SUMMARY
WNT signaling promotes the reprogramming of somatic cells to an induced pluripotent state. We provide genetic evidence that WNT signaling is a requisite step during the induction of pluripotency. Fibroblasts from individuals with Focal Dermal Hypoplasia (FDH), a rare genetic syndrome caused by mutations in the essential WNT processing enzyme PORCN, fail to reprogram using standard methods. This blockade in reprogramming is overcome by ectopic WNT signaling and by PORCN overexpression, thus demonstrating that WNT signaling is essential for reprogramming. The rescue of reprogramming is critically dependent on the level of WNT signaling: steady baseline activation of the WNT pathway yields karyotypically normal iPS cells, whereas daily stimulation with Wnt3a produces FDH-iPS cells with severely abnormal karyotypes. Therefore, although WNT signaling is required for cellular reprogramming, inappropriate activation of WNT signaling induces chromosomal instability, highlighting the precarious nature of ectopic WNT activation, and its tight relationship with oncogenic transformation.
INTRODUCTION
The process of converting, or reprogramming, a mature cell type to an embryonic stem cell-like state requires the establishment of a transcriptional regulatory network comprised of transcription factors including POU5F1/OCT4, SOX2 and NANOG (Boyer et al., 2005; Cole et al., 2008). In human and mouse embryonic stem cells, these factors maintain each other’s expression, and hence the pluripotent state, through regulatory feedback mechanisms. Disruption of this regulatory circuit causes cells to exit the pluripotent state and differentiate. Extracellular signals, such as FGF2 in human embryonic stem cells (hESCs) and LIF in mouse embryonic stem cells, influence and regulate the pluripotent state. In addition, the WNT signaling pathway critically influences the pluripotent state of embryonic stem cells (Blauwkamp et al., 2012; Jiang et al., 2013; Lyashenko et al., 2011; Sato et al., 2004; ten Berge et al., 2011; Wray et al., 2011; Yi et al., 2011). Although establishment of the OCT4-NANOG-SOX2 transcriptional regulatory network is clearly critical for the generation of induced pluripotent stem (iPS) cells, the role of extracellular signals, such as WNTs, in this process has not been examined extensively.
WNT and the WNT/β-catenin signaling pathway (also known as the canonical WNT signaling pathway) have been implicated in iPS cell generation, however, significant controversy surrounds their specific role in this process. First, in the original iPS cell studies, β-catenin was found to promote reprogramming, however, it was eliminated from the final reprogramming factor cocktail (Takahashi and Yamanaka, 2006). Second, addition of WNT proteins influences the induction of the pluripotent state (Aulicino et al., 2014; Ho et al., 2013; Marson et al., 2008; Zhang et al., 2014), however, one study found that WNT/β-catenin signaling was stimulatory (Zhang et al., 2014), whereas other studies found that it was inhibitory during early stages of reprogramming (Aulicino et al., 2014; Ho et al., 2013). Third, small molecules that inhibit GSK3—and hence activate WNT/β-catenin signaling—stimulate reprogramming efficiencies (Li et al., 2009; Silva et al., 2008) and can promote reprogramming with OCT4 as the only reprogramming factor (Li et al., 2011). However, GSK3 inhibitors, as well as purified WNT proteins, potently promote mesendodermal differentiation of hESCs (Bakre et al., 2007; Davidson et al., 2012), creating a conundrum over how pro-differentiation factors can also promote the induction of the pluripotent state. Finally, despite these established links between WNT signaling and the generation of iPS cells, a strict requirement for WNT signaling in this process has not been demonstrated. In this study, we employ fibroblasts from patients harboring mutations in an essential WNT processing enzyme, called PORCN, to establish that endogenous WNT signaling is required during the process of inducing a pluripotent stem cell state from fibroblasts.
The PORCN gene encodes an integral membrane resident ER protein that regulates processing of WNT proteins by catalyzing the covalent attachment of a lipid moiety to the WNT polypeptide backbone (Barrott et al., 2011; Biechele et al., 2011; Galli et al., 2007; Herr and Basler, 2012; Kadowaki et al., 1996; Proffitt and Virshup, 2012; van den Heuvel et al., 1993; Zhai et al., 2004). This lipid modification is essential for WNT activity, and, as demonstrated by the X-ray crystal structure of a WNT protein in complex with its receptor, is directly involved in receptor binding (Janda et al., 2012). Given the high degree of homology amongst members of the WNT gene family, it is generally accepted that disruption of PORCN activity either by mutation or with small molecule inhibitors impairs processing of all WNT proteins. Therefore, PORCN dysfunction will produce an “all-WNT” mutant phenotype.
Porcn knockout mice are early embryonic lethal and fail to enter early stages of embryonic induction as indicated by the absence of Bry/T expression at E6.5 (Barrott et al., 2011; Biechele et al., 2011; Liu et al., 2012), a nearly identical phenotype to that observed in Wnt3 knockout mice (Liu et al., 1999). In humans, PORCN mutations lead to a rare pleiotropic disorder called Focal Dermal Hypoplasia (FDH, also known as Goltz Syndrome) (Grzeschik et al., 2007; Wang et al., 2007), characterized by skin lesions and defects of the gastrointestinal, cardiovascular and central nervous system (Temple et al., 1990). In affected females, the severity of disease is influenced by the nature of the PORCN mutation and the extent to which the mutant PORCN allele is on the active X chromosome. Males carrying PORCN mutations are rare and have acquired somatic mutations during embryonic development and hence are mosaic with respect to the PORCN mutation and PORCN function. Since the mammalian genome encodes a single PORCN gene that specifically modifies WNT proteins, it offers a natural bottle-neck in WNT processing to explore the requirement of WNT proteins in any biological process.
