Abstract
Inhaled glucocorticoids acting via the glucocorticoid receptor are a mainstay treatment option for individuals with asthma. There is a consensus that the remedial actions of inhaled glucocorticoids are due to their ability to suppress inflammation by modulating gene expression. While inhaled glucocorticoids are generally effective in asthma, there are subjects with moderate-to-severe disease in whom inhaled glucocorticoids fail to provide adequate control. For these individuals, asthma guidelines recommend that a long-acting β2-adrenoceptor agonist (LABA) be administered concurrently with an inhaled glucocorticoid. This so-called “combination therapy” is often effective and clinically superior to the inhaled glucocorticoid alone, irrespective of dose. LABAs, and another class of drug known as phosphodiesterase 4 (PDE4) inhibitors, may also enhance the efficacy of inhaled glucocorticoids in chronic obstructive pulmonary disease (COPD). In both conditions, these drugs are believed to work by elevating the concentration of cyclic adenosine-3',5'-monophosphate (cAMP) in target cells and tissues. Despite the success of inhaled glucocorticoid/LABA combination therapy, it remains unclear how an increase in cAMP enhances the clinical efficacy of an inhaled glucocorticoid. In this report, we provide a state-of-the-art appraisal, including unresolved and controversial issues, of how cAMP-elevating drugs and inhaled glucocorticoids interact at a molecular level to deliver enhanced anti-inflammatory benefit over inhaled glucocorticoid monotherapy. We also speculate on ways to further exploit this desirable interaction. Critical discussion of how these two drug classes regulate gene transcription, often in a synergistic manner, is a particular focus. Indeed, because interplay between glucocorticoid receptor and cAMP signaling pathways may contribute to the superiority of inhaled glucocorticoid/LABA combination therapy, understanding this interaction may provide a logical framework to rationally design these multicomponent therapeutics that was not previously possible.
Introduction
Asthma is a complex inflammatory disorder of the airways and lungs for which inhaled glucocorticoids – commonly referred to as corticosteroids or simply steroids – are a recommended treatment option (www.ginasthma.org). Most patients with asthma are responsive to the remedial actions of inhaled glucocorticoids. However, a proportion of individuals with moderate-to-severe disease, in whom inflammation is pronounced, are not effectively managed by inhaled glucocorticoids regardless of dose. In these cases, asthma guidelines recommend that a LABA be administered concurrently with an inhaled glucocorticoid as a combination therapy [1,2]. This often provides asthma control and is clinically superior to the inhaled glucocorticoid alone using a variety of outcome measures, including lung function, symptoms, the need for rescue medication and the frequency of exacerbations [3–5]. Inhaled glucocorticoid/LABA combination therapy given in a single inhaler device has been extremely successful, with Advair®/Seretide® (fluticasone propionate plus salmeterol xinafoate) being ranked third in 2010 in the top 10 drugs based on sales [6]. This success has fuelled the development of second generation inhaled glucocorticoid/LABA combination therapy, which has a longer duration of action suitable for once-a-day dosing that may translate into improved patient compliance and, hence, asthma control [7]. Inflammation of the airways combined with systemic inflammation is also a cardinal feature of COPD, and there is evidence that in certain patient populations inhaled glucocorticoid/LABA combination therapy is clinically superior to both LABA and inhaled glucocorticoid monotherapy [8–11]. Indeed, patients of a severe bronchitic phenotype, who have pronounced inflammation, are most responsive to this intervention using several metrics, including frequency of hospitalizations, exacerbation rate, inflammatory markers, lung function, and quality of life [8–12]. Additional clinical benefit may also be produced by the PDE4 inhibitor, roflumilast, when combined with an inhaled glucocorticoid in patients with COPD of the same phenotype [13].
The mechanism(s) underlying the superiority of these multicomponent therapeutics is unknown. A widely held view is that a LABA, in addition to promoting long-lasting bronchodilatation, enhances the anti-inflammatory actions of an inhaled glucocorticoid in a cAMP-dependent manner [2,14–16]. PDE4 inhibitors are likely to work in a similar way [17]. It is believed that an inhaled glucocorticoid acting via the glucocorticoid receptor suppresses inflammation by modifying gene expression [16]. Two general paradigms have been proposed. One of these is called transrepression, where the glucocorticoid receptor binds to and inhibits the activity of transcription factors responsible for activating pro-inflammatory genes. The other process is transactivation, in which the glucocorticoid receptor promotes the transcription of anti-inflammatory genes [16,18–23]. Despite inhaled glucocorticoid/LABA combination therapy being available since 1998, unresolved and controversial issues remain. How activation of the β2-adrenoceptor on pro-inflammatory and immune cells in the lung augments glucocorticoid action and whether anything else can be done to further enhance clinical efficacy are particularly important areas of research. Certainly, β2-adrenoceptor agonists produce several, potentially unwanted, effects, including pro-inflammatory cytokine production, which are reduced or prevented by a glucocorticoid [2,16]. Equally, glucocorticoids can increase β2-adrenoceptor expression and arrest desensitization, which should preserve the beneficial actions of a LABA [2,16]. However, while these interactions are demonstrable in isolated cells and tissues, their clinical relevance is unclear and they are unlikely to explain the superiority of inhaled glucocorticoid/LABA combination therapy in asthma and COPD. In this report, we focus on the probability that cAMP directly enhances the anti-inflammatory activity of an inhaled glucocorticoid by up-regulating glucocorticoid receptor-mediated signaling leading to improved clinical outcomes. This tenet is consistent with clinical data showing improved therapeutic activity when pulmonary co-deposition of both drugs is maximised (vide infra).
How does a LABA enhance the clinical effects of an inhaled glucocorticoid?
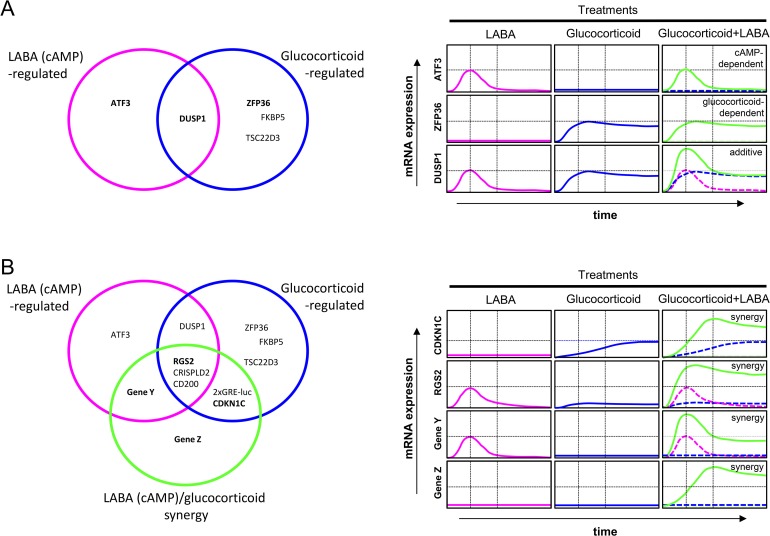
Conceptually, two mutually inclusive possibilities could explain the clinical superiority of inhaled glucocorticoid/LABA combination therapy. Simplistically, a LABA and a glucocorticoid may act in a purely additive manner by exerting a variety of independent, non-interacting responses that may or may not be sensitive to either drug alone (Figure 1A). The induction of DUSP1 (dual specificity phosphatase 1), a putative anti-inflammatory gene encoding a phosphatase responsible for inactivating mitogen-activated protein kinases that are typically “switched on” by pro-inflammatory stimuli [24], is a good example [25,26] (Figure 1A). However, while simple additive interactions undoubtedly occur, the clinical data also accommodate the idea of synergy between the glucocorticoid receptor and cAMP signaling pathways (Figure 1B). For example, RGS2 (regulator of G-protein signaling 2), like DUSP1, is a gene whose expression in airway smooth muscle and epithelial cells is induced by a LABA [27,28] and, to a lesser degree, a glucocorticoid [27–30]. However, unlike DUSP1, RGS2 expression is increased synergistically (defined here as a response produced by two drugs in a combination that is greater than the sum of their individual effects) when these agents are used concurrently [27] (Figure 1B). RGS2 is a GTPase-activating protein that terminates agonist-induced signaling mediated by G-protein-coupled receptors that work through Gq [31,32]. In inflammatory lung diseases, RGS2 exerts effects that may be interpreted as beneficial, such as bronchoprotection and the down-regulation of pro-inflammatory signaling, including those pathways that up-regulate mucus (MUC5AC) production [27,28,33,34]. Other genes that have potential anti-inflammatory activity, such as CD200 and CRISPLD2 [35–38], are also induced in a synergistic manner by a LABA and a glucocorticoid in combination [39], suggesting that this molecular interaction may be commonplace.
