Abstract
Objective
Although extracellular matrix (ECM)–derived scaffolds have been extensively studied and applied in a number of clinical applications, the use of ECM as a biomaterial for (osteo)chondral regeneration is less extensively explored. This study aimed at evaluating the chondrogenic potential of cells seeded on cartilage-derived matrix (CDM) scaffolds in vitro.
Design
Scaffolds were generated from decellularized equine articular cartilage and seeded with either chondrocytes or multipotent stromal cells (MSCs). After 2, 4, and 6 weeks of in vitro culture, CDM constructs were analyzed both histologically (hematoxylin and eosin, Safranin-O, collagen types I and II) and biochemically (glycosaminoglycan [GAG] and DNA content).
Results
After 4 weeks, both cell types demonstrated chondrogenic differentiation; however, the MSCs significantly outperformed chondrocytes in producing new GAG-containing cartilaginous matrix.
Conclusion
These promising in vitro results underscore the potency of CDM scaffolds in (osteo)chondral defect repair.
Keywords: cartilage tissue engineering, chondrocyte, extracellular matrix (ECM), mesenchymal stromal cell, scaffold
Introduction
The young and active population is often affected by traumatic injuries to the knee leading to cartilage or osteochondral defects.1-3 When untreated, cartilage defects develop into osteoarthritis (OA), which strongly affects the patient’s quality of life because of increased discomfort or pain as well as a strong decline in mobility.4 The ultimate salvage treatment option for OA is artificial knee replacement, which is not an appropriate option for young and active patients because of the relatively short life span of artificial joint replacements.
Ideally, to prevent the onset and progression of osteoarthritis, the body would have to initiate cartilage healing itself. However, the regeneration and repair of cartilage are hampered by insufficient nutrient supply and hypocellularity.5 To compensate for the incapacity of the body to heal cartilage defects, new techniques to guide articular cartilage regeneration are sought after.
A promising approach for cartilage reconstruction is tissue engineering, which uses scaffolds to aid in the delivery of cells and/or growth factors. Additionally, these scaffolds can serve as a mechanically stable platform for the deposition of newly formed extracellular matrix (ECM).6 Structural and biological cues, such as growth factors can be incorporated in biomaterials to stimulate the deposition of neo-tissue and/or to tailor the construct’s properties for specific tissue requirements. Through interactions between the resident cells and the surrounding ECM, chemotactic stimuli and specific gene expression result in constant remodeling of the tissue.7 Hence, the tissue-specific ECM drives cellular differentiation and differential functional adaptation8; therefore it seems logical to explore the application of ECM as a biomaterial for tissue engineering applications. Additional advantages of natural ECM-based scaffolds are their biodegradable nature and the potential to be applied cross-species, as ECM proteins are highly conserved across species.7,9 ECM-derived biomaterials are often decellularized to allow for optimal host incorporation and to prevent or modulate possible immunogenic responses after implantation.8,10,11 Indeed, these materials have already been pursued and were found effective in many different fields of reconstructive and regenerative medicine.10,12-15 For cartilage tissue, relatively more vigourous decellularization protocols are needed because of the dense nature of the cartilage matrix. This typically results in lower glycosaminoglycan (GAG) content and loss of biomechanical resilience of the cartilage-derived matrix (CDM).16 Nevertheless, scaffolds have been generated from decellularized cartilage matrix particles through lyophilization,17,18 and when inoculated with multipotent stromal cells (MSCs), hyaline cartilage formation was observed in a orthotopic rabbit model.19 However, despite this luring prospect of ECM-based scaffolds,20 the number of studies that report on the use of decellularized CDM to drive chondrogenic differentiation and eventually cartilage repair18,19,21-24 is limited. We therefore aimed at further exploring the potential of this approach by producing and characterizing CDM scaffolds and evaluating the in vitro chondrogenic potential of chondrocytes or MSCs when seeded on CDM scaffolds.