In this study, we show that fibroblasts from individuals carrying mutations in the PORCN gene fail to reprogram to an induced pluripotent state. This blockade to iPS cell formation is readily overcome through the ectopic activation of WNT signaling or by overexpression of PORCN. Additionally, we show that precise dosing of WNT activity is critical to generating karyotypically normal iPS cells, an observation that has important implications for WNT’s role in promoting genomic instability and hence in cancer progression.
RESULTS
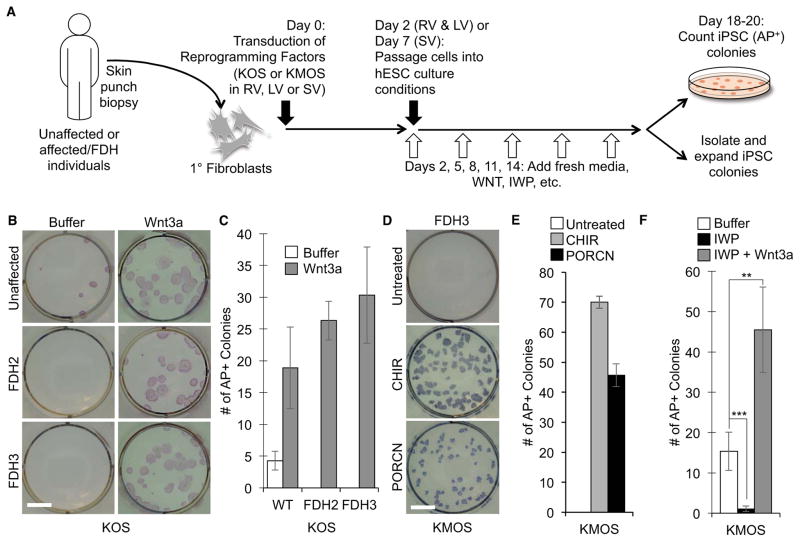
Focal dermal hypoplasia (FDH) is a rare genetic syndrome with many pleiotropic consequences caused by mutations in the X-linked gene PORCN. To develop a model for this complex disorder, we sought to develop a disease-in-a-dish by first reprogramming fibroblast cultures from FDH patients to induced pluripotent stem (iPS) cells (Figure 1A). To this end, we established fibroblast cultures from skin punch biopsies from individuals with clinical hallmarks of FDH and confirmed the presence of PORCN mutations by sequencing (summarized in Table S1). One of the mutations (FDH2) altered the highly conserved GT splice donor site after exon 2, which allowed us to demonstrate by reverse transcription PCR that the fibroblast culture expressed undetectable to very low levels of the wiltype PORCN gene (Figure S1A and B). Interestingly, in contrast to wild-type (WT) fibroblasts, FDH fibroblasts failed to yield iPS cell colonies (Figure 1B and C, Table S1). Since PORCN encodes an essential WNT processing enzyme, we reasoned that a defect in WNT secretion contributed to the observed reprogramming defect. Consistent with this hypothesis, FDH fibroblasts (FDH2 and FDH3) failed to secrete WNT proteins at levels observed for WT fibroblasts (an age-matched control FDH6 and FDH7), as monitored by secretion of the WNT protein encoded by the WNT5A gene (Figure S1C and D). (Note: other WNT proteins may be secreted by WT cells, however, we currently do not have a sensitive detection assay for other WNT proteins.) Treatment of WT fibroblasts with the PORCN inhibitor IWP2 (IWP)(Chen et al., 2009) greatly reduced levels of WNT5A protein in the CM, confirming that WNT secretion is dependent on PORCN function (Figure S1D).
Figure 1.
Ectopic WNT signaling and PORCN overexpression restores reprogramming of PORCN mutant fibroblasts. A. Flow chart of reprogramming experiments. Fibroblast cultures established from unaffected and affected FDH individuals were transduced with reprogramming factors (KLF4, MYC, OCT4, SOX2 [KMOS] or KLF4, OCT4, SOX2 [KOS]) via viral infections with either retro- (RV), lenti- (LV) or Sendai-viral (SV) vectors. After passaging to hESC culture conditions, culture media were supplemented with compounds that perturb WNT signaling. Once colonies were visible by eye, cultures were fixed and stained for Alkaline Phosphatase (AP) to quantify colony numbers per condition, or individual colonies were isolated and expanded as iPS cell lines. B. PORCN mutant fibroblasts (FDH2 and 3) fail to reprogram in the absence of exogenous WNT activation. Addition of Wnt3a during reprogramming restores generation of AP+ colonies. Buffer = WNT storage buffer. Shown are representative images of AP stained cultures. Scale bar = 10 mm. C. Quantitation of reprogramming experiments in FDH fibroblasts. (mean ± SD of four biological replicates for WT and of three biological replicates for FDH2 and 3). D. Overexpression of PORCN in FDH3 fibroblasts, as well as treatment with the GSK3 inhibitor CHIR98014 (CHIR), rescues the reprogramming defect of PORCN mutant cells. Scale bar = 10 mm. E. Quantitation of reprogramming experiments in FDH fibroblasts. (mean ± SD of three biological replicates for FDH3). F. A PORCN inhibitor blocks reprogramming. Fibroblasts undergoing reprogramming in the presence of the PORCN inhibitor IWP fail to yield AP positive (AP+) colonies. This inhibitory effect is overcome by addition of Wnt3a protein. (mean ± SD of three biological replicates. **P < 0.005; ***P < 0.001). See also Figure S1 and Table S1.