Figure 1. Interactions and effects on gene expression that may occur between long-acting β2-adrenoceptor agonists, or other cAMP-elevating agents, and glucocorticoids.
A: Additive effects of long-acting β2-adrenoceptor agonists (LABAs) and glucocorticoids.
LABAs and glucocorticoids each induce a set of responses. These sets overlap and responses in the intersection may reveal additivity. Thus, mRNA expression for the gene, ATF3, may be induced in a LABA-dependent, glucocorticoid-independent manner. Conversely, glucocorticoids induce the mRNA expression of multiple genes (e.g., ZFP36, aka tristetraprolin), and the expression of these may be unaffected by LABA. Finally, gene expression, for example DUSP1, may be up-regulated by both a LABA and a glucocorticoid and with concurrent treatment these two effects may show simple additivity.
B: Synergistic interactions between LABAs and glucocorticoids.
In addition to independent effects of LABAs and glucocorticoids on gene expression, there are a set of responses (genes) whose expression is synergistically enhanced by the combination of LABA plus glucocorticoid. In such situations there is synergy between these two pathways. Four general possibilities exist. Genes, such as CDKN1C, may show no material effect of the LABA, yet be induced by the glucocorticoid in a manner that is synergistically enhanced by the LABA. Genes, such as RGS2, are modestly induced by both LABAs and glucocorticoids, yet in combination there is very considerable enhanced mRNA expression. Conversely, it is theoretically possible that a LABA–inducible gene, depicted here as gene Y, may be enhanced by a glucocorticoid but is, nevertheless, insensitive to the glucocorticoid alone. Alternatively, other genes, here gene Z, may be induced only by an inhaled glucocorticoid and LABA in combination but not by either drug alone. With the exception of ATF3, gene expression data are taken from references 25 and 28. Genes Y and Z are hypothetical.
Abbreviations: ATF3, activating transcription factor 3; CDKN1C, cyclin-dependent kinase inhibitor 1C; DUSP1, dual specificity phosphatase 1.
Another, mechanistically distinct form of synergy has been demonstrated in cells transfected with luciferase reporters that respond only to glucocorticoids [25,40]. In human bronchial epithelial cells and airway myocytes, glucocorticoid receptor-mediated reporter activation is significantly enhanced by a LABA [25]. In these same cell types, this effect is also reproduced at the level of gene expression with CDKN1C, which encodes a cell cycle kinase inhibitor that could be beneficial in asthma and COPD [41,42], being a representative example [25,43]. Thus, there are glucocorticoid-induced responses that are up-regulated by a LABA despite the LABA alone being inactive (Figure 1B). Theoretically, the opposite profile could be displayed whereby a glucocorticoid augments the effect of a LABA but is inactive itself. Responses that have an absolute requirement for LABA and glucocorticoid are also possible (Figure 1B).
Currently, the molecular mechanism underlying the inhaled glucocorticoid/LABA interaction remains elusive, with multiple, potentially conflicting, ideas having been proposed. In terms of understanding this effect, most studies have focused on the ability of cAMP to enhance glucocorticoid receptor function. Indeed, data from the 1990s show that activation of the cAMP pathway enhances glucocorticoid receptor-mediated transcription [44–47]. However, at that time, there was a lack of clarity regarding the way in which cAMP and glucocorticoid receptors interact at a molecular level. Indeed, evidence for and against the idea that cAMP enhances the binding of the glucocorticoid receptor to DNA is available [44–47]. This controversy has been perpetuated with claims and counter-claims that cAMP-elevating drugs, including LABAs, enhance the translocation of the glucocorticoid receptor to the nucleus [45,48–53]. Variable effects of cAMP on glucocorticoid receptor expression levels and glucocorticoid receptor binding have also added to the confusion [45–47].
In considering how a LABA could enhance glucocorticoid receptor-mediated signaling, an appreciation of possible cell- and system-dependent effects should not be overlooked. Nevertheless, it is clear from data garnered in single cell types (e.g. airway epithelial cells) that some glucocorticoid-induced genes are not affected by a LABA, whereas other genes are enhanced in an additive or often synergistic manner [25]. Such observations correspondingly require explanations that accommodate gene-specific regulation rather than a global modification of glucocorticoid receptor function. For example, the RGS2 promoter has a putative glucocorticoid receptor binding region [54] and functional sites for the cAMP-regulated transcription factor (CREB) [55]. Thus, the concurrent binding of the glucocorticoid receptor and CREB to DNA in a target gene may very well lead to transcriptional synergy. Equally, a number of other transcription factors (e.g. CCAAT-enhancer-binding proteins) can be induced by glucocorticoid and cAMP, ultimately leading to transcriptional synergies at complex glucocorticoid receptor-binding sites in certain gene promoters [40]. Further potential for gene-specific transcriptional regulation is provided by the finding that cAMP modulates the behaviour of obligatory co-activators and/or co-repressors that are known to play roles in transcriptional programming mediated by a variety of nuclear hormone receptors, including the glucocorticoid receptor [56–60]. For example, cAMP augments progesterone receptor-mediated gene expression by a mechanism that involves the phosphorylation and dissociation of nuclear co-repressors 1 and 2, rendering the ligand-bound receptor more transcriptionally competent [60,61]. Similar regulation of the glucocorticoid receptor seems likely.
The importance of pharmacodynamics
Inhaled glucocorticoids acting through the glucocorticoid receptor exert a pleiotropic suppressive effect of many cell types that collectively promote the chronic inflammation that characterises asthma and COPD. However, glucocorticoid receptor density varies considerably between different human tissues [62–64] and this has important pharmacodynamic implications. The ability of an inhaled glucocorticoid to produce a response is, in its simplest form, the product of intrinsic efficacy (a sole property of the drug determined by its structure) and the number of functional glucocorticoid receptors in a given target (a tissue-dependent parameter). Therefore, glucocorticoid receptor density is a key determinant of the magnitude of gene transactivation and transrepression that can be produced by a given glucocorticoid [65–67]. Equally, at constant glucocorticoid receptor number in a given cell type, response will also be determined by the structure of the glucocorticoid and whether or not the glucocorticoid receptor forms an optimal or suboptimal conformation to effect transrepression or bind DNA and transactivate a particular gene. In pharmacological terms, these parameters will define a glucocorticoid as a full agonist, partial agonist or even antagonist in a tissue- and response-dependent manner. In considering gene transactivation as a functionally relevant, anti-inflammatory output, it is clear that the gene expression “fingerprint” produced by different glucocorticoid receptor agonists across a panel of target tissues will not be invariant and could provide a basis for the pharmaceutical industry to rationally design new inhaled glucocorticoid or inhaled glucocorticoid/LABA combination therapy [16]. Clearly, compounds would have to be screened in target and off-target human tissues and the physiological role(s) of genes in the “fingerprint” defined. With this knowledge, glucocorticoids could be identified and selected to preferentially induce genes with anti-inflammatory activity, while at the same time minimising those that mediate unwanted effects.