Methods
Scaffold Production
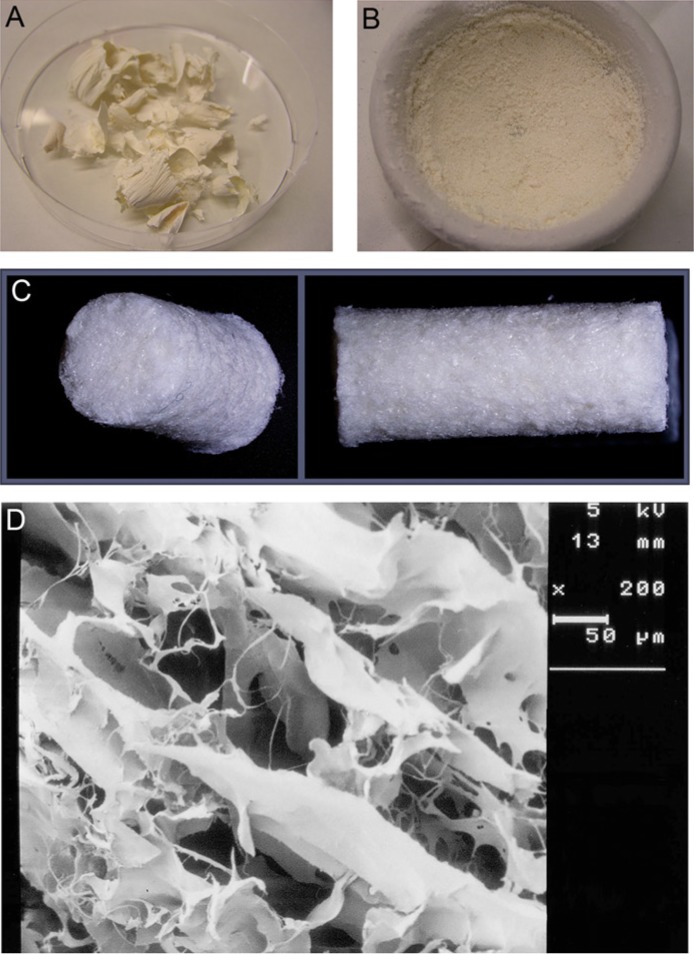
The CDM scaffolds were produced according to a protocol adapted from Yang et al.19 Briefly, full-thickness cartilage from the medial and lateral femoral condyles of the stifle (knee) joint of an equine donor was dissected using a scalpel, and washed in phosphate-buffered saline (PBS) (supplemented with penicillin, streptomycin, and fungizone [Invitrogen, Carlsbad, CA]). Next, the cartilage particles were snap-frozen in liquid nitrogen and lyophilized for 24 hours (Fig. 1A). Thereafter, the lyophilized tissue was ground for approximately 40 minutes under liquid nitrogen to obtain fine cartilage particles (Fig. 1B). Subsequently, the particles underwent 6 cycles of 0.25% trypsin–ethylenediamine tetraacetic acid (trypsin-EDTA; Invitrogen, Carlsbad, CA) treatment in 24 hours at 37°C under vigorous agitation. Next, the tissue was washed in PBS and treated with a nuclease solution of 50 U/mL deoxyribonuclease (Sigma, St. Louis, MO) and 1 U/mL ribonuclease A (Sigma, St. Louis, MO) in 10 mM Tris–HCl, pH 7.5, at 37°C under vigorous agitation. After 4 hours, the nuclease solution was removed and replaced by 10 mM hypotonic Tris–HCl for 20 hours on a roller plate at room temperature. Subsequently, the tissue was immersed in 1% (v/v) Triton X-100 in PBS for 24 hours on a roller plate at room temperature. To remove all remnants of the enzymatic treatments, the tissue was washed thoroughly in PBS in 6 cycles over the course of 48 hours. The supernatants of all described steps were stored at −20°C. After the decellularization process, the resulting matrix particles were inserted into 8 mm diameter cylindrical molds and lyophilized for 24 hours (Fig. 1C). To allow cross-linking, the scaffolds were subjected to ultraviolet light overnight. Prior to cell seeding, the scaffolds were sterilized using ethylene oxide gas. A number of scaffolds were sputter-coated (Cressington, Watford, UK) with a thin gold layer to study them with a scanning electron microscope (Zeiss, Oberkochen, Germany) (Fig. 1D).
Figure 1.
The production of the scaffolds starts with lyophilized cartilage shrapnels (A) that were ground into small particles (B) that are subjected to several enzymatic treatments. The produced scaffolds are porous (C), as was confirmed by scanning electron microscopy (D). The scale bar represents 50 µm.
Equine Chondrocyte and Equine Multipotent Stromal Cell Isolation
Full-thickness equine chondrocytes were obtained from macroscopically healthy cartilage of the load-bearing sites of the medial and lateral femoral condyles of skeletally mature donors (n = 3, age 3-10 years) that died of causes not related to their joints. Tissue was obtained with permission of the owners, in line with the institutional ethical regulations. Cartilage was obtained under aseptic conditions and digested overnight using 0.15% type II collagenase (Worthington Biochemical Corp., Lakewood, NJ) at 37°C. Next, the cell suspension was filtered, and washed thoroughly in PBS. Cells were resuspended in chondrocyte expansion medium consisting of DMEM (Dulbecco’s Modified Eagle Medium 41965, Invitrogen, Carlsbad, CA), 10% heat-inactivated fetal bovine serum (FBS; Biowhittaker, Walkersville, MD), 100 U/mL penicillin and 100 µg/mL streptomycin (Invitrogen, Carlsbad, CA), and 10 ng/mL FGF-2 (R&D Systems, Minneapolis, MN). The cells were expanded in a monolayer culture with a cell seeding density of 5.0 × 103 cells/cm2 until confluency was reached (approximately 10-14 days, ~3-4 population doublings).