Importantly, treatment with Wnt3a after transduction of the reprogramming factors restored iPS cell generation from FDH fibroblasts (Figure 1B and C, Table S1), suggesting that endogenous WNT secretion and signaling are required during the process of reprogramming from a fibroblast to an iPS cell. Reprogramming of FDH fibroblasts required exogenous Wnt3a irrespective of whether MYC, a known WNT/β-catenin target in several cell types (He et al., 1998; Zhang et al., 2012), was included as one of the reprogramming factors (KMOS versus KOS; Table S1). The fact that inclusion of MYC as a reprogramming factor fails to yield FDH-iPS cells indicates that transcriptional activation of MYC by WNT is insufficient in promoting reprogramming and that other downstream WNT targets are involved. In addition, we found that a GSK3 inhibitor, CHIR98014 (CHIR), rescued FDH fibroblast reprogramming (Table S1), suggesting that WNT acts through the stabilization of its key mediator, β-catenin. These data indicate that WNT acts through β-catenin (i.e. the canonical WNT pathway) to promote reprogramming to the induced pluripotent state.
The observation that exogenous Wnt3a rescues reprogramming of FDH fibroblasts implies that the mutant PORCN protein fails to properly process endogenously expressed WNT proteins. To address this point we transduced a PORCN transgene along with the KMOS reprogramming factors. As expected, overexpression of PORCN, like ectopic Wnt3a treatment, rescued the reprogramming defect of FDH fibroblasts (Figure 1D and E). Isolated PORCN-rescued FDH iPS cells carried the PORCN transgene (Figure S1E). Therefore, in FDH fibroblasts, PORCN is an essential reprogramming factor along with KMOS.
Consistent with this genetic evidence that PORCN function is required for reprogramming, we found that treatment of WT fibroblast cultures undergoing standard reprogramming assays with the PORCN inhibitor IWP significantly reduced the number alkaline phosphatase positive (AP+) colonies (Figure 1F). This reduction in reprogramming rates was previously reported by others (Ho et al., 2013). As with FDH fibroblasts, this blockade on reprogramming was overcome by addition of exogenous Wnt3a protein, suggesting that PORCN processing of endogenously produced WNT proteins is required for cellular reprogramming.
Isolation and expansion of iPS cell colonies generated from FDH fibroblasts required continuous ectopic stimulation of WNT signaling. When cultured in media containing CHIR at low concentrations (20nM), FDH-iPS cells expressed the pluripotency markers NANOG and POU5F1/OCT4 at levels comparable to WT iPS cells (Figure 2A). In the absence of CHIR, NANOG and POU5F1/OCT4 expression levels were unaffected in WT iPS cells but decreased in FDH-iPS cells, indicating that an endogenous WNT signaling loop maintains optimal expression levels of these pluripotency regulators. As expected, at high CHIR concentrations (200 to 1000nM), NANOG and POU5F1/OCT4 expression levels declined dramatically, indicative of cells exiting the pluripotent state. In addition, in the presence of low CHIR concentrations (20nM), FDH-iPS cells displayed morphologies most closely resembling those of human pluripotent stem cells (Figure 2B). Withdrawal of CHIR from FDH-iPS cells led to marked reduction in cell surface staining of the pluripotency markers SSEA4 and TRA1-81 (Figure 2C). Similar dosage effects were observed when Wnt3a protein was used in place of CHIR (Figure S2). Therefore, endogenous WNT signaling, as exists in WT iPS cells, or a low level of exogenous WNT signal activation with CHIR in FDH iPS cells is required to maintain normal expression of pluripotency-associated genes.
Figure 2.
WNT signaling is required to maintain the pluripotent state. A. WT iPS or FDH iPS (Clone 3.C.1) cells were cultured for 5 days with increasing doses of CHIR98014 (CHIR) and expression of POU5F1/OCT4 and NANOG was determined by qRT-PCR. See also Figure S2. B. Clone 3.C.1 requires low levels of WNT stimulation to maintain an optimal hPSC morphology. In the absence of CHIR, WT iPSCs exhibit a normal hPSC morphology whereas FDH-iPSCs fail to grow with the characteristic morphology associated with hPSCs. At CHIR concentrations of 20nM, FDH iPS cell colonies most closely resemble WT iPS cells. At concentrations of 200nM, both cell populations assume a cell morphology indicative of differentiation. Scale bar = 500μM. C. FDH iPS (Clone 3.C.4) cells were cultured for 5 days in the presence (left) or absence (right) of CHIR (20nM), stained for the cell surface markers SSEA4 and TRA1-81 and analyzed by flow cytometry. Significantly higher cell surface staining for these two pluripotency-associated markers is observed in the presence of CHIR (70%) than in the absence of CHIR (21%).
Additional characterization confirmed that FDH-iPS cells exhibit properties associated with bona-fide pluripotent stem cells, including the characteristic morphology associated with human embryonic stem cells (Figure 3A), and expression of pluripotency markers, such as OCT4, NANOG (Figure 3B) and TRA-1-81 (Figure 3C) and SSEA4 (Figure S3A). In addition, we confirmed their pluripotency by directing them to differentiate via embryoid bodies (EB) into cell populations of the three major germ layers, endo-, ecto-, and meso-derm. EBs from FDH-iPS cell clone 3.C.1 expressed genes indicative of definitive endoderm (DE, SOX17, FOXA2 and CXCR4 [Figure 3D and Figure S3B, C]), mesoderm (BRY/T, MESP1 and ACTC1 [Figure 3E and Figure S3D]), and ectoderm (PAX6, SOX1 and TUBB3 [Figure 3F]). Therefore, the FDH-iPS cells retain their ability to differentiate into cell types representative of the three major germ layers.
