Cumulative) |
|||
|---|---|---|---|
| Sheep Chondrocytes | Human Chondrocytes | P | |
| With FGF | |||
| P1 | 44.2 ± 8.6 | 37.4 ± 4.7 | >0.1 |
| P2 | 2030.9 ± 279.2 | 1599.9 ± 77.7 | <0.05 |
| P3 | 103782.6 ± 11513.8 | 65938.8 ± 10563.0 | <0.02 |
| Without FGF | |||
| P1 | 14.7 ± 2.9 | 12.7 ± 3.0 | >0.1 |
| P2 | 194.1 ± 36.1 | 117.8 ± 21.0 | <0.05 |
| P3 | 2931.5 ± 629.8 | 1083.9 ± 322.3 | <0.02 |
FGF = fibroblast growth factor.
Collagen Gene Expression
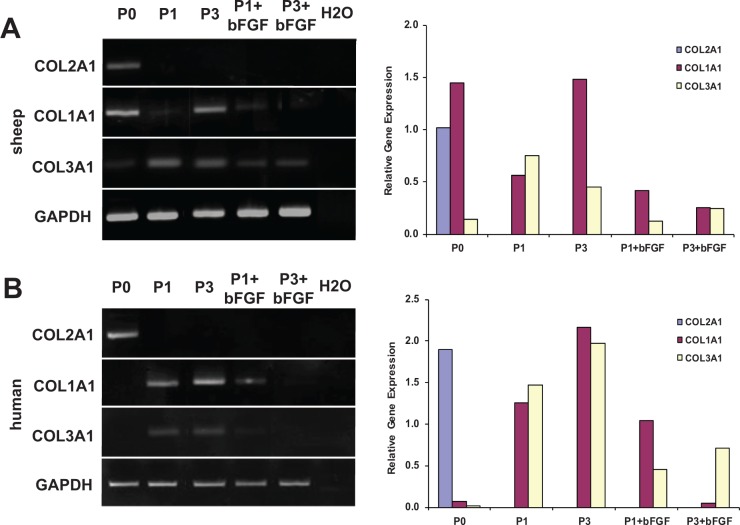
To assess changes in chondrocyte phenotype throughout expansion, COL1A1, COL2A1, and COL3A1 gene expression levels were measured using end point PCR (Fig. 5). P0 chondrocyte profiling from ovine and human sources revealed detectable levels of COL2A1. Expression profiles of high (P3) passage sheep and human chondrocytes expanded in standard conditions demonstrated an increased COL3A1 expression throughout 2D expansion as compared to primary (P0) chondrocytes. Conversely, expression of COL2A1 was downregulated past the threshold of detection. The upregulation of COL3A1 and downregulation of COL2A1 is indicative of chondrocyte dedifferentiation, confirming that our high expansion (P3) chondrocytes represent a dedifferentiated population.
Figure 5.
Gene expression profiles and relative gene expression of sheep and human auricular chondrocytes expanded with and without bFGF. (A) In sheep chondrocytes, detectable levels of COL2A1 were present only in P0 cells. COL1A1 and COL3A1 also had detectable levels. COL1A1 and COL3A1 expression increased in chondrocytes expanded in standard conditions and COL2A1 was downregulated past the threshold of detection. bFGF supplementation during expansion resulted in decreased COL1A1 and COL3A1 expression, but COL2A1 expression was not rescued. (B) In human P0 chondrocytes, only COL2A1 but not COL1A1 and COL3A1 were detected. Similar gene expression profiles were demonstrated in response to proliferation and bFGF supplementation.
Profiling of chondrocytes expanded in the presence of bFGF revealed downregulation of COL1A1 and 3A1 for both P1 and P3 chondrocytes as compared to cells expanded in standard conditions. It should also be noted that at P1, COL1A1 expression levels were comparable between medium conditions in sheep and human, whereas COL3A1 expression levels were much more varied. COL2A1 expression was not rescued by bFGF supplementation for either sheep or human cells.