Equine sternal bone marrow aspirate was obtained from healthy, living donors (n = 3, age 3-10 years), with approval of the local animal ethical committee. The mononuclear fraction (MNF) was isolated from the bone marrow aspirate by centrifuging on Ficoll-Paque. The MNF was resuspended in MSC expansion medium containing α-MEM (22561, Invitrogen, Carlsbad, CA) complemented with 10% heat-inactivated FBS, 0.2 mM l-ascorbic acid-2-phosphate (Sigma, St. Louis, MO), 100 U/mL penicillin, 100 µg/mL streptomycin, and 1 ng/mL FGF-2. Cells were expanded in a monolayer culture with an initial cell density of 2.5 × 105 cells/cm2. The cells were expanded to subconfluence before passaging.
To ensure that the cells isolated from the sternal bone marrow aspirate were MSCs, the multilineage potential was confirmed by differentiating the equine MSCs into the adipogenic, osteogenic, and chondrogenic lineages, as previously described.25 In brief, the MSCs were cultured in 3 different media. Osteogenic and adipogenic differentiation was stimulated in monolayer cultures once confluency was obtained. Osteogenic differentiation was stimulated in α-MEM, supplemented with 10% heat-inactivated FBS, 0.2 mM l-ascorbic acid-2-phosphate, 100 U/mL penicillin, 100 µg/mL streptomycin, 10 mM β-glycerophosphate (G9891, Sigma, St. Louis, MO), and 10 nM dexamethasone (Sigma, St. Louis, MO). Adipogenic medium consisted of α-MEM, supplemented with 10% inactivated FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, 1 µM dexamethasone, 0.5 mM IBMX (3-isobutyl-1-methylxanthine, Sigma, St. Louis, MO), 0.2 mM indomethacin (Sigma, St. Louis, MO), and 1.72 µM insulin (Sigma, St. Louis, MO). Chondrogenic differentiation was evaluated in a pellet culture of MSCs in chondrogenic MSC differentiation medium. Pellets were made by centrifuging 250,000 cells in a 15-mL tube. Chondrogenic MSC differentiation medium contained DMEM (31966, Invitrogen) supplemented with 0.2 mM l-ascorbic acid-2-phosphate, 1× ITS + premix (BD Biosciences, Franklin Lakes, NJ), 0.1 µM dexamethasone, 100 U/mL penicillin, 100 µg/mL streptomycin, and 10 ng/mL TGFβ-2 (R&D Systems, Minneapolis, MN).
Osteogenic differentiation was evaluated through alkaline phosphatase (ALP) activity of the differentiated MSCs. The monolayer was permeabilized with 0.2% Triton X-100 in tris-buffered saline (TBS). The presence of ALP was determined using the Fuchsin-Substrate-Chromogen kit (Dako, Glostrup, Denmark). Adipogenic differentiation was confirmed by an Oil-red O staining (Sigma, St. Louis, MO). The monolayer was fixed in formalin, washed in distilled water, washed with 60% isopropanol, and stained with Oil-red O for 10 to 20 minutes at room temperature. Chondrogenic differentiation was confirmed by the presence of GAGs (Safranin-O staining) and collagen type II (immunohistochemistry), as described below.
Scaffold Seeding and Culturing
Prior to cell seeding, the scaffolds were cut into discs of approximately 3 mm thickness. These discs were presoaked in DMEM (Invitrogen) for 1 hour. Next, 3.0 × 106 cells, either chondrocytes (P1) or MSCs (P1), were seeded onto the scaffolds (n = 6 per donor). For this, 1.5 × 106 cells were seeded on the top of the scaffold and after 60 minutes, the scaffold was turned and 1.5 × 106 cells were seeded on the bottom in order to improve cell seeding efficiency.
Scaffolds seeded with expanded chondrocytes were cultured for 2, 4, and 6 weeks (n = 3 per donor) in chondrogenic differentiation medium (DMEM [41965, Invitrogen] supplemented with 0.2 mM l-ascorbic acid-2-phosphate, 0.5% human serum albumin (SeraCare Life Sciences, Milford, MA), 1× ITS-X (Invitrogen), 100 U/mL penicillin and 100 µg/mL streptomycin, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; Invitrogen), and 5 ng/mL TGFβ-2).
The MSC-seeded scaffolds were first cultured for 1 week in MSC expansion medium and subsequently differentiated for either 4 or 6 (n = 3 per donor) weeks in MSC chondrogenic differentiation medium.