Figure 3.
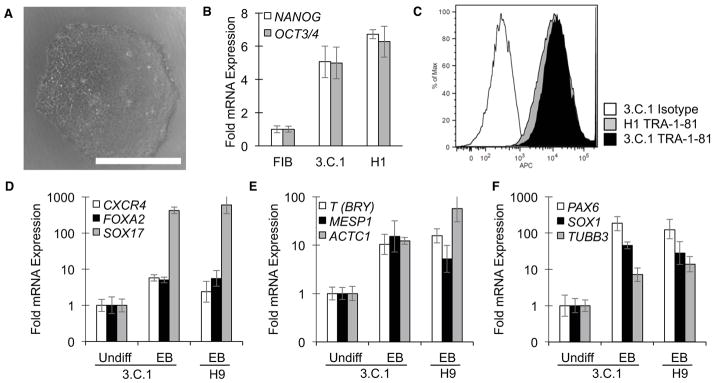
Characterization of FDH-iPS cell clone. A. A phase contrast image of a FDH-iPS cell clone (3.C.1) derived in the presence of CHIR exhibits characteristics of human pluripotent stem cells. Note: as shown in Figure 4D, these cells have a normal number of chromosomes. Scale bar = 500 μm. B. Quantitative reverse transcription PCR (qRT-PCR) confirms expression of the pluripotency markers NANOG and OCT4/POU5F1 at levels comparable to a human embryonic stem cell line (H1/WA01). C. Flow cytometry confirms presence of the cell surface pluripotency markers TRA1-81 at levels comparable to that found on H1 cells. D–F. Upon differentiation via embryoid body (EB) formation, cells express markers associated with endodermal (D), mesodermal (E), and ectodermal (F) lineages. See also Figure S3.
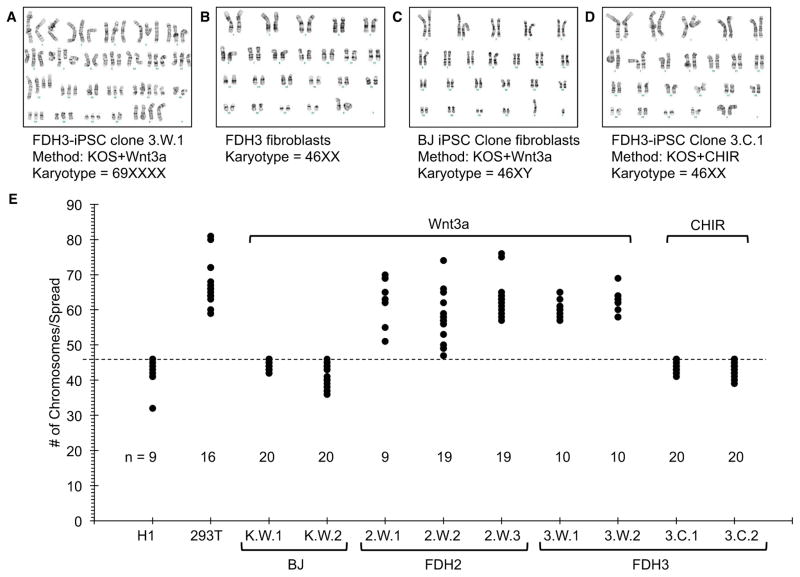
Surprisingly, FDH-iPS cell clones isolated in the presence of Wnt3a exhibited dramatically altered karyotypes, with cells containing more than 60 chromosomes and other chromosomal aberrations, including insertions, deletions, translocations and inversions (Figure 4A, Figure S4A–C). Since the FDH fibroblasts were euploid (Figure 4B), the acquisition of aberrant chromosome numbers likely occurred during reprogramming. Importantly, iPS cells derived from WT fibroblasts (BJ) in the presence of Wnt3a were karyotypically normal (Figure 4C), indicating that changes in chromosome numbers upon reprogramming are associated with ectopic WNT signaling only in a PORCN-deficient context. Karyotypic abnormalities in Wnt3a-derived FDH-iPS cells did not represent clonal expansions since individual metaphase spreads from the same iPS cell line exhibited distinct chromosome numbers (Figure 4E, Figure S4A–C). Therefore, reprogramming of FDH fibroblasts (and hence WNT-deficient cells) in the presence of Wnt3a is detrimental to genomic integrity.
Figure 4.
Ectopic Wnt3a stimulation yields karyotypically abnormal iPS cells. A. A FDH3-iPS cell derived in the presence of exogenous Wn3a (Clone 3.W.1) exhibits an abnormal chromosome number. Additional images of abnormal chromosome spreads highlighting chromosomal anomalies, such as insertions, deletions, translocations and inversions, are provided in the Supplemental Information (Figure S4). B. FDH3 fibroblasts exhibit a normal karyotype. C. An iPS cell derived from unaffected fibroblasts (BJ, ATCC CRL-2522) in the presence of Wnt3a is euploid. Additional characterization of this iPS cell clone is provided in Supplemental Information (Figure S5). D. A FDH-iPS cell (Clone 3.C.1) derived in the presence of the GSK3 inhibitor CHIR98014 (CHIR) is euploid. E. Quantitation of chromosome numbers in FDH-iPS cells. Chromosome numbers were obtained by counting condensed chromosomes of metaphase-arrested cells (for representative images see Supplemental Information Figure S4C). The dashed line marks 46 chromosomes. Like HEK-293T (293T) cells, FDH-iPS cells derived in the presence of exogenous Wnt3a carried significantly increased numbers of chromosomes. The hESC line H1, BJ-iPS cells derived in the presence of Wnt3a (K.W.1 and K.W.2) and FDH-iPS cells derived in the presence of CHIR (clones 3.C.1 and 3.C.2) have 46 chromosomes. Note: This method to quantify chromosome numbers is only reliable in determining the maximum number of chromosomes; metaphase spreads with less than 46 are most likely due to loss of chromosomes during the preparation of the samples. See also Figure S4.