The ability of a LABA to enhance anti-inflammatory glucocorticoid activity will also be governed by the same pharmacodynamic principles that apply to a glucocorticoid receptor, as well as the efficiency with which the β2-adrenoceptor can generate cAMP. There are ~30,000-40,000 β2-adrenoceptors on a human airway smooth muscle cell [68], whereas, on human neutrophils and T-lymphocytes, functional receptor expression is considerably (30 to 50 fold) lower [69,70]. Logic dictates that the enhancement of anti-inflammatory glucocorticoid activity by a LABA may be modest, or even absent, in a cell where β2-adrenoceptor number is limiting. Weak receptor-adenylyl cyclase coupling in a particular pro-inflammatory or immune cell type may also render a LABA with low intrinsic efficacy, such as salmeterol, unable to enhance glucocorticoid activity. For example, although eosinophils express a moderate number of β2-adrenoceptors (~4300 sites/cell) [71], their ability to couple to adenylyl cyclase is apparently weak, such that salmeterol behaves as an antagonist [72,73]. Based on the aforementioned discussion, it may not be unreasonable to speculate that the relatively poor activity of an inhaled glucocorticoid or an inhaled glucocorticoid/LABA combination therapy in COPD is due, in part, to the involvement of immune and pro-inflammatory cells that express, intrinsically, a low density of functional glucocorticoid receptors. Similar insensitivity may also result if the β2-adrenoceptor population on those same cells is low and/or are coupled inefficiently to adenylyl cyclase (vide infra). However, the possibility that exposure of target cells and tissues to cigarette smoke, a primary cause of COPD, reduces the expression of, and signaling mediated by, these receptors is also plausible and should be considered.
Enhancing the anti-inflammatory effects of glucocorticoids with PDE inhibitors
While asthma is often controlled with an inhaled glucocorticoid or an inhaled glucocorticoid/LABA combination therapy, COPD is considerably less responsive to the anti-inflammatory actions of these drugs. Accordingly, there is a clear unmet clinical need for more effective therapies. The appreciation of distinct COPD phenotypes [74–77] allows patients to be classified using a variety of criteria, including “response to treatment”. Patients with severe disease, who experience frequent exacerbations and have a history of productive cough or expectoration, represent a COPD phenotype that may respond to PDE4 inhibitors, such as roflumilast (as well as inhaled glucocorticoid/LABA combination therapy). Indeed, patients with this form of COPD often exhibit pronounced inflammation [78], suggesting that they are most likely to respond to anti-inflammatory drugs [79]. Moreover, because LABAs and PDE4 inhibitors act on the same signaling pathway (i.e. they increase cAMP synthesis and block cAMP degradation, respectively), they may act synergistically. Thus, conceptually, the high level of inflammation seen in severe, bronchitic COPD [78] may be more responsive to an inhaled glucocorticoid, LABA and PDE4 inhibitor if used together rather than individually. This assumption, which is being tested in phase IV clinical trials [80], is reflected in current treatment guidelines, which recommend that roflumilast be given to patients already taking an inhaled glucocorticoid/LABA combination therapy (www.goldcopd.org).
In the previous section, we speculated that the inability of a LABA alone to optimally enhance glucocorticoid activity in patients with COPD in whom inflammation is prevalent may be due to a dominant participation of immune and/or pro-inflammatory cell types where β2-adrenoceptor number is limiting. If this is true then, theoretically, this insensitivity could be overcome, partially or even totally, with a PDE4 inhibitor in the form of a triple combination therapy [17]. Indeed, a PDE4 inhibitor, by blocking cAMP degradation, might transform a cell that exhibits weak sensitivity to a LABA into one that now generates a cAMP signal of sufficient magnitude to enhance anti-inflammatory glucocorticoid activity above the level produced by an inhaled glucocorticoid/LABA combination therapy alone.
Recently, there has been renewed interest in targeting PDE3, another family of cAMP PDEs, in inflammatory lung diseases [81]. Tight-binding PDE3 inhibitors, such as the highly selective compound RPL554 [82], have been evaluated in clinical trials of asthma and COPD with promising results [83]. Although it is unclear if a PDE3 inhibitor can enhance the anti-inflammatory effects of an inhaled glucocorticoid in the clinical setting (vide infra), it has been shown that combining a PDE3 and PDE4 inhibitor sensitizes human airway epithelial cells to a LABA and extends the duration of glucocorticoid- and LABA-induced gene expression, relative to a PDE4 inhibitor alone [84]. Such data, if reproduced in vivo, might prolong anti-inflammatory activity and give rise to inhaled glucocorticoid/LABA combination therapy being administered concurrently with inhibitors of multiple PDEs.
Novel combination therapies
The ability of LABAs and PDE4 inhibitors to enhance glucocorticoid receptor-mediated gene expression is reproduced by several but not all agents that increase cAMP [25,85–89]. Agonists of the EP2-, EP4- and IP prostanoid receptors and the adenosine A2B-receptor subtype all display this property [87,89], whereas inhibitors of PDE2, PDE3 and PDE7 do not [39,90]. Theoretically, a more generic effect of cAMP-elevating drugs on glucocorticoid receptor-mediated gene expression could be exploited to therapeutic advantage in inflammatory diseases where perhaps β2-adrenoceptor agonists are poorly effective, due to low functional receptor expression or weak coupling to adenylyl cyclase in target cells and tissues. In this context, detailed knowledge of G protein-coupled receptor expression could allow various cAMP-elevating agonists to be “added-on” to an inhaled glucocorticoid, depending on the specific inflammatory disease of interest. Such effects could be further enhanced by identifying receptors that may be less prone to agonist-induced desensitization and/or by “adding on” one or more PDE inhibitors.
Maximising efficacy by ensuring drug co-deposition: development of conjugated pro-drugs and bifunctional ligands
The ability of a LABA (and PDE4 inhibitor) to augment the anti-inflammatory actions of an inhaled glucocorticoid requires that these drugs reach the same target cells and tissues at concentrations that allow them to interact at a molecular level (vide supra). This is not a trivial point. Biophysical analyses have revealed significantly greater pulmonary co-deposition of an inhaled glucocorticoid and LABA when delivered by a combination meter dose inhaler than if each drug is given separately [91]. Moreover, this optimised delivery translates into a significantly greater improvement in lung function [92,93]. Thus, the co-deposition of both components of an inhaled glucocorticoid/LABA combination therapy is necessary if maximum therapeutic benefit is to be realised. Intuitively, this is more likely to occur when both drugs are inhaled in a single breath from a single inhaler device, as this would facilitate the ability of the LABA and inhaled glucocorticoid to interact. However, even this method of delivery is unlikely to provide optimal drug co-deposition, necessitating the development of alternative approaches to achieve this goal. Two possibilities are discussed here: conjugated pro-drugs and bifunctional ligands.