Histology
Samples were cut in half and dehydrated through a graded ethanol series, cleared in xylene and embedded in paraffin. The paraffin-embedded samples were sectioned into 5-µm slices and stained with hematoxylin and eosin for cell detection, and a triple stain of hematoxylin, fast green, and Safranin-O to identify GAG deposition (all from Sigma). The stained sections were examined using a light microscope (Olympus BX51) and representative images were taken from sections of the centre of the constructs.
Immunohistochemistry
Paraffin-embedded sections were deparaffinized through a graded ethanol series and washed in PBS with 0.1% Tween 20 for 5 minutes prior to immunolocalization of collagen types I and II. Antigen retrieval steps involved exposure to hyaluronidase for 30 minutes (Sigma; 10 mg/mL in PBS), and to pronase for 30 minutes (Roche, Basel, Switzerland; 1 mg/mL in PBS), both at 37°C. Next, the sections were blocked with 5% bovine serum albumin in PBS for 30 minutes at room temperature, and incubated overnight at 4°C with antibodies either against collagen type I (1:50; I-8H5, Calbiochem, Darmstadt, Germany) or type II (1:100; II-6B3II, Developmental Studies Hybridoma Bank). Then, the samples were incubated with a biotinylated anti-mouse antibody (1:200; GE Healthcare, Fairfield, CT) and streptavidin/peroxidase (1:400; Beckman Coulter, Indianapolis, IN), or a secondary anti-mouse antibody conjugated with peroxidase (Dako, Glostrup, Denmark), respectively, all for 60 minutes at room temperature. Antibody binding in all of the sections was visualized using 3,3′-diaminobenzidine solution (Sigma, St. Louis, MO) for up to 10 minutes. Nuclei were counterstained with Mayer’s hematoxylin.
Glycosaminoglycan and DNA Quantification
The remaining half of each of the samples was digested overnight in papain solution (0.01 M cysteine, 250 µg/mL papain, 0.2 M NaH2PO4 and 0.01 M EDTA) at 60°C. After reaction with dimethylmethylene blue (DMMB; Sigma, St. Louis, MO), GAG content was measured spectrophotometrically in a microplate reader (Bio-Rad, Hercules, CA) by determining the ratio of absorbances at 540 and 595 nm. GAG content per scaffold was quantified using a chondroitin sulfate (Sigma) standard.
DNA content was quantified on the papain digests using a Picogreen DNA assay (Invitrogen) according to the manufacturer’s instructions.
Statistical Analysis
To compare the GAG per DNA of chondrocyte- and MSC-seeded CDM scaffolds, 2-tailed unpaired Student’s t tests were performed. Significance was set at P < 0.05.
Results
Scaffold Characterization
The produced CDM scaffolds had a diameter of 8 mm and were approximately 2 to 3 mm in height. Scanning electron microscopy demonstrated that the scaffolds were highly porous and contained randomly aligned extracellular matrix particles (Fig. 1D). Successful decellularization was confirmed, as no cells were observed in hematoxylin and eosin–stained sections (Fig. 2A). Moreover, the scaffolds did not contain any residual GAGs, as was demonstrated by the Safranin-O staining and confirmed by the quantitative DMMB analysis (Figs. 2C and 5). Quantitative DMMB analysis revealed that the majority of GAGs were lost in the decellularization process during the first enzymatic treatment steps (trypsin) (Fig. 2E). In contrast to the loss of GAGs, the scaffolds did show intense staining for collagen type II (Fig. 2B), while no staining for collagen type I was observed (Fig. 2D).
Figure 2.
The cartilage-derived matrix (CDM) scaffold is porous with no remaining cells (A, hematoxylin and eosin staining), no glycosaminoglycans (GAGs) (C, Safranin-O staining), and no collagen type I (D). All scaffold material was positive for collagen type II (B). Scale bars represent 500 µm. During the decellularization process, the majority of GAGs was lost during the first trypsin treatment steps as confirmed by quantitative GAG analysis (E); error bars indicate 95% confidence intervals.
Figure 5.

Quantitative analysis of glycosaminoglycan (GAG) production expressed as GAG/DNA. The amount of GAG/DNA increased for both the chondrocyte-seeded (C) and multipotent stromal cell (MSC)–seeded (M) conditions compared with the empty scaffolds, but significantly more in the MSC-seeded condition after 4 weeks of culture (*P = 0.002).
ND = not determined at that time point. Error bars indicate 95% confidence intervals.