Discussion
Wider clinical use of engineered cartilage is contingent on availability of a sufficient cell source with preserved cartilage-forming ability. As a part of our ongoing effort to develop a living replacement ear,22-24 we explored the possibility of engineering auricular cartilage in vivo using chondrocytes that underwent clinically relevant expansion, using methodologies that could be readily translated into healthcare practice.
Our data confirm results of earlier in vitro studies performed by two separate groups with dedifferentiated P2 bovine articular chondrocytes mixed with cryopreserved P0 chondrocytes to induce redifferentiation.6-10 On the contrary, a third group showed that the quality of engineered cartilage did not improve with increased ratio of P0 rabbit articular chondrocytes in P0 and P3 pellet coculture.26 The authors suggested that the adult age of the P0 chondrocyte donors (skeletally mature 18- to 24-month-old rabbits) could have a negative impact on differentiation. Indeed, no neocartilage formed from bovine articular P0 and P2 chondrocyte cocultures in vitro when P0 chondrocytes originated from 18- to 24-month-old animals; however, the age of P2 cell source had no effect on their responsiveness to P0 chondrocytes originating from 6- to 9-month-old animals.8 The exact mechanisms and soluble factors responsible for dedifferentiated chondrocyte phenotype recovery after addition of P0 chondrocytes remain undetermined. In our study, human auricular chondrocytes failed to redifferentiate in the presence of cryopreserved freshly isolated human chondrocytes, which is similar to prior in vitro studies using human articular chondrocytes.8,9
We did not attempt to mix sheep P0 chondrocytes with expanded human P3 chondrocytes to induce redifferentiation9; such an approach would be problematic for clinical use because of the xenogeneic nature of the P0 cells. In vitro transwell experiments demonstrated that no cell-to-cell contact was required between P0 and passaged chondrocytes, suggesting that soluble factors inducing redifferentiation were secreted into the culture medium.8,10 In support of this approach, stem cell differentiation toward chondrogenic lineage has been achieved by growing stem cells in chondrocyte-conditioned medium.27,28 Freshly isolated xenogeneic chondrocytes potentially could be used as a source of conditioned medium with secreted factors to redifferentiate human cells. For example, xenogeneic cells (proliferation-arrested 3T3 mouse fibroblasts) are used as a feeder layer in the production of a commercial wound dressing, cultured epidermal autograft (Epicel, Genzyme).
Human articular P2 chondrocytes have been redifferentiated in side-by-side culture with bovine P0 chondrocytes,8,9 suggesting that they are able to respond to the factors secreted by xenogeneic P0 chondrocytes. However, in our experiments, the concentration of these factors in medium conditioned by sheep chondrocytes appears be insufficient to redifferentiate human auricular chondrocytes. Whereas the ability of freshly isolated chondrocytes to secrete these factors depends on the donor age and species,8,29 it is also possible that this ability is significantly reduced after chondrocytes undergo population doublings.10 Cell feeder layers replaced weekly with a subconfluent population of primary juvenile chondrocytes resulted in superior qualities of engineered cartilage in vitro.10 Further studies are needed to identify these factors and optimize the cell source for them.
Low-dose bFGF supplementation in serum-containing medium has been used to expand bovine, porcine, and human articular chondrocytes20,30,31 and human auricular chondrocytes32-34 without loss of chondrogenic potential. Significant suppression of type II and III collagen gene expression was reported for human auricular chondrocytes expanded with bFGF supplementation, similar to our findings.32 Other groups reported that levels of collagen type II mRNA expressed by human auricular P2 chondrocytes expanded in standard medium or with growth factor supplementation (a mixture of transforming growth factor-β [TGF-β], bFGF, and platelet-derived growth factor bb) were not statistically different.34 Therefore, bFGF supplementation failed to rescue COL2A1 expression levels, suggesting that neither redifferentiation nor stimulation of chondrogenic activity was achieved during bFGF-supplemented 2D culture.