Since purified WNT proteins are highly unstable (Dhamdhere et al., 2014; Green et al., 2013), we reasoned that daily addition of Wnt3a protein may produce non-physiological spikes in WNT signaling activity that may contribute to this chromosomal instability. Previous studies have linked aberrant WNT signaling to chromosomal instability (Aoki et al., 2007; Fodde et al., 2001; Hadjihannas and Behrens, 2006; Hadjihannas et al., 2006; Kaplan et al., 2001; Tighe et al., 2007). To circumvent this problem, we isolated FDH-iPS cell colonies from reprogrammed cultures derived in the presence of CHIR, which can be carefully dosed and is more stable over longer periods than Wnt3a protein (Figure S4D). Importantly, iPS cell clones isolated, expanded and maintained in the presence of low CHIR concentrations that do not promote differentiation of hESCs exhibited normal chromosome numbers (Figure 4D and E, Figure S4C), indicating that a continuous and low level of WNT signaling activity, as provided by CHIR, is essential for the generation of euploid iPS cell colonies from PORCN-mutant fibroblasts.
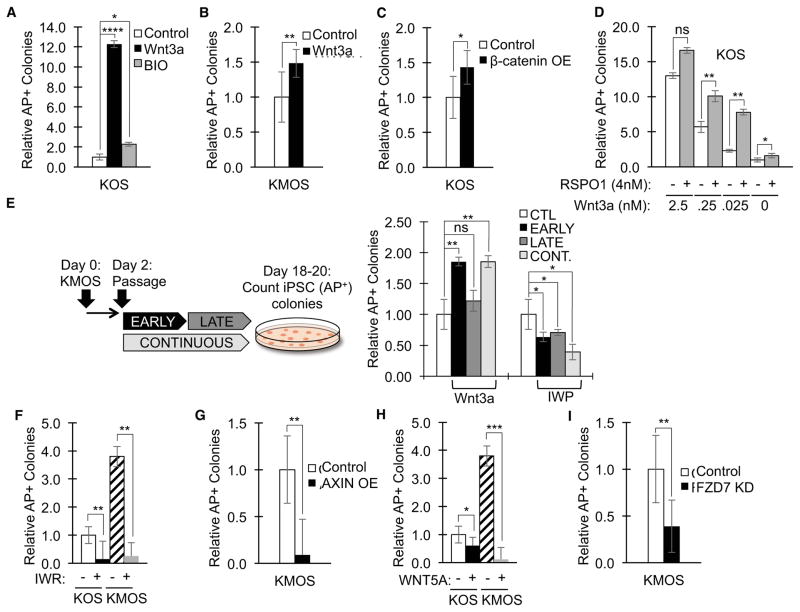
To gain a better understanding of the role of the WNT signaling pathway during reprogramming, we perturbed the pathway using a number of manipulations. Addition of Wnt3a protein during reprogramming significantly increased the number of AP+ colonies (Figure 5A), consistent with findings by other groups (Ho et al., 2013; Marson et al., 2008; Zhang et al., 2014). IPS cells derived from WT fibroblasts in the presence of Wnt3a displayed all the hallmarks of bona-fide iPS cells, including a normal karyotype of 46 chromosomes (Figure 4C), the characteristic cell morphology of hPSCs (Figure S5A), and expression of the pluripotency markers OCT4, NANOG (Figure S5B), TRA-1-81 (Figure S5C), and SSEA4 (Figure S5D). In addition, these iPS cells were pluripotent, as determined by their ability to differentiate into endodermal (expression of FOXA2 and SOX17 [Figure S5E] and CXCR4 ([Figure S5E and F]), ectodermal (expression of PAX6, SOX1 and MAP2 [Figure S5G]), and mesodermal lineages (expression of T (BRY), MESP1 and ACTC1 [Figure S5H]).
Figure 5.