In Vitro Tissue Formation: Chondrocyte-Seeded CDM Scaffolds
Cartilage-derived matrix scaffolds seeded with equine chondrocytes were differentiated for 2, 4, and 6 weeks. During the culture period, limited matrix deposition was observed. Nevertheless, after 4 weeks of culture, the chondrocytes were dispersed throughout the entire volume of the scaffolds (Fig. 3A). The newly formed matrix did show positive staining for collagen type II (Fig. 3B), as well as some positive staining for collagen type I in the pericellular matrix (Fig. 3D), as was demonstrated by immunolocalization. However, Safranin-O staining revealed that hardly any GAGs were deposited (Fig. 3C). The chondrocyte-seeded constructs degraded during the culture period (2-4 weeks). After 6 weeks, the culture had fully disintegrated and samples could not be further analyzed.
Figure 3.
Chondrocyte-seeded scaffolds after 4 weeks of culture, showing cells on remaining scaffold particles (A, hematoxylin and eosin staining), with collagen type II positive matrix (stains brown in B), which hardly contains glycosaminoglycans (GAGs) (stains red in C). In the pericellular matrix, some staining for collagen type I was noted (stains brown in D). Scale bars represent 200 µm, S = scaffold, arrows indicate cells.
In Vitro Tissue Formation: MSC-Seeded CDM Scaffolds
Cartilage-derived matrix scaffolds with MSCs were cultured for 4 and 6 weeks in chondrogenic differentiation medium. After 4 and 6 weeks of culture, cells were observed throughout the scaffold structure (Fig. 4A and 4E, respectively) and the deposited matrix stained more intensely for GAGs (Fig. 4C and 4G, respectively) than the matrix in the corresponding chondrocyte-seeded CDM constructs (Fig. 3C). After culture, the MSC-seeded tissue constructs were stable on handling and had macroscopically a cartilage-like appearance. In addition, the produced matrix showed intense staining for collagen type II both after 4 weeks (Fig. 4B) and 6 weeks (Fig. 4F). However, at both time points staining for collagen type I was noted at the periphery of the constructs (Fig. 4D and 4H).
Figure 4.

Multipotent stromal cells (MSC)–seeded scaffolds after 4 weeks of culture (top row) and 6 weeks of culture (bottom row). Abundant cartilage matrix production was already noted after 4 weeks and was further increased at 6 weeks of culture (A and E, hematoxylin and eosin staining). The newly formed matrix was positive for glycosaminoglycan (GAG) (C and G, Safranin-O stains GAGs red), collagen type II (brown in B and F) and was in the periphery also positive for collagen type I (brown in D and H). Scale bars represent 200 µm, S = scaffold.
Glycosaminoglycan and DNA analysis
Quantitative GAG and DNA analyses demonstrated an increase in GAG per DNA after culturing both chondrocytes and MSCs, confirming the histological findings (Fig. 5). However, a significant difference was observed between the chondrocyte-seeded group and MSC-seeded group after 4 weeks of culture (P = 0.002), with the latter showing more GAG/DNA.
Discussion
This study aimed to evaluate the chondrogenic potential of chondrocytes and MSCs that were seeded CDM scaffolds that can ultimately be applied to osteochondral defect repair. Previous studies in other fields of regenerative medicine, including reconstructive skin surgery, heart valve replacement, and bladder repair have shown great regenerative potential of ECM-based materials.7-10,12-15,23,26 While the treatment of cartilage defects remains a significant clinical challenge, approaches based on ECM-derived scaffolds have thus far not been extensively studied for this application.20
Our results demonstrate that porous scaffold structures can be generated from decellularized cartilage matrix. Moreover, the outcomes underscore the chondrogenic potential of CDM scaffolds when seeded with MSCs. In contrast, we showed that chondrocyte-seeded constructs do not result in the production of abundant new cartilaginous matrix. Previous studies performed using a similar chondrocyte cell source and expanded (passage 1) under the exact same comditions, confirms the capacity of these cells to form abundant cartilaginous matrix in pellet cultures.27 While chondrocytes are the resident cells of the cartilage tissue and are clinically applied in regenerative approaches for cartilage repair (e.g., via autologous chondrocyte implantation28), our results thus question the presumption that they are efficacious in producing matrix when seeded on scaffolds that potentially resembling their collagen-rich natural habitat. Although some differences in GAG deposition and collagen production have been observed between chondrocytes and MSCs on ECM-based scaffolds.29 MSCs performed substantially better on CDM scaffolds.
Multipotent stromal cells are generally known to have a higher proliferative potential and higher capability to retain their differentiation capacity than chondrocytes,30 and it has been suggested that MSCs intrinsically secrete a higher number of matrix anabolic agents after expansion,30 enhancing their capacity to form new cartilage matrix. Nevertheless, the observed difference is likely also related to the specific composition of the collagen type II–rich CDM scaffolds. Chondrocytes are indeed known to produce catabolic factors, such as matrixmetallo proteinase (MMP)1, MMP3, and MMP13 in response to exposure to collagen type II fragments.31,32 Also, these cells release increased levels of catabolic agents in response to exposure to cartilage ECM components.30 This may have led to a disruption of the delicate balance of anabolic and catabolic cues, resulting in the more rapid scaffold degradation and disintegration observed in the current study. Chondrocyte-seeded scaffolds could hence only be evaluated up to 4 weeks of culture, while MSC-seeded scaffolds did not disintegrate and formed stable tissue constructs during 6 weeks of in vitro culture. The exact mechanisms behind the superior production of cartilage matrix by MSCs on this scaffold have not been addressed yet, as is the generally observed tendency of hypertrophic differentiation by the MSCs. This will be the focus of future investigation.