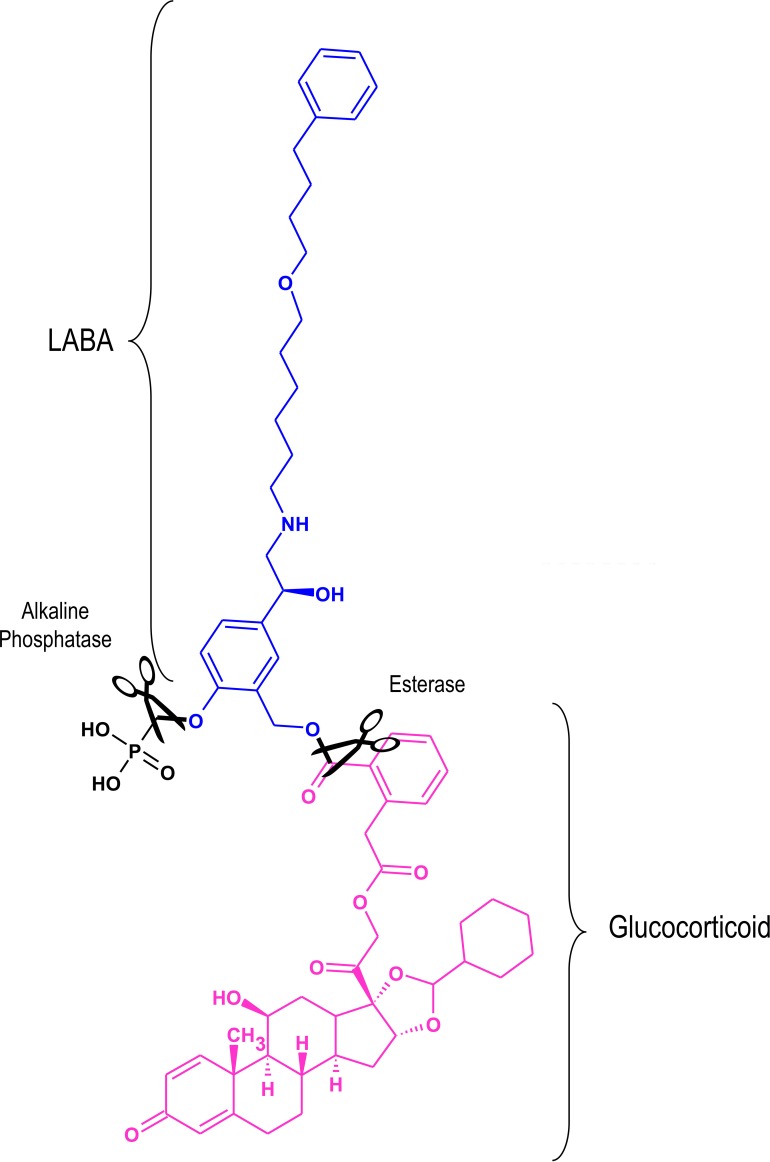
A pro-drug is an inactive or weakly active molecule that typically undergoes enzymatic activation in vivo [94]. The patent literature contains several claims for mutual inhaled pro-drugs consisting of a glucocorticoid and a LABA [95,96]. An example is shown in Figure 2 in which salmeterol is conjugated to a derivative of the inhaled glucocorticoid, desisobutyryl-ciclesonide. This particular pro-drug is activated by alkaline phosphatase and an esterase, which are enriched in the lung relative to saliva and plasma. In theory, conjugated pro-drugs of this form would ensure co-deposition and selective activation in the lung with an improved side-effect profile.
Figure 2. An example of a conjugated inactive long-acting β2-adrenoceptor agonist glucocorticoid pro-drug.
A phosphorylated form of salmeterol (blue) is shown conjugated to a derivative of the glucocorticoid, desisobutyryl-ciclesonide (pink). In vivo, the phosphate and ester bonds (in black) are cleaved (scissors) by alkaline phosphatase and esterases respectively to yield the active components in a 1:1 ratio. Adapted from [97].
Abbreviations: LABA, long-acting β2-adrenoceptor agonists.
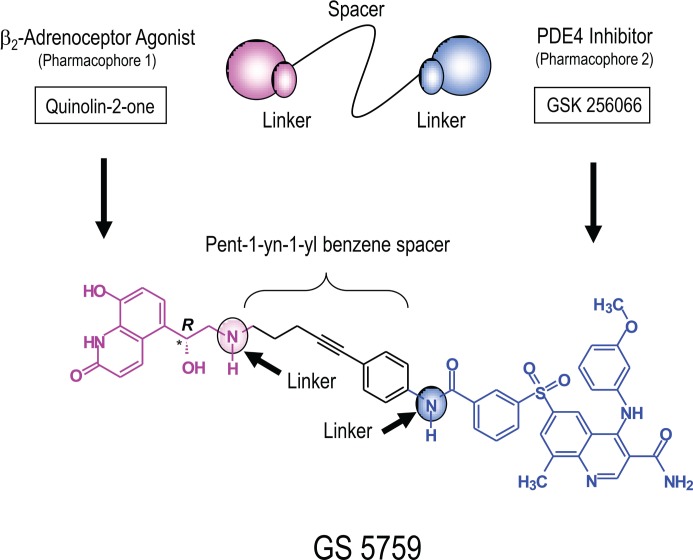
In contrast, bifunctional ligands are single chemical entities that contain two pharmacophores joined covalently by an optimally designed “spacer” at, so-called, linker sites (Figure 2) [97,98,99]. Typically, such ligands have a high molecular weight. For an inhaled drug, this often translates into greater lung retention, low oral bioavailability and reduced systemic exposure [98]. Moreover, the clinical development of bifunctional ligands is simplified in terms of matched pharmacokinetics, formulation and, critically, identical deposition characteristics [99]. GS-5759 is a novel, heterobifunctional ligand developed by Gilead Sciences [100] for COPD in which the exceptionally potent PDE4 inhibitor, GlaxoSmithKline (GSK) 256066 [101], is conjugated to the active head group of the LABAs, indacaterol and carmoterol, via a pent-1-yn-1yl benzene spacer (Figure 3) [100,102,103]. This molecule has a similar potency at both targets and has been optimised for inhaled delivery with potential as a first-line therapy in COPD or combined with an inhaled glucocorticoid as part of a triple combination therapy within a single inhaler device. Clearly, the cAMP signal generated by concurrent PDE4 inhibition and β2-adrenoceptor activation produced by a single molecule in the same cell over a similar concentration range would allow a greater therapeutic benefit of a glucocorticoid to be realised, especially in target tissues in which β2-adrenoceptor number is limiting.
Figure 3. Structure of the heterobifunctional ligand GS-5759.
Bifunctional ligands contain two pharmacophores that are joined covalently by an appropriately designed “spacer” at, so-called, linker sites (cartoon). GS-5759 is composed of the quinolin-2-one present in the long-acting β2-adrenoceptor agonists (LABAs) indacaterol and carmoterol (pink), and the phosphodiesterase 4 (PDE4) inhibitor GlaxoSmithKline (GSK) 256066 (blue) that have been linked covalently by a pent-1-yn-1-yl benzene spacer (black). The asterisk indicates chiral centre. Adapted from [97].
Concluding remarks
The use of inhaled glucocorticoid/LABA combination therapy in the control of asthma and COPD is entrenched in all national and international treatment guidelines. While the mechanism of action of combination therapy is not understood, the balance of evidence supports the idea that the regulation of glucocorticoid-induced transcription by cAMP is gene-specific rather than a global, non-selective effect on the expression of the “glucocorticoid transcriptome”. Pharmacodynamic analyses predict that gene expression will differ (maybe considerably) in both a tissue and glucocorticoid/LABA-dependent manner. While considerable investigation is still necessary to achieve a systematic characterization of these effects, such knowledge will provide significant opportunity to rationally design new inhaled glucocorticoid/LABA combination therapy perhaps with a more optimised, effective and safer gene expression profile. Gene transrepression is also believed to contribute to the remedial actions of glucocorticoids in inflammatory lung diseases. However, the relationship between this process and transactivation remains unclear [19,20] and, currently, there is little direct evidence that a LABA and a glucocorticoid transrepress gene expression in a synergistic manner. Nevertheless, the ability of glucocorticoids to repress inflammatory gene expression can often require glucocorticoid receptor-dependent transactivation. Therefore, under these circumstances, beneficial interactions between an inhaled glucocorticoid and a LABA are a real possibility. Based on the information presented herein, we propose that a detailed molecular appreciation and pharmacodynamic understanding of the changes in gene expression induced by glucocorticoids and LABAs in relevant target and off-target cells and tissues will provide considerable new opportunities to improve the clinical efficacy of inhaled glucocorticoid/LABA combination therapy in asthma and COPD.