The mechanisms of chondrogenic phenotype preservation by bFGF supplementation during cell expansion remain unknown. The ability of chondrocytes to differentiate in response to exogenous factors has been correlated with the cytoskeleton organization, and bFGF has been shown to prevent the development of thick F-actin fibers.20 bFGF has been also shown to inactivate TGF-β signaling,35 and TGF-β inhibition increased the chondrogenic redifferentiation capacity of human articular chondrocytes.36 TGF-β is introduced into cell culture with serum that reportedly contains 10 to 20 ng/mL of TGF-β and a trace of bFGF (40 pg/mL).
Interestingly, in our studies, elastin fibers appeared mature and were more abundant resembling elastin fibers in native auricular cartilage. It has been reported that bFGF causes significant elastin gene downregulation in auricular chondrocytes during in vitro expansion.32 However, similarly to collagen type II, elastin expression was able to be rescued after chondrocytes were seeded on a scaffold, cultured for 2 weeks in vitro, and implanted for 6 weeks into nude mice. Auricular chondrocyte differentiation potential was better maintained during expansion in the presence of bFGF similar to earlier published studies with bovine articular chondrocytes20; however, the exact mechanisms remain unknown.21
In conclusion, we successfully demonstrated for the first time formation of elastic neocartilage engineered in immunocompromised rodents from auricular chondrocytes that underwent clinical-scale expansion. Both animal and human auricular chondrocytes expanded with low-concentration bFGF supplementation formed high-quality elastic neocartilage on porous collagen scaffolds after implantation in nude mice. This approach will be validated in a large immunocompetent animal model in which ear-shaped neocartilage will be generated from autologous chondrocytes that underwent clinically relevant expansion. The stability of this neocartilage and of the resulting engineered auricle will be evaluated over an extended period of time. The present work represents an important step toward the development of an engineered elastic neocartilage for auricular reconstruction in clinical practice.
Footnotes
Acknowledgments and Funding: This research was sponsored by the U.S. Army Medical Research and Materiel Command Award Number W81XWH-12-1-0334 and by the Armed Forces Institute of Regenerative Medicine Award Number W81XWH-08-2-0034. The content of this article does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred. The fibrous collagen scaffolds were generously provided by the DSM Biomedical.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The animal research protocol (2012N000144) was reviewed and approved by the Institutional Animal Care and Use Committee for the Massachusetts General Hospital, Boston, Massachusetts.
References
- 1. Bichara DA, O’Sullivan NA, Pomerantseva I, Zhao X, Sundback CA, Vacanti JP, et al. The tissue-engineered auricle: past, present, and future. Tissue Eng Part B Rev. 2012;18:51-61. [DOI] [PubMed] [Google Scholar]
- 2. Van Osch GJ, Mandl EW, Jahr H, Koevoet W, Nolst-Trenité G, Verhaar JA. Considerations on the use of ear chondrocytes as donor chondrocytes for cartilage tissue engineering. Biorheology. 2004;41:411-21. [PubMed] [Google Scholar]
- 3. Lin Z, Willers C, Xu J, Zheng MH. The chondrocyte: biology and clinical application. Tissue Eng. 2006;12:1971-84. [DOI] [PubMed] [Google Scholar]
- 4. Shieh SJ, Terada S, Vacanti JP. Tissue engineering auricular reconstruction: in vitro and in vivo studies. Biomaterials. 2004;25:1545-57. [DOI] [PubMed] [Google Scholar]
