Abstract
Background
The purpose of this study was to report the treatment outcomes of patients with advanced oropharyngeal cancer treated with transoral laser microsurgery (TLM) followed by radiation therapy (RT) at Mayo Clinic in Arizona.
Methods
A retrospective study of 80 patients treated from January 1, 2000 to November 7, 2011 was performed. All patients had stage III/IV oropharyngeal tumors and underwent TLM with neck dissection. Adjuvant RT was then given. Thirty-seven patients received concurrent adjuvant chemotherapy. The primary outcome was locoregional control.
Results
Median follow-up was 47.3 months (range, 9.7–139.2 months). The 3-year locoregional control, recurrence-free survival, and overall survival rates were 98.6% (95% confidence interval [CI], 91% to 100%), 91.1% (95% CI, 81% to 96%), and 93.7% (95% CI, 84% to 98%), respectively. There were a total of 5 treatment failures, 1 regional and 4 distant. Twenty-six patients underwent neck only RT with exclusion of the primary site.
Conclusion
TLM followed by RT for advanced oropharyngeal cancer results in excellent locoregional control rates.
Keywords: transoral laser microsurgery, transoral, radiation, head neck cancer, oropharyngeal
INTRODUCTION
The incidence of squamous cell carcinomas of the oropharynx is rising. This trend is because of the association of human papillomavirus (HPV) and oropharyngeal cancers.1 Transoral laser microsurgery (TLM) is an emerging modality that has been reported in the treatment of base of tongue cancer as early as 2003.2 The advantage of this approach is that it is less invasive than the traditional lip split mandibulotomy approach and allows a faster return to swallowing function. Traditional open surgical approaches carry a significant risk of morbidity and mortality.3 TLM is a natural-orifice surgery that decreases patient morbidity by decreasing swelling, faster return of swallowing function, and decreasing blood loss, which translates into a reduced hospital stay. Multiple institutional studies report the efficacy of TLM with better functional stability compared with traditional approaches.4–7
Adjuvant therapy in the form of radiation therapy (RT) and/or chemotherapy are used in the management of locally advanced oropharyngeal cancer.8 The benefit of chemotherapy in addition to RT in the adjuvant setting is shown with certain pathologic risk factors.9 TLM provides pathologic data regarding risk factors allowing for tailoring of patients’ adjuvant therapy. Theoretically, if surgical resection of the tumor is performed and adjuvant RT is deintensified by decreases in the dose and volume of treatment, the incidence of radiation-related toxicity, such as late dysphagia, would be reduced.
As a center with a long history in the use of transoral surgical management of oropharyngeal cancer, we present our 10-year experience with the use of TLM combined with modern adjuvant RT for the treatment of locally advanced oropharyngeal cancer. A portion of our experience was presented in a multicenter study with encouraging locoregional control rates.5 We now present our expanded experience with more follow-up, toxicity data, and details regarding adjuvant therapy.
MATERIALS AND METHODS
Study design
An Institutional Review Board approved retrospective inquiry of the Mayo Clinic Arizona Cancer Registry was performed for patients receiving TLM and adjuvant RT for oropharyngeal cancer from January 1, 2000, to November 7, 2011. Of the 334 patients with oropharyngeal cancer that have undergone TLM, 195 patients were excluded because their disease was pathologically staged T1 or T2 node negative and, therefore, they did not receive adjuvant therapy or they declined RT. One hundred thirty-nine patients were identified that also received adjuvant RT at the Mayo Clinic in Arizona. A total of 59 patients were excluded from the analysis because of the primary site being the oral cavity, recurrent status of tumor, the patient declined RT, and/or the patient was receiving adjuvant therapy outside of our institution. Therefore, a total of 80 patients represented our final cohort for the study. The median patient age was 58.0 years (range, 31–82 years). Pretreatment evaluation included a complete history and physical examination, fiber-optic endoscopic examination, dental evaluation, chest x-ray, and CT scan and/or MRI of the neck. In some cases, a CT scan of the chest/abdomen and/or positron emission tomography scan was performed before treatment.