The major ECM components of native cartilage matrix are collagen type II and GAGs.5 The decellularization protocol used in this study resulted in a predominantly collagen-based scaffold in which the GAGs were not retained. To accomplish full and consistent decellularization of dense tissues such as cartilage, the procedure involves the use of multiple agents. This will inevitably lead to a greater deprivation of ECM and structural integrity than in less dense tissues, such as small intestinal submucosa or urinary bladder walls.7,8,33 For example, the extensive use of the enzyme trypsin, and detergent Triton X-100 have previously been reported to lead to a loss of GAGs.8,34 Using these agents not only leads to effective decellularization, but it also results in extensive GAG loss compared with previously reported decellularization protocols for cartilage.18,19 Whereas in the present study these circumstances did not hamper satisfactory matrix formation in the MSC-seeded scaffolds, it remains to be evaluated whether remnants of GAG in the scaffold could contribute to enhanced chondrogenic differentiation.
Besides affecting cartilage repair, the encouraging in vitro results observed for MSC-seeded constructs may also offer opportunities for regenerative approaches to treat osteochondral defects. These defects are in direct contact with the bone marrow and MSCs could repopulate the scaffold in vivo through cell homing rather than through cell-delivery. This suggests a possible cell-free, “off-the-shelf” application for this scaffold, which might strongly facilitate therapeutic translation. Nevertheless, the potential catabolic effect of the implanted scaffold on the surrounding cartilage tissue should be carefully assessed, despite the fact that preliminary equine pilot studies have not indicated that such damaging effects on the surrounding tissue would occur.20 Moreover, MSCs have the ability to differentiate toward the osteogenic lineage, either directly or via the endochondral route35,36 and can, therefore, also regenerate the bone phase in an osteochondral defect, thus serving a dual purpose. As stated previously, the challenge will be to restrict hypertrophic differentiation and endochondral ossification to the osteogenic layer. The presence of bioactive and bioinductive cues is considered to be the main contributor to the success of biological ECM scaffolds.7,19,20,33 The ECM of small intestinal submucosa, for example, is often used as a biological scaffold material and has been characterized extensively.37-40 This material has been shown to retain endogenous growth factors that remain bioactive after decellularization and sterilization.41-43 Also, the presence of collagen and other structural and functional molecules has been proposed as a contributing factor for cell proliferation, migration, and differentiation.33
The tissue and cells that were used in the present study were all of equine origin, since previous studies have shown that there are clear similarities between equine and human cartilage in both thickness, as well as biochemical composition.44-46 These similarities make the equine model the large animal model of choice to perform in vivo preclinical translational research on osteochondral defect repair.47-49 Moreover, equine patients often develop cartilage or osteochondral lesions due to congenital disorders or traumatic events similar to human patients.50-52 Hence, the development of a new and ECM-based treatment modality may benefit both veterinary and human patients.
We have demonstrated the excellent chondrogenic differentiation capacity of MSCs in CDM scaffolds. The MSCs outperformed chondrocytes in cartilage matrix production when seeded on this scaffold. In addition, the use of CDM scaffolds has the potential to surpass the issues of biodegradability and biocompatibility that may arise with synthetic scaffolds. Also, the natural ECM environment might provide bioactive cues that initiate natural regeneration.
Footnotes
Acknowledgments and Funding: The authors thank Prof. J. Groll (University of Würzburg) for assistance with the electron microscopy. The antibody against collagen type II (II-II6B3), developed by T.F. Linsenmayer, was obtained from the DSHB developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, Iowa. K.B. is supported by the Alexandre Suerman Stipendium from the University Medical Center Utrecht, J.M. is supported by the Dutch Arthritis Foundation and D.G. is supported by a VENI fellowship from the Dutch Technology Foundation, STW, Applied Science Division of NOW, and the Technology Program of the Ministry of Economic Affairs.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by our institutional review board.