- 5. Schulze-Tanzil G. Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair. Ann Anat. 2009;191:325-38. [DOI] [PubMed] [Google Scholar]
- 6. Gan L, Kandel RA. In vitro cartilage tissue formation by co-culture of primary and passaged chondrocytes. Tissue Eng. 2007;13:831-42. [DOI] [PubMed] [Google Scholar]
- 7. Ahmed N, Gan L, Nagy A, Zheng J, Wang C, Kandel RA. Cartilage tissue formation using redifferentiated passaged chondrocytes in vitro. Tissue Eng Part A. 2009;15:665-73. [DOI] [PubMed] [Google Scholar]
- 8. Taylor DW, Ahmed N, Gan L, Gross AE, Kandel RA. Proteoglycan and collagen accumulation by passaged chondrocytes can be enhanced through side-by-side culture with primary chondrocytes. Tissue Eng Part A. 2010;16:643-51. [DOI] [PubMed] [Google Scholar]
- 9. Ahmed N, Taylor DW, Wunder J, Nagy A, Gross AE, Kandel RA. Passaged human chondrocytes accumulate extracellular matrix when induced by bovine chondrocytes. J Tissue Eng Regen Med. 2010;4:233-41. [DOI] [PubMed] [Google Scholar]
- 10. Tan AR, Dong EY, Andry JP, Bulinski JC, Ateshian GA, Hung CT. Coculture of engineered cartilage with primary chondrocytes induces expedited growth. Clin Orthop Relat Res. 2011;469:2735-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Sayed K, Haisch A, John T, Marzahn U, Lohan A, Müller RD, et al. Heterotopic autologous chondrocyte transplantation—a realistic approach to support articular cartilage repair? Tissue Eng Part B Rev. 2010;16:603-16. [DOI] [PubMed] [Google Scholar]
- 12. Hellingman CA, Verwiel ET, Slagt I, Koevoet W, Poublon RM, Nolst-Trenité GJ, et al. Differences in cartilage-forming capacity of expanded human chondrocytes from ear and nose and their gene expression profiles. Cell Transplant. 2011;20:925-40. [DOI] [PubMed] [Google Scholar]
- 13. Bobick BE, Chen FH, Le AM, Tuan RS. Regulation of the chondrogenic phenotype in culture. Birth Defects Res C Embryo Today. 2009;87:351-71. [DOI] [PubMed] [Google Scholar]
- 14. Yanaga H, Koga M, Imai K, Yanaga K. Clinical application of biotechnically cultured autologous chondrocytes as novel graft material for nasal augmentation. Aesthetic Plast Surg. 2004;28:212-21. [DOI] [PubMed] [Google Scholar]
- 15. Yanaga H, Yanaga K, Imai K, Koga M, Soejima C, Ohmori K. Clinical application of cultured autologous human auricular chondrocytes with autologous serum for craniofacial or nasal augmentation and repair. Plast Reconstr Surg. 2006;117:2019-30. [DOI] [PubMed] [Google Scholar]
- 16. Yanaga H, Imai K, Fujimoto T, Yanaga K. Generating ears from cultured autologous auricular chondrocytes by using two-stage implantation in treatment of microtia. Plast Reconstr Surg. 2009;124:817-25. [DOI] [PubMed] [Google Scholar]
- 17. Yanaga H, Imai K, Yanaga K. Generative surgery of cultured autologous auricular chondrocytes for nasal augmentation. Aesthetic Plast Surg. 2009;33:795-802. [DOI] [PubMed] [Google Scholar]
- 18. Yanaga H, Imai K, Koga M, Yanaga K. Cell-engineered human elastic chondrocytes regenerate natural scaffold in vitro and neocartilage with neoperichondrium in the human body post-transplantation. Tissue Eng Part A. 2012;18:2020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fulco I, Miot S, Haug MD, Barbero A, Wixmerten A, Feliciano S, et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet. Epub 2014. April 9. [DOI] [PubMed] [Google Scholar]
- 20. Martin I, Vunjak-Novakovic G, Yang J, Langer R, Freed LE. Mammalian chondrocytes expanded in the presence of fibroblast growth factor 2 maintain the ability to differentiate and regenerate three-dimensional cartilaginous tissue. Exp Cell Res. 1999;253:681-8. [DOI] [PubMed] [Google Scholar]