Acknowledgments
Robert Newton is an Alberta Innovates – Health Solutions Senior Scholar. Mark A. Giembycz holds a Tier 1 Canada Research Chair in Pulmonary Pharmacology. Work in the laboratories of Robert Newton and Mark A. Giembycz is/was supported by operating grants awarded by the Canadian Institutes of Health Research (MOP 68828 and MOP 93742 respectively), the Lung Association, Alberta and North West Territories. A grant from the Canadian Fund for Innovation and the Alberta Science and Research Authority provided equipment and infrastructure for performing real-time PCR.
Abbreviations
- cAMP
cyclic adenosine-3',5'-monophosphate
- COPD
chronic obstructive pulmonary disease
- LABA
long-acting β2-adrenoceptor agonist
- PDE
phosphodiesterase
Disclosures
Robert Newton's and Mark A. Giembycz's laboratories are/was supported by operating grants awarded by Astra Zeneca, Gilead Sciences, GlaxoSmithKline and Takeda/Nycomed.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/7/16
References
- 1.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. The European respiratory journal. 2008;31:143–78. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. The European respiratory journal. 2002;19:182–91. doi: 10.1183/09031936.02.00283202. [DOI] [PubMed] [Google Scholar]
- 3.Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. Allen & Hanburys Limited UK Study Group. Lancet. 1994;344:219–24. doi: 10.1016/S0140-6736(94)92996-3. [DOI] [PubMed] [Google Scholar]
- 4.Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. American journal of respiratory and critical care medicine. 1996;153:1481–8. doi: 10.1164/ajrccm.153.5.8630590. [DOI] [PubMed] [Google Scholar]
- 5.Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, Ullman A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. The New England journal of medicine. 1997;337:1405–11. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 6.Arrowsmith J. A decade of change. Nature reviews. Drug discovery. 2012;11:17–8. doi: 10.1038/nrd3630. [DOI] [PubMed] [Google Scholar]
- 7.Cazzola M, Calzetta L, Matera MG. β2-adrenoceptor agonists: current and future direction. British journal of pharmacology. 2011;163:4–17. doi: 10.1111/j.1476-5381.2011.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. The Cochrane database of systematic reviews. 2012;9:CD006829. doi: 10.1002/14651858.CD006829.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717958451
- 9.Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. The Cochrane database of systematic reviews. 2014;3:CD010844. doi: 10.1002/14651858.CD010844.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718326329
- 10.Barnes NC, Qiu Y, Pavord ID, Parker D, Davis PA, Zhu J, Johnson M, Thomson NC, Jeffery PK. Antiinflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. American journal of respiratory and critical care medicine. 2006;173:736–43. doi: 10.1164/rccm.200508-1321OC. [DOI] [PubMed] [Google Scholar]
- 11.Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, Vestbo J, Knobil K, Yates JC, Calverley Peter MA. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. American journal of respiratory and critical care medicine. 2008;178:332–8. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 12.Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden C. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 13.Rennard SI, Calverley Peter MA, Goehring UM, Bredenbröker D, Martinez FJ. Reduction of exacerbations by the PDE4 inhibitor roflumilast—the importance of defining different subsets of patients with COPD. Respiratory research. 2011;12:18. doi: 10.1186/1465-9921-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giembycz MA, Kaur M, Leigh R, Newton R. A Holy Grail of asthma management: toward understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids. British journal of pharmacology. 2008;153:1090–104. doi: 10.1038/sj.bjp.0707627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung KF, Caramori G, Adcock IM. Inhaled corticosteroids as combination therapy with beta-adrenergic agonists in airways disease: present and future. European journal of clinical pharmacology. 2009;65:853–71. doi: 10.1007/s00228-009-0682-z. [DOI] [PubMed] [Google Scholar]
- 16.Newton R, Leigh R, Giembycz MA. Pharmacological strategies for improving the efficacy and therapeutic ratio of glucocorticoids in inflammatory lung diseases. Pharmacology & therapeutics. 2010;125:286–327. doi: 10.1016/j.pharmthera.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Giembycz MA, Newton R. How phosphodiesterase 4 inhibitors work in patients with chronic obstructive pulmonary disease of the severe, bronchitic, frequent exacerbator phenotype. Clinics in chest medicine. 2014;35:203–17. doi: 10.1016/j.ccm.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 18.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocrine reviews. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 19.Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacology & therapeutics. 2012;134:54–67. doi: 10.1016/j.pharmthera.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Newton R, Holden NS. Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Molecular pharmacology. 2007;72:799–809. doi: 10.1124/mol.107.038794. [DOI] [PubMed] [Google Scholar]
- 21.Clark AR. Anti-inflammatory functions of glucocorticoid-induced genes. Molecular and cellular endocrinology. 2007;275:79–97. doi: 10.1016/j.mce.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 22.King EM, Chivers JE, Rider CF, Minnich A, Giembycz MA, Newton R. Glucocorticoid repression of inflammatory gene expression shows differential responsiveness by transactivation- and transrepression-dependent mechanisms. PloS one. 2013;8:e53936. doi: 10.1371/journal.pone.0053936. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717977187
- 23.Kelly MM, King EM, Rider CF, Gwozd C, Holden NS, Eddleston J, Zuraw B, Leigh R, O'Byrne PM, Newton R. Corticosteroid-induced gene expression in allergen-challenged asthmatic subjects taking inhaled budesonide. British journal of pharmacology. 2012;165:1737–47. doi: 10.1111/j.1476-5381.2011.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham SM, Clark AR. Dual-specificity phosphatase 1: a critical regulator of innate immune responses. Biochemical Society transactions. 2006;34:1018–23. doi: 10.1042/BST0341018. [DOI] [PubMed] [Google Scholar]
- 25.Kaur M, Chivers JE, Giembycz MA, Newton R. Long-acting β2-adrenoceptor agonists synergistically enhance glucocorticoid-dependent transcription in human airway epithelial and smooth muscle cells. Molecular pharmacology. 2008;73:203–14. doi: 10.1124/mol.107.040121. [DOI] [PubMed] [Google Scholar]
- 26.Manetsch M, Ramsay EE, King EM, Seidel P, Che W, Ge Q, Hibbs DE, Newton R, Ammit AJ. Corticosteroids and β2-agonists upregulate mitogen-activated protein kinase phosphatase 1: in vitro mechanisms. British journal of pharmacology. 2012;166:2049–59. doi: 10.1111/j.1476-5381.2012.01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holden NS, Bell MJ, Rider CF, King EM, Gaunt DD, Leigh R, Johnson M, Siderovski DP, Heximer SP, Giembycz MA, Newton R. β2-Adrenoceptor agonist-induced RGS2 expression is a genomic mechanism of bronchoprotection that is enhanced by glucocorticoids. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19713–8. doi: 10.1073/pnas.1110226108. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13408991