Surgery
Each patient underwent TLM using the principles of Steiner and Ambrosch.2 A detailed description of the TLM procedure has been described earlier.5 In brief, exposure by specialized laryngoscopes allow for a piecemeal resection with meticulous tumor mapping and frozen section margin analysis. A bilateral selective or modified radical neck dissection was performed for oropharyngeal tumors with midline extension and a unilateral neck dissection was performed for well lateralized tumors.
Radiation therapy
All patients underwent adjuvant RT. RT commenced before 12 weeks from the date of surgery with most patients starting before 8 weeks from the date of surgery. All patients underwent CT simulation using a thermoplastic mask for head, neck, and shoulder immobilization. Seventy-two patients (90%) received intensity-modulated radiation therapy, with the rest receiving 3-dimensional (3D) conformal therapy. A dose painting technique was used in most patients allowing for delivery of different dose levels to different targets simultaneously. Clinical target volumes (CTV) consisted of the primary tumor bed and dissected and undissected necks. The median prescribed dose was 60 Gray (Gy) in 30 fractions using 2 Gy per fraction to the high-risk CTV. Areas deemed low risk, at the discretion of the treating physician, received 54 Gy in 30 fractions. If there was concern for close/positive margins and/or extracapsular extension, then 63 to 66 Gy in 30 fractions was delivered to the area of concern. Of note, 26 patients received RT to the neck alone with exclusion of the primary postoperative site after discussion between the surgeon and radiation oncologist in certain low-risk patients.
Chemotherapy
Thirty-seven patients (46%) received concurrent adjuvant chemotherapy with the RT. Indications for chemotherapy administration included positive/close margins, extracapsular extension (ECE), multiple positive lymph nodes, lymphovascular space invasion (LVSI) and/or perineural invasion (PNI). Single-agent cisplatin was the most commonly administered agent usually delivered in 2 to 3 cycles at 100 mg/m2 dose on days 1, 22, and 43 to 30 patients. Seven patients received cetuximab because of toxicity considerations secondary to medical comorbidities. Patients receiving cetuximab received an initial loading dose of 400 mg/m2 followed by a weekly dose of 250 mg/m2 during the radiation.
Follow-up
Pathological data including ECE, LVSI, PNI, margin status, and HPV status (p16 status) were gathered from the surgical pathology report. Moreover, documented discussion among the treating radiation oncologist, surgeon, and/or pathologist was used to help determine final margin status. A close margin was defined as being within 1 mm. Weekly evaluations during RT were performed for each patient. After RT completion, patients were followed every 2 to 3 months for the first 2 years, every 6 months until 5 years, then yearly thereafter. Toxicities were graded using the Common Terminology Criteria for Adverse Events, version 4.0. Of note, percutaneous endoscopic gastrostomy (PEG) tubes were being placed at the discretion of the treating radiation oncologist. In the recent past, there has been a shift in practice to not place PEG tubes prophylactically. The PEG tube dependence rate was calculated at 1 year.
Statistical methods
Patient, disease, and treatment characteristics were described using means, medians, and ranges for continuous variables, and frequencies and relative frequencies for categorical variables. Overall, survival was calculated as the time from cancer diagnosis to date of death because of any cause. Recurrence-free survival was calculated as the time from cancer diagnosis to earliest date of recurrence or death because of any cause. Time to event endpoints were described overall and by group using Kaplan–Meier estimates. Overall and recurrence-free survivals were compared between patient groups using log-rank tests and Cox regression analysis. For each toxicity, the maximum grade per patient was compared between patients whose primary site was included in the RT field versus excluded from the RT field using the Jonckheere–Terpstra test. All reported p values are 2-sided unless otherwise noted and p values < .05 were considered statistically significant throughout.
RESULTS
Patient characteristics are summarized in Table 1. Median age at presentation was 58.0 years (range, 31–82 years) with the majority being men (87.5%). The majority of patients presented with stage IVA disease (86.3%). The median follow-up for surviving patients is 47.3 months (range, 9.7–139.2 months). The p16 tumor status was available for 72 patients (90%). Of the remaining 8 patients with unknown p16 status, 6 patients had less than a 10 pack-year smoking history.