Modulation of WNT signaling influences reprogramming efficiencies. Fibroblasts were reprogrammed with factors KOS or KMOS in the presence of the indicated compounds or transgenes. A. Wnt3a and the GSK3 inhibitor BIO increase KOS reprogramming of wild-type fibroblasts. (Quantification represents mean number of AP+ colonies ± SD, normalized to untreated condition [CTL]; n=7 for CTL, n=6 for Wnt3a, and n=3 for BIO. * P ≤ 0.05, **** P ≤ 0.0001). B. Wnt3a increases KMOS reprogramming of wild-type fibroblasts. (Quantification represents mean number of AP+ colonies ± SD, normalized to untreated condition [CTL]; n=23 for CTL and n=6 for Wnt3a. ** P ≤ 0.01). C. Overexpression (OE) of constitutively active β-catenin (β-catenin 4A) increases reprogramming efficiencies. (Quantification represents mean number of AP+ colonies ± SD, normalized to untreated condition [no Wnt3a and no RSPO1]; n=13 for KOS, n=3 for all other conditions. * P ≤ 0.05). D. RSPO1 sensitizes cells to low levels of Wnt3a. (Quantification represents mean number of AP+ colonies ± SD, normalized to untreated condition [no Wnt3a and no RSPO1]; n=13 for KOS, n=3 for all other conditions. P-values are between corresponding Wnt3a concentrations ± RSPO1, ns = not significant [p-value for 2.5nM Wnt3a data points = 0.061], * P ≤ 0.05 [p-value for 0nM Wnt3a data points = 0.022], ** P ≤ 0.01). E. Addition of Wnt3a increases AP+ colony numbers when added early during the reprogramming process, whereas addition of IWP reduces AP+ colony numbers when added early or late. (Quantification represents mean number of AP+ colonies, normalized to untreated condition [CTL]; n=3 for all conditions). CONT. = continuous treatment with Wnt3a or IWP. F. The Tankyrase inhibitor IWR, which induces AXIN stabilization, reduces numbers of AP+ colonies. (Quantification represents mean number of AP+ colonies ± SD, normalized to untreated KOS condition; n=7 for KOS, n=5 for KOS+IWR, n=13 for KMOS, and n=3 for KMOS+IWR; ** P ≤ 0.01). G. AXIN overexpression (OE) by lenti-viral gene transduction reduces numbers of AP+ colonies. (Quantification represents mean number of AP+ colonies ± SD, normalized to KMOS condition [CTL]; n=23 for CTL and n=9 for AXIN; ** P ≤ 0.01). H. Treatment of reprogramming cultures with Wnt5a, which antagonizes WNT/β-catenin signaling, reduces numbers of AP+ colonies. (Quantification represents mean number of AP+ colonies ± SD, normalized to untreated KOS condition; n=7 for KOS, n=4 for KOS+Wnt5a, n=23 for KMOS, and n=4 for KMOS+Wnt5a; * P ≤ 0.05, *** P ≤ 0.001). I. FZD7 knockdown (KD) by lenti-viral transduction of a FZD7-specific shRNA reduces numbers of AP+ colonies. (Quantification represents mean number of AP+ colonies ± SD, normalized to standard condition [CTL]; n=13 for CTL and n=8 for FZD7 knockdown, ** P ≤ 0.01). See also Figure S5.
The enhancement of AP+ colonies was particularly pronounced in the absence of MYC (KOS versus KMOS, Figure 5A versus 5B). Likewise, addition of the GSK3 inhibitor BIO increased the number of iPS cell colonies (Figure 5A). Overexpression of constitutively active β-catenin (β-catenin 4A) increased reprogramming efficiencies by 1.4-fold (Figure 5C), thus confirming the findings by several other groups, including the original description of iPS cell generation (Takahashi and Yamanaka, 2006). Addition of RSPO1, a WNT agonist and LGR4/5/6 ligand (Carmon et al., 2011; de Lau et al., 2011; Ruffner et al., 2012), sensitized cells to low levels of exogenously added Wnt3a (Figure 5D). In the absence of exogenous Wnt3a, RSPO1-treated cells exhibited a subtle and reproducible increase in reprogramming efficiency. Since RSPO1 itself fails to activate WNT/β-catenin signaling and only acts to augment WNT signaling, potentially through WNT receptor turnover by ZNRF3 (Hao et al., 2012), this result is consistent with the model that an endogenous WNT signaling pathway is present and is uncovered by RSPO1.
Several recent publications examined at what stage WNT signaling most significantly influenced the rate of reprogramming. These studies reached opposite conclusions with two studies demonstrating that early WNT activation inhibited (Aulicino et al., 2014; Ho et al., 2013) and another study demonstrating that early WNT activation stimulated reprogramming rates (Zhang et al., 2014). We do not have an explanation for this discrepancy, however, our findings strongly argue that WNT signaling not only enhances reprogramming, but is also required for reprogramming. In addition, in our system, which differs from these other studies in that it is human and does not involve the use of secondary iPS cells, we find that early activation of WNT signaling promotes reprogramming rates while late activation has no impact (Figure 5E). Treatment with IWP during any stage of reprogramming diminishes the number of AP+ colonies. We therefore speculate that WNT signaling is required early to establish the pluripotent state.
In contrast to activation of WNT signaling, inhibition of the WNT signaling pathway reduced reprogramming efficiencies. Like IWP (Figure 1F and Figure 5E), blocking WNT/β-catenin signaling with IWR (Chen et al., 2009), which acts to stabilize AXIN (a component of the β-catenin degradation complex), reduced reprogramming efficiencies (Figure 5F). Consistent with the effect of IWR, overexpression of AXIN by lentiviral transduction reduced reprogramming efficiencies (Figure 5G). Treatment with purified Wnt5a, which antagonizes WNT/β-catenin signaling (Ishitani et al., 2003; Mikels and Nusse, 2006), also interfered with reprogramming (Figure 5H), indicating that this non-canonical WNT signaling pathway blocks reprogramming. Therefore, canonical, rather than non-canonical WNT signaling promotes reprogramming.
Several studies have implicated the WNT receptor encoded by the FZD7 gene to be critical to the maintenance of the pluripotent stem cell state (Fernandez et al., 2014; Melchior et al., 2008). Furthermore, FZD7 is the most abundantly expressed FZD gene (the mammalian genome encodes 10 FZD genes) in hESCs (Fernandez et al., 2014). We therefore examined to what extent knockdown of FZD7 expression affected reprogramming rates. Lenti-viral transduction of a FZD7-specific short hairpin RNA (shRNA) significantly reduced reprogramming rates (Figure 5I), suggesting a potential role for FZD7 in reprogramming. However, since an endogenous WNT-FZD signaling loop is essential to the maintenance of the pluripotent state, it is difficult to distinguish whether this negative effect of FZD7 knockdown is due to an effect on the reprogramming process or due to an effect on the maintenance and expansion of iPS cells. Nonetheless, these WNT signal pathway perturbations provide strong evidence that reprogramming requires an active endogenous WNT/FZD7/β-catenin signal.