- 28.Holden NS, George T, Rider CF, Chandrasekhar A, Shah S, Kaur M, Johnson M, Siderovski DP, Leigh R, Giembycz MA, Newton R. Induction of regulator of G-protein signaling 2 expression by long-acting β2-adrenoceptor agonists and glucocorticoids in human airway epithelial cells. The Journal of pharmacology and experimental therapeutics. 2014;348:12–24. doi: 10.1124/jpet.113.204586. [DOI] [PubMed] [Google Scholar]
- 29.Chivers JE, Gong W, King EM, Seybold J, Mak JC, Donnelly LE, Holden NS, Newton R. Analysis of the dissociated steroid RU24858 does not exclude a role for inducible genes in the anti-inflammatory actions of glucocorticoids. Molecular pharmacology. 2006;70:2084–95. doi: 10.1124/mol.106.025841. [DOI] [PubMed] [Google Scholar]
- 30.Wang JC, Derynck MK, Nonaka DF, Khodabakhsh DB, Haqq C, Yamamoto KR. Chromatin immunoprecipitation (ChIP) scanning identifies primary glucocorticoid receptor target genes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15603–8. doi: 10.1073/pnas.0407008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR. RGS2/G0S8 is a selective inhibitor of Gqα function. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14389–93. doi: 10.1073/pnas.94.26.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heximer SP. RGS2-mediated regulation of Gqα. Methods in enzymology. 2004;390:65–82. doi: 10.1016/S0076-6879(04)90005-5. [DOI] [PubMed] [Google Scholar]
- 33.Xie Y, Jiang H, Nguyen H, Jia S, Berro A, Panettieri RA, Wolff DW, Abel PW, Casale TB, Tu Y. Regulator of G protein signaling 2 is a key modulator of airway hyperresponsiveness. The Journal of allergy and clinical immunology. 2012;130:968–76.e3. doi: 10.1016/j.jaci.2012.05.004. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/724863048
- 34.Liu C, Li Q, Zhou X, Kolosov VP, Perelman JM. Regulator of G-protein signaling 2 inhibits acid-induced mucin5AC hypersecretion in human airway epithelial cells. Respiratory physiology & neurobiology. 2013;185:265–71. doi: 10.1016/j.resp.2012.10.003. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/722337125
- 35.Vásárhelyi V, Trexler M, Patthy L. Both LCCL-domains of human CRISPLD2 have high affinity for lipid A. Biochimie. 2014;97:66–71. doi: 10.1016/j.biochi.2013.09.021. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718132690
- 36.Wang Z, Xing W, Fan H, Wang K, Zhang H, Wang Q, Qi J, Yang H, Yang J, Ren Y, Cui S, Zhang X, Liu F, Lin D, Wang W, Hoffmann MK, Han Z. The novel lipopolysaccharide-binding protein CRISPLD2 is a critical serum protein to regulate endotoxin function. Journal of immunology (Baltimore, Md.: 1950) 2009;183:6646–56. doi: 10.4049/jimmunol.0802348. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/719459521
- 37.Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nature immunology. 2008;9:1074–83. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1120707
- 38.Himes BE, Jiang X, Wagner P, Hu R, Wang Q, Klanderman B, Whitaker RM, Duan Q, Lasky-Su J, Nikolos C, Jester W, Johnson M, Panettieri RA, Tantisira KG, Weiss ST, Lu Q. RNA-Seq transcriptome profiling identifies CRISPLD2 as a glucocorticoid responsive gene that modulates cytokine function in airway smooth muscle cells. PloS one. 2014;9:e99625. doi: 10.1371/journal.pone.0099625. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718448277
- 39.Moodley T, Wilson SM, Joshi T, Rider CF, Sharma P, Yan D, Newton R, Giembycz MA. Phosphodiesterase 4 inhibitors augment the ability of formoterol to enhance glucocorticoid-dependent gene transcription in human airway epithelial cells: a novel mechanism for the clinical efficacy of roflumilast in severe chronic obstructive pulmonary disease. Molecular pharmacology. 2013;83:894–906. doi: 10.1124/mol.112.083493. [DOI] [PubMed] [Google Scholar]
- 40.Yeagley D, Quinn PG. 3',5'-cyclic adenosine monophosphate response element-binding protein and CCAAT enhancer-binding protein are dispensable for insulin inhibition of phosphoenolpyruvate carboxykinase transcription and for its synergistic induction by protein kinase A and glucocorticoids. Molecular endocrinology (Baltimore, Md.) 2005;19:913–24. doi: 10.1210/me.2004-0281. [DOI] [PubMed] [Google Scholar]
- 41.Samuelsson MK, Pazirandeh A, Davani B, Okret S. p57Kip2, a glucocorticoid-induced inhibitor of cell cycle progression in HeLa cells. Molecular endocrinology (Baltimore, Md.) 1999;13:1811–22. doi: 10.1210/mend.13.11.0379. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/722636654
- 42.Chang T, Kim MJ, Ryoo K, Park J, Eom S, Shim J, Nakayama KI, Nakayama K, Tomita M, Takahashi K, Lee M, Choi E. p57KIP2 modulates stress-activated signaling by inhibiting c-Jun NH2-terminal kinase/stress-activated protein Kinase. The Journal of biological chemistry. 2003;278:48092–8. doi: 10.1074/jbc.M309421200. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718974832
- 43.Rider CF, King EM, Holden NS, Giembycz MA, Newton R. Inflammatory stimuli inhibit glucocorticoid-dependent transactivation in human pulmonary epithelial cells: rescue by long-acting β2-adrenoceptor agonists. The Journal of pharmacology and experimental therapeutics. 2011;338:860–9. doi: 10.1124/jpet.111.181016. [DOI] [PubMed] [Google Scholar]
- 44.Rangarajan PN, Umesono K, Evans RM. Modulation of glucocorticoid receptor function by protein kinase A. Molecular endocrinology (Baltimore, Md.) 1992;6:1451–7. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- 45.Gruol DJ, Altschmied J. Synergistic induction of apoptosis with glucocorticoids and 3',5'-cyclic adenosine monophosphate reveals agonist activity by RU 486. Molecular endocrinology (Baltimore, Md.) 1993;7:104–13. doi: 10.1210/mend.7.1.8383286. [DOI] [PubMed] [Google Scholar]
- 46.Moyer ML, Borror KC, Bona BJ, DeFranco DB, Nordeen SK. Modulation of cell signaling pathways can enhance or impair glucocorticoid-induced gene expression without altering the state of receptor phosphorylation. The Journal of biological chemistry. 1993;268:22933–40. [PubMed] [Google Scholar]
- 47.Zhang H, Li YC, Young AP. Protein kinase A activation of glucocorticoid-mediated signaling in the developing retina. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3880–4. doi: 10.1073/pnas.90.9.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eickelberg O, Roth M, Lörx R, Bruce V, Rüdiger J, Johnson M, Block LH. Ligand-independent activation of the glucocorticoid receptor by β2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. The Journal of biological chemistry. 1999;274:1005–10. doi: 10.1074/jbc.274.2.1005. [DOI] [PubMed] [Google Scholar]
- 49.Usmani OS, Ito K, Maneechotesuwan K, Ito M, Johnson M, Barnes PJ, Adcock IM. Glucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapy. American journal of respiratory and critical care medicine. 2005;172:704–12. doi: 10.1164/rccm.200408-1041OC. [DOI] [PubMed] [Google Scholar]
- 50.Lovén J, Svitacheva N, Jerre A, Miller-Larsson A, Korn SH. Anti-inflammatory activity of β2-agonists in primary lung epithelial cells is independent of glucocorticoid receptor. The European respiratory journal. 2007;30:848–56. doi: 10.1183/09031936.00129606. [DOI] [PubMed] [Google Scholar]
- 51.Essilfie-Quaye S, Ito K, Ito M, Kharitonov SA, Barnes PJ. Comparison of Symbicort® versus Pulmicort® on steroid pharmacodynamic markers in asthma patients. Respiratory medicine. 2011;105:1784–9. doi: 10.1016/j.rmed.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 52.Haque R, Hakim A, Moodley T, Torrego A, Essilfie-Quaye S, Jazrawi E, Johnson M, Barnes PJ, Adcock IM, Usmani OS. Inhaled long-acting β2 agonists enhance glucocorticoid receptor nuclear translocation and efficacy in sputum macrophages in COPD. The Journal of allergy and clinical immunology. 2013;132:1166–73. doi: 10.1016/j.jaci.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 53.Rider CF, Yan D, Miller-Larsson A, Giembycz MA, Newton R. Long-acting β2-adrenoceptor agonists do not enhance glucocorticoid activity by increasing glucocorticoid receptor expression or translocation in BEAS-2B cells. Am J Respir Crit Care Med. 2014;189:A4890. [Google Scholar]