- 21. Martin I, Suetterlin R, Baschong W, Heberer M, Vunjak-Novakovic G, Freed LE. Enhanced cartilage tissue engineering by sequential exposure of chondrocytes to FGF-2 during 2D expansion and BMP-2 during 3D cultivation. J Cell Biochem. 2001;83:121-8. [DOI] [PubMed] [Google Scholar]
- 22. Zhou L, Pomerantseva I, Bassett EK, Bowley CM, Zhao X, Bichara DA, et al. Engineering ear constructs with a composite scaffold to maintain dimensions. Tissue Eng Part A. 2011;17:1573-81. [DOI] [PubMed] [Google Scholar]
- 23. Cervantes TM, Bassett EK, Tseng A, Kimura A, Roscioli N, Randolph MA, et al. Design of composite scaffolds and three-dimensional shape analysis for tissue-engineered ear. J R Soc Interface. 2013;10:20130413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bichara DA, Pomerantseva I, Zhao X, Zhou L, Kulig KM, Tseng A, et al. Successful creation of tissue-engineered autologous auricular cartilage in an immunocompetent large animal model. Tissue Eng Part A. 2014;20:303-12. [DOI] [PubMed] [Google Scholar]
- 25. Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281-94. [DOI] [PubMed] [Google Scholar]
- 26. Huey DJ, Hu JC, Athanasiou KA. Chondrogenically tuned expansion enhances the cartilaginous matrix-forming capabilities of primary, adult, leporine chondrocytes. Cell Transplant. 2013;22:331-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hwang NS, Varghese S, Puleo C, Zhang Z, Elisseeff J. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2007;212:281-4. [DOI] [PubMed] [Google Scholar]
- 28. Hwang NS, Im SG, Wu PB, Bichara DA, Zhao X, Randolph MA, et al. Chondrogenic priming adipose-mesenchymal stem cells for cartilage tissue regeneration. Pharm Res. 2011;28:1395-405. [DOI] [PubMed] [Google Scholar]
- 29. Giannoni P, Crovace A, Malpeli M, Maggi E, Arbicò R, Cancedda R, et al. Species variability in the differentiation potential of in vitro-expanded articular chondrocytes restricts predictive studies on cartilage repair using animal models. Tissue Eng. 2005;11:237-48. [DOI] [PubMed] [Google Scholar]
- 30. Jakob M, Démarteau O, Schäfer D, Hintermann B, Dick W, Heberer M, et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368-77. [DOI] [PubMed] [Google Scholar]
- 31. Jin RL, Park SR, Choi BH, Min BH. Scaffold-free cartilage fabrication system using passaged porcine chondrocytes and basic fibroblast growth factor. Tissue Eng Part A. 2009;15:1887-95. [DOI] [PubMed] [Google Scholar]
- 32. Shasti M, Jacquet R, McClellan P, Yang J, Matsushima S, Isogai N, et al. Effects of FGF-2 and OP-1 in vitro on donor source cartilage for auricular reconstruction tissue engineering. Int J Pediatr Otorhinolaryngol. 2014;78:416-22. [DOI] [PubMed] [Google Scholar]
- 33. Mandl EW, van der Veen SW, Verhaar JA, van Osch GJ. Multiplication of human chondrocytes with low seeding densities accelerates cell yield without losing redifferentiation capacity. Tissue Eng. 2004;10:109-18. [DOI] [PubMed] [Google Scholar]
- 34. Tay AG, Farhadi J, Suetterlin R, Pierer G, Heberer M, Martin I. Cell yield, proliferation, and postexpansion differentiation capacity of human ear, nasal, and rib chondrocytes. Tissue Eng. 2004;10:762-70. [DOI] [PubMed] [Google Scholar]
- 35. Ito T, Sawada R, Fujiwara Y, Tsuchiya T. FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-beta signaling. Cytotechnology. 2008;56:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Narcisi R, Signorile L, Verhaar JA, Giannoni P, van Osch GJ. TGFβ inhibition during expansion phase increases the chondrogenic redifferentiation capacity of human articular chondrocytes. Osteoarthritis Cartilage. 2012;20:1152-60. [DOI] [PubMed] [Google Scholar]