DISCUSSION
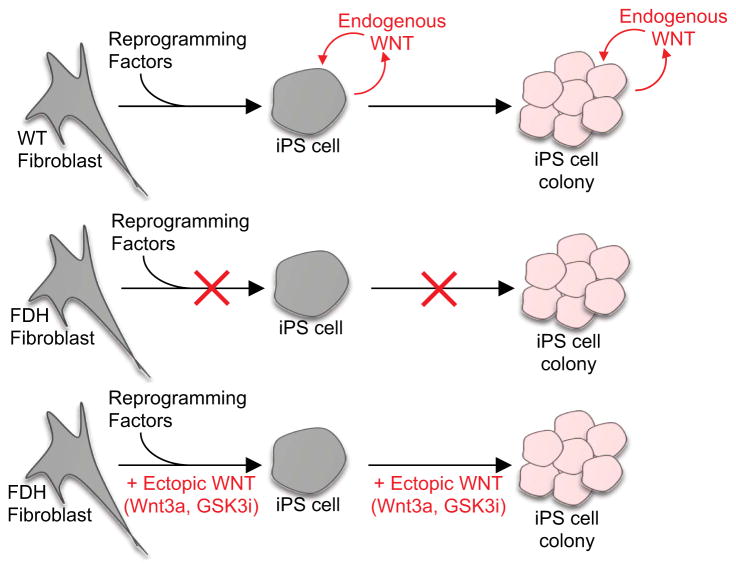
Here we show that WNT signaling is required for the generation of iPS cells from fibroblasts. Mutation of the PORCN gene or chemical inhibition of this essential WNT processing enzyme potently blocks iPS cell generation, a blockade that is readily overcome through the ectopic activation of the WNT/β-catenin signaling pathway. This effect of WNT signaling can be attributed to two possible, and not necessarily mutually exclusive, modes of action (summarized in Figure 6). First, activation of WNT signaling is a requisite step to convert a differentiated cell to an iPS cell. Second, continuous WNT signaling is required to promote expansion of a single iPS cell to an iPS cell colony.
Figure 6.
A model summarizing the role of WNT signaling during the reprogramming process. An endogenous WNT signaling loop is required for acquisition of the pluripotent state and for the expansion of iPS cells.
Our experiments, as well as studies by several other groups, indicate that endogenous WNT signaling is required to maintain the pluripotent state. Blocking endogenous WNT signaling with PORCN inhibitors, such as IWP2, leads to a decrease in expression of WNT target genes AXIN2 and SP5 and a concomitant reduction in expression of the pluripotency regulators NANOG and OCT4 (Fernandez et al., 2014). Consistent with these observations, WNT signal activation is required to generate iPS cells in which endogenous WNT signaling is disrupted through PORCN mutation or inhibition. Interestingly, expression of pluripotency genes is sensitive to the level of WNT signal activation in FDH-iPS cells but not in wild-type iPS cells, again consistent with the notion that WNT signaling is required to maintain expression of pluripotency components. We also found that the WNT co-factor RSPO1, which augments WNT signaling activity, increases the efficiency of reprogramming, suggesting that it uncovers an endogenous WNT signaling activity necessary to promote the reprogramming process. Together, these data support the model that endogenous WNT signaling is required to maintain pluripotency and consequently is essential to induce pluripotency in fibroblasts.
Our experiments to address the timing requirement for WNT signaling during reprogramming indicate that WNT signal activation is more critical during the early rather than later stages of reprogramming, consistent with the results of others (Zhang et al., 2014). However, other groups found that activation of WNT signaling during early stages inhibits reprogramming while inhibition of WNT signaling during early stages promotes reprogramming (Aulicino et al., 2014; Ho et al., 2013). We currently do not have an explanation for this discrepancy. However, it is important to stress that WNT signaling exhibits distinct dosage effects, with low levels promoting the pluripotent state, as discussed above, and high levels promoting mesendodermal differentiation (Bakre et al., 2007; Davidson et al., 2012). Treatment of undifferentiated human embryonic stem cells with Wnt3a or a GSK3 inhibitor is an established method to direct definitive endoderm differentiation (Brafman et al., 2013; D’Amour et al., 2005; Kroon et al., 2008; Schulz et al., 2012). Therefore, over-stimulation of WNT signaling may drive pluripotent stem cells into a mesendodermal lineage which will lack expression of pluripotency-associated genes and hence will not be observed as iPS cells.