References
- 1. Flanigan BD, Harris JD, Trinh TQ, Siston RA, Brophy RH. Prevalence of chondral defects in athletes’ knees: a systematic review. Med Sci Sports Exerc. 2010;42(10):1795-801. [DOI] [PubMed] [Google Scholar]
- 2. Fitzpatrick K, Tokish JM. A military perspective to articular cartilage defects. J Knee Surg. 2011;24(3):159-66. [DOI] [PubMed] [Google Scholar]
- 3. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730-4. [DOI] [PubMed] [Google Scholar]
- 4. Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema T, Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22(2):351-84. [DOI] [PubMed] [Google Scholar]
- 6. Martin I, Miot S, Barbero A, Jakob M, Wendt D. Osteochondral tissue engineering. J Biomech. 2007;40(4):750-65. [DOI] [PubMed] [Google Scholar]
- 7. Gilbert TW. Strategies for tissue and organ decellularization. J Cell Biochem. 2012;113(7):2217-22. [DOI] [PubMed] [Google Scholar]
- 8. Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turner NJ, Yates AJ, Jr, Weber DJ, Qureshi IR, Stolz DB, Gilbert TW, et al. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng Part A. 2010;16(11):3309-17. [DOI] [PubMed] [Google Scholar]
- 10. Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023-30. [DOI] [PubMed] [Google Scholar]
- 11. Haykal S, Zhou Y, Marcus P, Salna M, Machuca T, Hofer SO, et al. The effect of decellularization of tracheal allografts on leukocyte infiltration and of recellularization on regulatory T cell recruitment. Biomaterials. 2013;34(23):5821-32. [DOI] [PubMed] [Google Scholar]
- 12. Armitage S, Seman EI, Keirse MJ. Use of surgisis for treatment of anterior and posterior vaginal prolapse. Obstet Gynecol Int. 2012;2012:376251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D’Onofrio A, Cresce GD, Bolgan I, Magagna P, Piccin C, Auriemma S, et al. Clinical and hemodynamic outcomes after aortic valve replacement with stented and stentless pericardial xenografts: a propensity-matched analysis. J Heart Valve Dis. 2011;20(3):319-25. [PubMed] [Google Scholar]
- 14. Meyer T, Schwarz K, Ulrichs K, Hocht B. A new biocompatible material (Lyoplant) for the therapy of congenital abdominal wall defects: first experimental results in rats. Pediatr Surg Int. 2006;22(4):369-74. [DOI] [PubMed] [Google Scholar]
- 15. Vardanian AJ, Clayton JL, Roostaeian J, Shirvanian V, Da Lio A, Lipa JE, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128(5):403e-410e. [DOI] [PubMed] [Google Scholar]
- 16. Elder BD, Eleswarapu SV, Athanasiou KA. Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials. 2009;30(22):3749-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwarz S, Koerber L, Elsaesser A, Goldberg-Bockhorn E, Seitz AM, Durselen L, et al. Decellularized cartilage matrix as a novel biomatrix for cartilage tissue-engineering applications. Tissue Eng Part A. 2012;18(21-22):2195-209. [DOI] [PubMed] [Google Scholar]
- 18. Yang Q, Peng J, Guo Q, Huang J, Zhang L, Yao J, et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29(15):2378-87. [DOI] [PubMed] [Google Scholar]
- 19. Yang Z, Shi Y, Wei X, He J, Yang S, Dickson G, et al. Fabrication and repair of cartilage defects with a novel acellular cartilage matrix scaffold. Tissue Eng Part C Methods. 2010;16(5):865-76. [DOI] [PubMed] [Google Scholar]
- 20. Benders KEM, van Weeren PR, Badylak SF, Saris DBF, Dhert WJA, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31(3):169-76. [DOI] [PubMed] [Google Scholar]
- 21. Jin CZ, Choi BH, Park SR, Min BH. Cartilage engineering using cell-derived extracellular matrix scaffold in vitro. J Biomed Mater Res A. 2010;92(4):1567-77. [DOI] [PubMed] [Google Scholar]
- 22. Jin CZ, Park SR, Choi BH, Park K, Min BH. In vivo cartilage tissue engineering using a cell-derived extracellular matrix scaffold. Artif Organs. 2007;31(3):183-92. [DOI] [PubMed] [Google Scholar]
- 23. Gong YY, Xue JX, Zhang WJ, Zhou GD, Liu W, Cao Y. A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials. 2011;32(9):2265-73. [DOI] [PubMed] [Google Scholar]
- 24. Jia S, Liu L, Pan W, Meng G, Duan C, Zhang L, et al. Oriented cartilage extracellular matrix-derived scaffold for cartilage tissue engineering. J Biosci Bioeng. 2012;113(5):647-53. [DOI] [PubMed] [Google Scholar]
- 25. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-7. [DOI] [PubMed] [Google Scholar]
- 26. Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29(8):690-700. [DOI] [PubMed] [Google Scholar]