TABLE 1.
Patient characteristics.
| Characteristic | No. of patients (%) |
|---|---|
| Age, y | |
| ≤60 | 51 (64.8) |
| >60 | 29 (36.3) |
| Sex | |
| Male | 70 (87.5) |
| Female | 10 (12.5) |
| ECOG Performance Status | |
| 0 | 54 (67.5) |
| 1 | 22 (27.5) |
| 2 | 4 (5) |
| Primary site | |
| Base of tongue | 45 (56.3) |
| Tonsil | 35 (43.8) |
| T classification | |
| T1 | 42 (52.5) |
| T2 | 31 (38.8) |
| T3 | 7 (8.8) |
| N classification | |
| N0 | 1 (1.3) |
| N1 | 9 (11.3) |
| N2a | 9 (11.3) |
| N2b | 49 (61.3) |
| N2c | 10 (12.5) |
| N3 | 2 (2.5) |
| Stage | |
| 3 | 9 (11.3) |
| 4a | 69 (86.3) |
| 4b | 2 (2.5) |
| Smoking history (>10 pack-years) | |
| Yes | 40 (50) |
| No | 40 (50) |
| p16 status | |
| Positive | 59 (73.8) |
| Negative | 13 (16.3) |
| Not available | 8 (10) |
| Margins | |
| Positive | 8 (10) |
| Close | 6 (7.5) |
| Negative | 66 (82.5) |
| ECE | |
| Positive | 42 (52.5) |
| Negative | 38 (47.5) |
| LVSI | |
| Positive | 7 (8.8) |
| Negative | 73 (91.3) |
| PNI | |
| Positive | 6 (7.5) |
| Negative | 74 (92.5) |
| Chemotherapy | |
| Cisplatin | 30 (37.5) |
| Cetuximab | 7 (19.4) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ECE, extracapsular extension; LVSI, lymphovascular space invasion; PNI, perineural invasion.
Survival
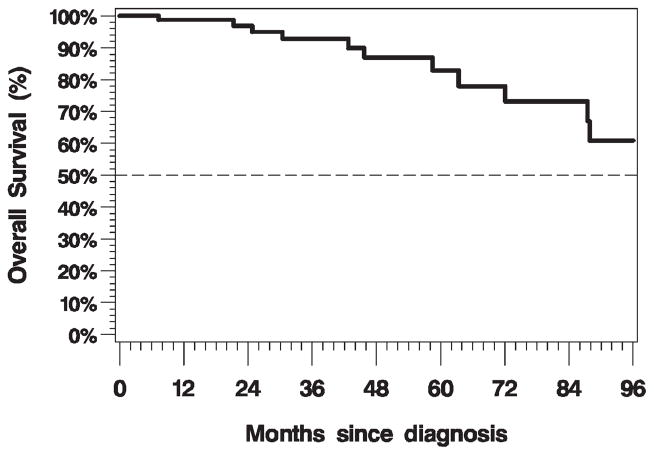
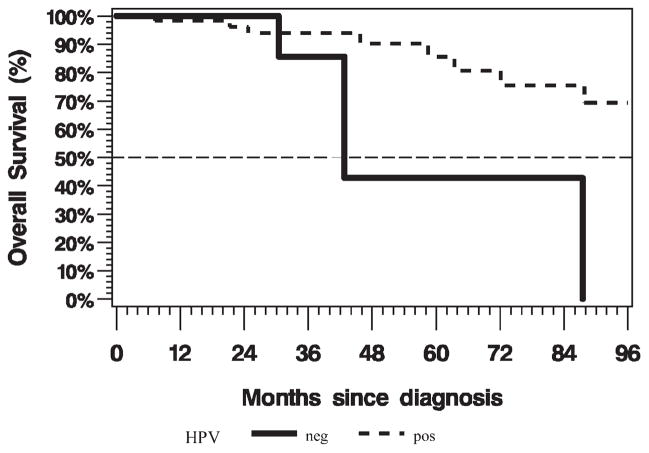
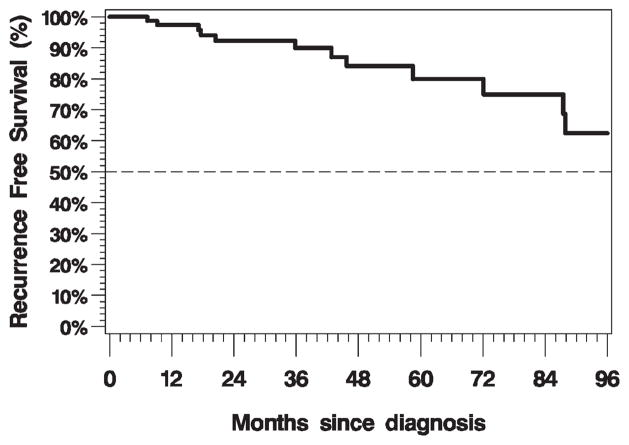
The 3-year overall survival rate was 93.7% (95% confidence interval [CI], 84% to 98%; Figure 1). There was no impact on overall survival by margin status (positive vs negative, log-rank p = .67), ECE (log-rank p = .90), LVSI (log-rank p = .89), PNI (log-rank p = .44), or inclusion of the primary site in the RT field (log-rank p = .69) on univariate analysis. There was a trend toward longer survival for patients with p16-positive disease compared with patients with p16-negative disease (log-rank p = .05; Cox regression hazard ratio [HR] = 0.27; 95% CI, 0.07–1.12). Overall survival by p16 status is illustrated in Figure 2. The 3-year recurrence-free survival rate was 91.1% (95% CI, 81% to 96%; Figure 3). There was no impact on recurrence-free survival by margin status (positive vs negative, log-rank p = .17), ECE (log-rank p = .90), LVSI (log-rank p = .99), PNI (log-rank p = .40), or inclusion of the primary site in the RT field (log-rank p = .47) on univariate analysis. Patients with p16-positive disease compared with patients with p16-negative disease had statistically significantly longer recurrence-free survival (log-rank p = .02; Cox regression HR = 0.24; 95% CI, 0.07–0.87).
FIGURE 1.
Kaplan–Meier plot of overall survival (n = 80, events = 11).
FIGURE 2.
Kaplan–Meier plot of overall survival by human papillomavirus (HPV) status (positive [pos], n = 59; events = 8; negative [neg], n = 13; events = 3; log-rank p = .07; hazard ratio [HR] = 0.29; 95% confidence interval [CI], 0.07–1.21).
FIGURE 3.
Kaplan–Meier plot of recurrence-free survival (n = 80, events = 12).
Locoregional control/patterns of failure
The 3-year locoregional control rate was 98.6% (95% CI, 91% to 100%). The patterns of failure are illustrated in Table 2. There were no local failures, 1 regional failure, and 4 distant failures. The patient with the regional failure initially presented with a T2N2bM0 grade IV squamous cell carcinoma. He had p16-negative disease and had a positive margin after TLM. He received concurrent cisplatin chemotherapy with RT. Neck recurrence occurred 12.6 months after completion of RT with multiple positive lymph nodes on neck dissection. Of note, 5 months later, this patient was found to have lung metastases.
TABLE 2.
Patterns of failure.
| Patient | Primary site | T classification | N classification | p16 status | Smoking history | Margins | ECE | LVSI | PNI | Adjuvant | Recurrence site | Time to recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BOT | 2 | 2b | pos | Yes | neg | neg | neg | neg | RT | Lung | 36 mo |
| 2 | Tonsil | 2 | 2b | neg | Yes | neg | neg | neg | neg | RT | Lung | 17.7 mo |
| 3 | Tonsil | 1 | 2b | pos | Yes | neg | neg | neg | neg | RT | Bone | 9.2 mo |
| 4 | Tonsil | 3 | 2b | pos | Yes | pos | pos | pos | neg | RT, C | Lung | 20.5 mo |
| 5 | Tonsil | 2 | 2b | neg | No | pos | pos | neg | neg | RT, C | Neck | 17.2 mo |
Abbreviations: ECE, extracapsular extension; LVSI, lymphovascular space invasion; PNI, perineural invasion; BOT, base of tongue; pos, positive; RT, radiation therapy; neg, negative; C, chemotherapy.