- 54.So AY, Cooper SB, Feldman BJ, Manuchehri M, Yamamoto KR. Conservation analysis predicts in vivo occupancy of glucocorticoid receptor-binding sequences at glucocorticoid-induced genes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5745–9. doi: 10.1073/pnas.0801551105. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/723923717
- 55.Xie Z, Liu D, Liu S, Calderon L, Zhao G, Turk J, Guo Z. Identification of a cAMP-response element in the regulator of G-protein signaling-2 (RGS2) promoter as a key cis-regulatory element for RGS2 transcriptional regulation by angiotensin II in cultured vascular smooth muscles. The Journal of biological chemistry. 2011;286:44646–58. doi: 10.1074/jbc.M111.265462. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718906040
- 56.Rowan BG, Garrison N, Weigel NL, O'Malley BW. 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Molecular and cellular biology. 2000;20:8720–30. doi: 10.1128/MCB.20.23.8720-8730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Constantinescu A, Wu M, Asher O, Diamond I. cAMP-dependent protein kinase type I regulates ethanol-induced cAMP response element-mediated gene expression via activation of CREB-binding protein and inhibition of MAPK. The Journal of biological chemistry. 2004;279:43321–9. doi: 10.1074/jbc.M406994200. [DOI] [PubMed] [Google Scholar]
- 58.Hoang T, Fenne IS, Cook C, Børud B, Bakke M, Lien EA, Mellgren G. cAMP-dependent protein kinase regulates ubiquitin-proteasome-mediated degradation and subcellular localization of the nuclear receptor coactivator GRIP1. The Journal of biological chemistry. 2004;279:49120–30. doi: 10.1074/jbc.M409746200. [DOI] [PubMed] [Google Scholar]
- 59.Fenne IS, Hoang T, Hauglid M, Sagen JV, Lien EA, Mellgren G. Recruitment of coactivator glucocorticoid receptor interacting protein 1 to an estrogen receptor transcription complex is regulated by the 3',5'-cyclic adenosine 5'-monophosphate-dependent protein kinase. Endocrinology. 2008;149:4336–45. doi: 10.1210/en.2008-0037. [DOI] [PubMed] [Google Scholar]
- 60.Wagner BL, Norris JD, Knotts TA, Weigel NL, McDonnell DP. The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor. Molecular and cellular biology. 1998;18:1369–78. doi: 10.1128/mcb.18.3.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L, Lei K, Malawana J, Yulia A, Sooranna SR, Bennett PR, Liang Z, Grammatopoulos D, Johnson MR. Cyclic AMP enhances progesterone action in human myometrial cells. Molecular and cellular endocrinology. 2014;382:334–43. doi: 10.1016/j.mce.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 62.Pujols L, Mullol J, Torrego A, Picado C. Glucocorticoid receptors in human airways. Allergy. 2004;59:1042–52. doi: 10.1111/j.1398-9995.2004.00635.x. [DOI] [PubMed] [Google Scholar]
- 63.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6062–7. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pujols L, Mullol J, Roca-Ferrer J, Torrego A, Xaubet A, Cidlowski JA, Picado C. Expression of glucocorticoid receptor alpha- and beta-isoforms in human cells and tissues. American journal of physiology. Cell physiology. 2002;283:C1324–31. doi: 10.1152/ajpcell.00363.2001. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/722354347
- 65.Zhang S, Jonklaas J, Danielsen M. The glucocorticoid agonist activities of mifepristone (RU486) and progesterone are dependent on glucocorticoid receptor levels but not on EC50 values. Steroids. 2007;72:600–8. doi: 10.1016/j.steroids.2007.03.012. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1086932
- 66.Zhao Q, Pang J, Favata MF, Trzaskos JM. Receptor density dictates the behavior of a subset of steroid ligands in glucocorticoid receptor-mediated transrepression. International immunopharmacology. 2003;3:1803–17. doi: 10.1016/j.intimp.2003.08.005. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/725308609
- 67.Joshi T, Johnson M, Newton R, Giembycz MA. An analysis of glucocorticoid receptor-mediated gene expression in BEAS-2B human airway epithelial cells identifies distinct, ligand-directed, transcription profiles with implications for asthma therapeutics. British journal of pharmacology. 2015 doi: 10.1111/bph.13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson M. Effects of beta2-agonists on resident and infiltrating inflammatory cells. The Journal of allergy and clinical immunology. 2002;110:S282–90. doi: 10.1067/mai.2002.129430. [DOI] [PubMed] [Google Scholar]
- 69.Galant SP, Underwood S, Duriseti L, Insel PA. Characterization of high-affinity beta2-adrenergic receptor binding of (-)-[3H]-dihydroalprenolol to human polymorphonuclear cell particulates. The Journal of laboratory and clinical medicine. 1978;92:613–8. [PubMed] [Google Scholar]
- 70.Martinsson A, Larsson K, Hjemdahl P. Studies in vivo and in vitro of terbutaline-induced beta-adrenoceptor desensitization in healthy subjects. Clinical science (London, England: 1979) 1987;72:47–54. doi: 10.1042/cs0720047. [DOI] [PubMed] [Google Scholar]
- 71.Yukawa T, Ukena D, Kroegel C, Chanez P, Dent G, Chung KF, Barnes PJ. Beta2-adrenergic receptors on eosinophils. Binding and functional studies. The American review of respiratory disease. 1990;141:1446–52. doi: 10.1164/ajrccm/141.6.1446. [DOI] [PubMed] [Google Scholar]
- 72.Rabe KF, Giembycz MA, Dent G, Perkins RS, Evans P, Barnes PJ. Salmeterol is a competitive antagonist at beta-adrenoceptors mediating inhibition of respiratory burst in guinea-pig eosinophils. European journal of pharmacology. 1993;231:305–8. doi: 10.1016/0014-2999(93)90466-U. [DOI] [PubMed] [Google Scholar]
- 73.Muñoz NM, Rabe KF, Vita AJ, McAllister K, Mayer D, Weiss M, Leff AR. Paradoxical blockade of beta adrenergically mediated inhibition of stimulated eosinophil secretion by salmeterol. The Journal of pharmacology and experimental therapeutics. 1995;273:850–4. [PubMed] [Google Scholar]
- 74.Rennard SI, Vestbo J. The many “small COPDs”: COPD should be an orphan disease. Chest. 2008;134:623–7. doi: 10.1378/chest.07-3059. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/725308610
- 75.Vestbo J. COPD: definition and phenotypes. Clinics in chest medicine. 2014;35:1–6. doi: 10.1016/j.ccm.2013.10.010. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718292925
- 76.Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, Trigueros JA, Piñera P, Simón A, Riesco JA, Ancochea J, Soriano JB. A new approach to grading and treating COPD based on clinical phenotypes: summary of the Spanish COPD guidelines (GesEPOC) Primary care respiratory journal: journal of the General Practice Airways Group. 2013;22:117–21. doi: 10.4104/pcrj.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725308611
- 77.Miravitlles M, Soler-Cataluña JJ, Calle M, Soriano JB. Treatment of COPD by clinical phenotypes: putting old evidence into clinical practice. The European respiratory journal. 2013;41:1252–6. doi: 10.1183/09031936.00118912. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/722034788