- 27. Schuurman W, Harimulyo EB, Gawlitta D, Woodfield TB, Dhert WJA, van Weeren PR, et al. Three-dimensional assembly of tissue-engineered cartilage constructs results in cartilaginous tissue formation without retainment of zonal characteristics. J Tissue Eng Regen Med. Epub 2013. April 18. [DOI] [PubMed] [Google Scholar]
- 28. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. [DOI] [PubMed] [Google Scholar]
- 29. Giavaresi G, Bondioli E, Melandri D, Giardino R, Tschon M, Torricelli P, et al. Response of human chondrocytes and mesenchymal stromal cells to a decellularized human dermis. BMC Musculoskelet Disord. 2013;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polacek M, Bruun JA, Elvenes J, Figenschau Y, Martinez I. The secretory profiles of cultured human articular chondrocytes and mesenchymal stem cells: implications for autologous cell transplantation strategies. Cell Transplant. 2011;20(9):1381-93. [DOI] [PubMed] [Google Scholar]
- 31. Fichter M, Korner U, Schomburg J, Jennings L, Cole AA, Mollenhauer J. Collagen degradation products modulate matrix metalloproteinase expression in cultured articular chondrocytes. J Orthop Res. 2006;24(1):63-70. [DOI] [PubMed] [Google Scholar]
- 32. Klatt AR, Paul-Klausch B, Klinger G, Kuhn G, Renno JH, Banerjee M, et al. A critical role for collagen II in cartilage matrix degradation: collagen II induces pro-inflammatory cytokines and MMPs in primary human chondrocytes. J Orthop Res. 2009;27(1):65-70. [DOI] [PubMed] [Google Scholar]
- 33. Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5(1):1-13. [DOI] [PubMed] [Google Scholar]
- 34. Gilbert T, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675-83. [DOI] [PubMed] [Google Scholar]
- 35. Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(Suppl 1):S58-S65. [DOI] [PubMed] [Google Scholar]
- 36. Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54(10):3254-66. [DOI] [PubMed] [Google Scholar]
- 37. Hodde J, Janis A, Ernst D, Zopf D, Sherman D, Johnson C. Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J Mater Sci Mater Med. 2007;18(4):537-43. [DOI] [PubMed] [Google Scholar]
- 38. Hodde J, Janis A, Hiles M. Effects of sterilization on an extracellular matrix scaffold: part II. Bioactivity and matrix interaction. J Mater Sci Mater Med. 2007;18(4):545-50. [DOI] [PubMed] [Google Scholar]
- 39. Hodde J, Janis A, Hiles M. Fibronectin peptides mediate HMEC adhesion to porcine-derived extracellular matrix. Biomaterials. 2002;23(8):1841-8. [DOI] [PubMed] [Google Scholar]
- 40. Hodde JP, Badylak SF, Brightman AO, Voytik-Harbin SL. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng. 1996;2(3):209-17. [DOI] [PubMed] [Google Scholar]
- 41. McDevitt CA, Wildey GM, Cutrone RM. Transforming growth factor-beta1 in a sterilized tissue derived from the pig small intestine submucosa. J Biomed Mater Res A. 2003;67(2):637-40. [DOI] [PubMed] [Google Scholar]
- 42. Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8(1):11-24. [DOI] [PubMed] [Google Scholar]
- 43. Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67(4):478-91. [PubMed] [Google Scholar]
- 44. Frisbie DD, Cross MW, McIlwraith CW. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. 2006;19(3):142-6. [PubMed] [Google Scholar]
- 45. Shepherd DE, Seedhom BB. Thickness of human articular cartilage in joints of the lower limb. Ann Rheum Dis. 1999;58(1):27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Malda J, Benders KEM, Klein TJ, de Grauw JC, Kik MJ, Hutmacher DW, et al. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthritis Cartilage. 2012;20(10):1147-51. [DOI] [PubMed] [Google Scholar]
- 47. Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16(1):105-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hurtig MB, Buschmann MD, Fortier LA, Hoemann CD, Hunziker EB, Jurvelin JS, et al. Preclinical studies for cartilage repair: recommendations from the international cartilage repair society. Cartilage. 2011;2(2):137-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McIlwraith CW, Fortier LA, Frisbie DD, Nixon AJ. Equine models of articular cartilage repair. Cartilage. 2011;2(4):317-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Weeren PR. Osteochondrosis. In: Auer JA, Stick JA, editors. Equine surgery. St Louis, MO: Saunders; 2006. p. 1166-78. [Google Scholar]
- 51. Voute LC, Henson FM, Platt D, Jeffcott LB. Osteochondrosis lesions of the lateral trochlear ridge of the distal femur in four ponies. Vet Rec. 2011;168(10):265. [DOI] [PubMed] [Google Scholar]
- 52. Ytrehus B, Carlson CS, Ekman S. Etiology and pathogenesis of osteochondrosis. Vet Pathol. 2007;44(4):429-48. [DOI] [PubMed] [Google Scholar]