Subgroup analysis
Twenty-six of the 80 patients received RT without inclusion of their primary site in the CTV. RT was directed to the bilateral necks only. Although the primary operative bed was not included as a clinical target purposely for radiation, there was incidental dose delivered to this region in the range of 40 to 45 Gy. The amount of incidental radiation dose varied, depending on the primary tumor site (base of tongue vs tonsil). There were no local or regional relapses in this subgroup. All of these patients had negative surgical margins and no lymphovascular space invasion. One patient had perineural invasion. Sixteen of the patients had ECE on pathology and 5 of the patients did not receive adjuvant chemotherapy with their RT. One of these patients who had no adverse factors on pathology developed a distant relapse.
Toxicity
The average length of hospitalization after TLM and neck dissection was 3.74 days. Table 3 summarizes the acute toxicity during RT. There was 1 grade V toxicity. The patient developed progressive hepatic failure secondary to fulminant hepatitis B 1 month after completion of adjuvant chemotherapy and RT. Twenty-one patients (26.3%) experienced grade III toxicity during their adjuvant therapy. For patients that had their primary site excluded in the RT field, there was a statistically significant lower grade of dysphagia (Jonckheere–Terpstra 1-sided p = .06) and mucositis (Jonckheere–Terpstra 1-sided p = .005). Excluding the primary site did not have an impact on xerostomia (Jonckheere–Terpstra 1-sided p = .23). Thirty-one patients (38.8%) had a preradiation PEG tube, whereas 11 patients (13.8%) had a PEG tube placed during RT. Five patients had a PEG tube at 1 year, yielding a PEG tube dependence rate of 6.3%.
TABLE 3.
Acute toxicity.
| No. of patients by grade of toxicity
|
||||||
|---|---|---|---|---|---|---|
| Grade 0 | Grade I | Grade II | Grade III | Grade IV | Grade V | |
| Dysphagia | 2 | 10 | 47 | 20 | 1 | 0 |
| Xerostomia | 0 | 15 | 61 | 4 | 0 | 0 |
| Mucositis | 0 | 17 | 50 | 13 | 0 | 0 |
| Other | 0 | 0 | 0 | 0 | 0 | 1* |
Progressive fulminant hepatitis B.
DISCUSSION
Our study represents one of the largest experiences with long follow-up on the use of TLM with contemporary adjuvant RT for locally advanced oropharyngeal cancer. Our analyzed cohort consisted of a majority of T1 to T3 primaries with nodal metastasis. Our excellent locoregional control, recurrence-free survival, and overall survival rates are consistent with previously reported series.4–6 Rich et al4 reported on the Washington University St. Louis experience on TLM for patients with locally advanced oropharyngeal carcinoma with a very similar recurrence pattern. They reported only 1 local recurrence (with concurrent regional and distant recurrence) in a patient with a T3N1M0 and HPV-negative that received adjuvant chemotherapy and RT. Regional only recurrence was uncommon, occurring only in a T3N2M0 base of tongue primary with ECE of disease in the neck node.4 Grant et al6 reported on a cohort of base of tongue cancers treated with TLM. The 3 reported locoregional recurrences in his study all had declined adjuvant RT. Compared with transoral robotic surgery, our results show similar outcomes with high locoregional control rates.10 With the increasing use of transoral surgery for oropharyngeal cancers, there have been concerns whether the treatment outcomes are inferior to definitive concurrent chemotherapy and radiation. Setton et al11 recently reported the Memorial Sloan–Kettering Cancer Center long-term experience using intensity-modulated radiation therapy. The majority of their patients were T1 and T2 primary with N2 nodal disease. Their 3-year overall survival rate was 84.9% and local and regional failure rates were 5.4% and 5.6%, respectively, against which our results compare favorably. Two caveats with this comparison are that the HPV status is unknown in the Setton et al11 series and their series did include more patients with T3/T4 primary carcinoma.