- 78.Perera WR, Hurst JR, Wilkinson TMA, Sapsford RJ, Müllerova H, Donaldson GC, Wedzicha JA. Inflammatory changes, recovery and recurrence at COPD exacerbation. The European respiratory journal. 2007;29:527–34. doi: 10.1183/09031936.00092506. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/722036575
- 79.Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC medicine. 2013;11:181. doi: 10.1186/1741-7015-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calverley Peter MA, Martinez FJ, Fabbri LM, Goehring U, Rabe KF. Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol. International journal of chronic obstructive pulmonary disease. 2012;7:375–82. doi: 10.2147/COPD.S31100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abbott-Banner KH, Page CP. Dual PDE3/4 and PDE4 inhibitors: novel treatments for COPD and other inflammatory airway diseases. Basic & clinical pharmacology & toxicology. 2014;114:365–76. doi: 10.1111/bcpt.12209. [DOI] [PubMed] [Google Scholar]
- 82.Boswell-Smith V, Spina D, Oxford AW, Comer MB, Seeds EA, Page CP. The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]isoquinolin-4-one] The Journal of pharmacology and experimental therapeutics. 2006;318:840–8. doi: 10.1124/jpet.105.099192. [DOI] [PubMed] [Google Scholar]
- 83.Franciosi LG, Diamant Z, Banner KH, Zuiker R, Morelli N, Kamerling Ingrid MC, de Kam Marieke L, Burggraaf J, Cohen AF, Cazzola M, Calzetta L, Singh D, Spina D, Walker Michael JA, Page CP. Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: findings from four clinical trials. The Lancet. Respiratory medicine. 2013;1:714–27. doi: 10.1016/S2213-2600(13)70187-5. [DOI] [PubMed] [Google Scholar]
- 84.BinMahfouz H, Borthakur B, Yan D, George T, Giembycz MA, Newton R. Superiority of combined phosphodiesterase PDE3/PDE4 inhibition over PDE4 inhibition alone on glucocorticoid- and long-acting β2-adrenoceptor agonist-iduced gene expression in human airway epithelial cells. Molecular pharmacology. 2015;87:64–76. doi: 10.1124/mol.114.093393. [DOI] [PubMed] [Google Scholar]
- 85.Nordeen SK, Moyer ML, Bona BJ. The coupling of multiple signal transduction pathways with steroid response mechanisms. Endocrinology. 1994;134:1723–32. doi: 10.1210/endo.134.4.8137736. [DOI] [PubMed] [Google Scholar]
- 86.Ballard PL, Lee JW, Fang X, Chapin C, Allen L, Segal MR, Fischer H, Illek B, Gonzales LW, Kolla V, Matthay MA. Regulated gene expression in cultured type II cells of adult human lung. American journal of physiology. Lung cellular and molecular physiology. 2010;299:L36–50. doi: 10.1152/ajplung.00427.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. American journal of physiology. Lung cellular and molecular physiology. 2002;283:L940–51. doi: 10.1152/ajplung.00127.2002. [DOI] [PubMed] [Google Scholar]
- 88.Greer S, Page CW, Joshi T, Yan D, Newton R, Giembycz MA. Concurrent agonism of adenosine A2B and glucocorticoid receptors in human airway epithelial cells cooperatively induces genes with anti-inflammatory potential: a novel approach to treat chronic obstructive pulmonary disease. The Journal of pharmacology and experimental therapeutics. 2013;346:473–85. doi: 10.1124/jpet.113.206284. [DOI] [PubMed] [Google Scholar]
- 89.Wilson SM, Shen P, Rider CF, Traves SL, Proud D, Newton R, Giembycz MA. Selective prostacyclin receptor agonism augments glucocorticoid-induced gene expression in human bronchial epithelial cells. Journal of immunology (Baltimore, Md.: 1950) 2009;183:6788–99. doi: 10.4049/jimmunol.0902738. [DOI] [PubMed] [Google Scholar]
- 90.Patel BS, Prabhala P, Oliver BG, Ammit AJ. Inhibitors of PDE4, but Not PDE3, Increase β2-agonist-induced Expression of Anti-inflammatory MKP-1 in Airway Smooth Muscle Cells. American journal of respiratory cell and molecular biology. 2014 doi: 10.1165/rcmb.2014-0344OC. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/720328787
- 91.Theophilus A, Moore A, Prime D, Rossomanno S, Whitcher B, Chrystyn H. Co-deposition of salmeterol and fluticasone propionate by a combination inhaler. International journal of pharmaceutics. 2006;313:14–22. doi: 10.1016/j.ijpharm.2006.01.018. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/722743179
- 92.Nelson HS, Chapman KR, Pyke SD, Johnson M, Pritchard JN. Enhanced synergy between fluticasone propionate and salmeterol inhaled from a single inhaler versus separate inhalers. The Journal of allergy and clinical immunology. 2003;112:29–36. doi: 10.1067/mai.2003.1558. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/724866013
- 93.Huchon G, Magnussen H, Chuchalin A, Dymek L, Gonod FB, Bousquet J. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respiratory medicine. 2009;103:41–9. doi: 10.1016/j.rmed.2008.09.002. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/722619734
- 94.Testa B, Mayer JM. Design of intramolecularly activated prodrugs. Drug metabolism reviews. 1998;30:787–807. doi: 10.3109/03602539808996330. [DOI] [PubMed] [Google Scholar]
- 95.Baker WR, Kim M, Phillips G, Rudolph A, Stasiak M. Corticosteroid beta-agonist-linked compounds for use in therapy. Gilead Sciences, Inc. 2010 Patent number: WO/2010/126953. [Google Scholar]
- 96.Baker WR, Girton BC, Stasiak M. Substituted phenylphosphates as mutual pro-drugs of steroids and beta-agonists for the treatment of pulmonary inflammation and bronchoconstriction. Corus Pharma Inc., 2006. Patent number: WO/2006/138212. [Google Scholar]
- 97.Giembycz MA, Maurice DH. Cyclic nucleotide-based therapeutics for chronic obstructive pulmonary disease. Current opinion in pharmacology. 2014;16:89–107. doi: 10.1016/j.coph.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Phillips G, Salmon M. From Bifunctional compounds for the treatment of COPD. In: Desai MC, editor. Annual reports in medicinal chemistry. Oxford: Elsevier; 2012:. pp. 209–21. [DOI] [Google Scholar]
- 99.Shonberg J, Scammells PJ, Capuano B. Design strategies for bivalent ligands targeting GPCRs. ChemMedChem. 2011;6:963–74. doi: 10.1002/cmdc.201100101. [DOI] [PubMed] [Google Scholar]
- 100.Baker WR, Cai S, Kaplan JA, Kim M, Loyer-Drew JA, Perrault S, Phillips G, Purvis LJ, Stasiak M, Steven KL, van Veldhuizen J. Bifunctional quinoline derivatives. Gilead Sciences, Inc. Patient number: WO/2011/143105. [Google Scholar]
- 101.Tralau-Stewart CJ, Williamson RA, Nials AT, Gascoigne M, Dawson J, Hart GJ, Angell Anthony DR, Solanke YE, Lucas FS, Wiseman J, Ward P, Ranshaw LE, Knowles RG. GSK256066, an exceptionally high-affinity and selective inhibitor of phosphodiesterase 4 suitable for administration by inhalation: in vitro, kinetic, and in vivo characterization. The Journal of pharmacology and experimental therapeutics. 2011;337:145–54. doi: 10.1124/jpet.110.173690. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/719106238
- 102.Tannheimer SL, Sorensen EA, Cui Z, Kim M, Patel L, Baker WR, Phillips GB, Wright CD, Salmon M. The in vitro pharmacology of GS-5759, a novel bifunctional phosphodiesterase 4 inhibitor and long acting β2-adrenoceptor agonist. The Journal of pharmacology and experimental therapeutics. 2014;349:85–93. doi: 10.1124/jpet.113.210997. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718273009
- 103.Salmon M, Tannheimer SL, Gentzler TT, Cui Z, Sorensen EA, Hartsough KC, Kim M, Purvis LJ, Barrett EG, McDonald JD, Rudolph K, Doyle-Eisele M, Kuehl PJ, Royer CM, Baker WR, Phillips GB, Wright CD. The in vivo efficacy and side effect pharmacology of GS-5759, a novel bifunctional phosphodiesterase 4 inhibitor and long-acting β2-adrenoceptor agonist in preclinical animal species. Pharmacol Res Perspect. 2014;2 doi: 10.1002/prp2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]