The majority of our patients are p16-positive, consistent with the recent epidemiological trend of increasing HPV-associated oropharyngeal cancers. Our excellent outcomes support the strong independent prognostic value of HPV. This has been shown irrespective of treatment modality, such as in the report by Hong et al12 in which a patient with HPV predicted for improved locoregional control and overall survival was treated primarily by surgery or radiation. Of note, 30 of the 59 patients who were p16-positive had a more than 10 pack-year smoking history, which would have classified them as an intermediate risk as described by Ang et al.13
Our acute toxicity profile did not reveal an excessive level of grade III or higher toxicities. Although acute dysphagia was present as expected, long-term swallowing difficulties were not apparent in most patients. With only 6.3% of patients needing a PEG tube at 1 year, this is comparable to both transoral surgical and contemporary definitive chemoradiation series.4,11,14 Our series lacks detailed information regarding long-term functional outcomes. Better description of toxicity can be achieved through the collection of prospective quality of life data and swallowing assessments pretreatment and posttreatment, such as reported by Feng et al.15 Efforts to collect these data are currently underway at our institution in the form of a prospective study. This would allow for more robust comparative studies in regard to functional outcomes between upfront surgical and radiation approaches for the management of oropharyngeal cancer.
Many questions remain on how to further deintensify RT in the setting of transoral surgery. Does lowering the RT dose by only 10 Gy after transoral surgery result in acceptable functional outcomes? Feng et al15 used a pharyngeal constrictor-sparing approach in their primary chemoradiation series resulting in improved swallowing outcomes. Moreover, Levendag et al16 showed a dose-response relationship for dysphagia and dose to the superior constrictor muscle, suggesting a significant increase in the risk of swallowing problems after 55 Gy. Theoretically, RT in the adjuvant setting allows for lower doses to the swallowing structures potentially improving long-term swallowing outcomes. Can we lower our RT dose to the low-risk postoperative bed below 57.6 Gy in the HPV era, a postoperative dose level established by Fletcher and Evers?17 Can we safely exclude the primary site from our radiation field in certain situations, as has been suggested by Quon et al?18 We have previously reported on 69 patients with oropharyngeal cancer that were treated with TLM alone.19 Forty-four of the 69 patients were stage III or IV and there were only 3 local recurrences. Twenty-five patients had declined RT to the neck and 4 patients developed a regional recurrence. In our current series, 26 patients had their primary site excluded from their radiation field. Most of these patients had negative margins and no perineural invasion. When evaluating margin status, it is important for the radiation oncologist to communicate with the surgeon and the pathologist. The overall experience of the surgical team with transoral surgery would also need to be taken into account when deciding whether to exclude the primary site for adjuvant irradiation. Furthermore, it is important to consider the anatomic location of the tumor. For example, the lateral pharyngeal wall can be as thin as 2.4 mm in the average healthy adult (Michael Hinni, MD, personal written communication). At our institution, we examined 128 tonsil cancers and found the average closest margin to be 1.98 mm (Michael Hinni, MD, personal written communication). This challenges the classic dogma of a close margin being within 2 mm and needs to be studied to refine adjuvant RT fields. The goals of deintensification of RT are to reduce the long-term toxicity while maintaining excellent cancer control. This RT volume reduction approach is being planned as a prospective protocol in our institution.
Another consideration when using multiple treatment modalities is cost. Are the added costs of surgery including hospitalization justified in this setting? Cost benefit analyses are difficult and involve significant complexity. Moore et al20 did report a cost analysis comparing trans-oral surgery alone, transoral surgery plus adjuvant radiation, transoral surgery plus adjuvant chemoradiation, and primary chemoradiation therapy. Interestingly, they found that the mean cost of transoral surgery alone, transoral surgery plus RT, and transoral surgery plus chemoradiation therapy were all less than the mean cost of definitive chemoradiation therapy, suggesting that treatment tailored by pathology obtained by surgery is more cost conscious. One must acknowledge that there are limitations to this type of study, which include selection bias based on patient comorbidities and in treatment based on stage.
TLM followed by adjuvant RT for locally advanced oropharyngeal cancers achieves very high locoregional control rates with acceptable rates of acute toxicity. Further delineation of the late toxicity profile and deintensification of RT will be examined through prospective study.
Acknowledgments
We thank Kyle E. Coppola for assistance in data collection.
References
- 1.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2.Steiner W, Fierek O, Ambrosch P, Hommerich CP, Kron M. Transoral laser microsurgery for squamous cell carcinoma of the base of the tongue. Arch Otolaryngol Head Neck Surg. 2003;129:36–43. doi: 10.1001/archotol.129.1.36. [DOI] [PubMed] [Google Scholar]
- 3.Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94:2967–2980. doi: 10.1002/cncr.10567. [DOI] [PubMed] [Google Scholar]
- 4.Rich JT, Milov S, Lewis JS, Jr, Thorstad WL, Adkins DR, Haughey BH. Transoral laser microsurgery (TLM) +/− adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope. 2009;119:1709–1719. doi: 10.1002/lary.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haughey BH, Hinni ML, Salassa JR, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head Neck. 2011;33:1683–1694. doi: 10.1002/hed.21669. [DOI] [PubMed] [Google Scholar]
- 6.Grant DG, Salassa JR, Hinni ML, Pearson BW, Perry WC. Carcinoma of the tongue base treated by transoral laser microsurgery, part one: untreated tumors, a prospective analysis of oncologic and functional outcomes. Laryngoscope. 2006;116:2150–2155. doi: 10.1097/01.mlg.0000244159.64179.f0. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen H, Hermann RM, Martin A, et al. Long-term follow-up after transoral laser microsurgery and adjuvant radiotherapy for advanced recurrent squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2006;65:1067–1074. doi: 10.1016/j.ijrobp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Pradier O, Christiansen H, Schmidberger H, et al. Adjuvant radiotherapy after transoral laser microsurgery for advanced squamous carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2005;63:1368–1377. doi: 10.1016/j.ijrobp.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein GS, O’Malley BW, Jr, Cohen MA, Quon H. Transoral robotic surgery for advanced oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136:1079–1085. doi: 10.1001/archoto.2010.191. [DOI] [PubMed] [Google Scholar]
- 11.Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan–Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82:291–298. doi: 10.1016/j.ijrobp.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 12.Hong AM, Dobbins TA, Lee CS, et al. Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br J Cancer. 2010;103:1510–1517. doi: 10.1038/sj.bjc.6605944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol. 2012;22:128–142. doi: 10.1016/j.semradonc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein GS, Quon H, O’Malley BW, Jr, Kim GG, Cohen MA. Selective neck dissection and deintensified postoperative radiation and chemotherapy for oropharyngeal cancer: a subset analysis of the University of Pennsylvania transoral robotic surgery trial. Laryngoscope. 2010;120:1749–1755. doi: 10.1002/lary.21021. [DOI] [PubMed] [Google Scholar]
- 15.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28:2732–2738. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: A dose-effect relationship. Radiother Oncol. 2007;85:64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher GH, Evers WT. Radiotherapeutic management of surgical recurrences and postoperative residuals in tumors of the head and neck. Radiology. 1970;95:185–188. doi: 10.1148/95.1.185. [DOI] [PubMed] [Google Scholar]
- 18.Quon H, O’Malley BW, Jr, Weinstein GS. Postoperative adjuvant therapy after transoral robotic resection for oropharyngeal carcinomas: rationale and current treatment approach. ORL J Otorhinolaryngol Relat Spec. 2011;73:121–130. doi: 10.1159/000319890. [DOI] [PubMed] [Google Scholar]
- 19.Grant DG, Hinni ML, Salassa JR, Perry WC, Hayden RE, Casler JD. Oropharyngeal cancer: a case for single modality treatment with transoral laser microsurgery. Arch Otolaryngol Head Neck Surg. 2009;135:1225–1230. doi: 10.1001/archoto.2009.185. [DOI] [PubMed] [Google Scholar]
- 20.Moore EJ, Hinni ML, Olsen KD, Price DL, Laborde RR, Inman JC. Cost considerations in the treatment of oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. 2012;146:946–951. doi: 10.1177/0194599812437534. [DOI] [PubMed] [Google Scholar]