The dosing of WNT activity not only affects the choice between self renewal and differentiation, but also influences genomic integrity. In our reprogramming experiments we found that ectopic WNT signal activation with Wnt3a promoted genomic instability during reprogramming of PORCN mutant cells. The association between ectopic WNT signaling and chromosomal instability had been described previously but only in cells with compromised genomic integrity, such as HCT116, SW480 and HeLa cells (Hadjihannas and Behrens, 2006; Tighe et al., 2007). Additional studies found that mouse embryonic stem cells harboring mutations in the APC gene, a negative regulator of WNT/β-catenin signaling, exhibit defects in chromosome segregation, leading to highly variable chromosome numbers (Aoki et al., 2007; Fodde et al., 2001; Kaplan et al., 2001). We extend these studies by showing that cells harboring PORCN mutations, and are consequently deficient for endogenous WNT protein processing, are exquisitely sensitive to the level of exogenous WNT signaling activity, with low and stable levels of WNT activation promoting iPS cell generation without inducing genomic instability. In contrast, higher levels of WNT signal activation invariably yielded iPS cells with grossly aberrant chromosome numbers. These findings on WNT’s effects on genomic integrity serve as a cautionary note for the manipulation of WNT signaling in the generation of cell populations intended for cell replacement therapies since such genomic aberrations may promote tumorigenesis. Our observation that ectopic WNT signaling promotes chromosome mis-segregation and results in aneuploidy may be related to WNT’s function as a locally acting polarity ligand as previously demonstrated with bead-immobilized WNT protein (Habib et al., 2013).
The process of reprogramming a mature cell type to an embryonic stem cell-like state shares similarities with the processes of cellular and tissue regeneration, especially as it occurs in vertebrates with high regenerative potential, such as Axolotl and Zebrafish. As these organisms repair damaged tissues, such as an amputated limb, a cell population, called the blastema, is formed that orchestrates the regenerative process. Formation of the blastema is thought to involve cellular de-differentiation in a manner that may resemble cellular reprogramming and the formation of induced pluripotent stem (iPS) cells. WNT genes are required for blastema formation (Kawakami et al., 2006; Stoick-Cooper et al., 2007). In addition, several studies have linked WNT signaling to tissue repair and wound healing, including in skin and bone (Chen et al., 2007; Chua et al., 2011; Fathke et al., 2006; Lim et al., 2013; Whyte et al., 2013). It is tempting to speculate that the observed requirement for WNT signaling during the induction of the pluripotent state represents a cell culture equivalent to these in vivo regenerative processes. Additional studies are needed to address the extent to which WNT’s role in reprogramming resembles its role in regenerative processes, such as wound healing.
EXPERIMENTAL PROCEDURES
Collection of FDH patient biopsies and establishment of fibroblast cultures
Skin punch biopsies were collected from consented individuals under Protocol H-21291 (Principal Investigator: Ignatia Barbara Van Den Veyver; Protocol Title: “Pathogenesis Of Focal Dermal Hypoplasia Or Goltz Syndrome And Related Disorders, And The Role Of PORCN In Focal Dermal Hypoplasia”), which was approved by the Institutional Review Board for Baylor College of Medicine and Affiliated Hospitals. Fibroblast cultures were established as described in the Supplemental Information.
Cells and culture conditions
Cells were cultured as described in Supplemental Information. Human ES cell lines (H1/WA01/NIH Registration Number 0043; H9/WA09/NIH Registration Number 0062; HUES 9/NIH Registration Number 0022) and iPS cell lines were cultured as described in (Chen et al., 2011). Experiments involving human embryonic and induced pluripotent stem cells were approved by UCSD Human Research Protections Program: Principal Investigator: Karl Willert, Protocols #120799: “A model for focal dermal hypoplasia using human induced pluripotent stem cells”, and #100210: “Role of Wnt signaling in human ES cell proliferation and differentiation”.
Plasmids, recombinant proteins, small molecules and antibodies
Sources of plasmids, small molecules and antibodies are provided in Supplemental Text. WNT proteins were purified as described (Willert, 2008). RSPO1 was purified as previously described (Wei et al., 2007) with the modifications described in Supplemental Information.
Reprogramming and differentiation protocols
Details of the reprogramming protocols, quantitation of iPS cell colonies, and differentiation into EBs or specific lineages are provided in the Supplemental Information.
Gene expression analysis
Gene expression was analyzed by reverse transcription quantitative polymerase chain reaction (qRT-PCR) using the protocols and primers provided in the Supplemental Information.
Flow cytometry
Flow cytometry was performed in the UCSD Human Embryonic Stem Cell Core Facility with experimental details and antibodies provided in the Supplemental Information.
Karyotyping
Karyotyping with G-banding was performed by Cell Line Genetics, Inc. (Madison, WI). Chromosome counts shown in Figures 4E and S4C were performed as described in the Supplemental Information.
WNT protein detection
WNT5A protein was detected by immunoblotting of Blue Sepharose-precipitates of fibroblast conditioned media, as shown in Figure S1D. A detailed protocol is provided in Supplemental Information.
Supplementary Material
Acknowledgments
We are grateful to our colleagues Dr. Larry Goldstein (UCSD, La Jolla, CA, USA) and Dr. Roel Nusse (Stanford School of Medicine, CA, USA) for critically reading the manuscript prior to submission, to Dr. Xi He (Children’s Hospital Boston, Harvard Medical School, MA, USA) for kindly providing HEK293 cells expressing human RSPO1, Dr. Roel Nusse for providing Wnt5a-specific antibody, Dr. Randall T. Moon (University of Washington, Seattle, WA, USA) for providing the Super-TOP-Flash vector, and Dr. Tomas Bos (UCSD, La Jolla, CA) for providing reagents and advice for retro- and lenti-virus production. Many tanks to the National Foundation for Ectodermal Dysplasias for providing support for these studies, and to the families of affected individuals for participating in this study. This work was supported by grants to KW from the California Institute for Regenerative Medicine (CIRM, RB1-01406), the National Institute of Health (1R01GM110304-01) and by the UCSD Stem Cell Program, and was made possible in part by the CIRM Major Facilities grant (FA1-00607) to the Sanford Consortium for Regenerative Medicine. This work was supported in part by cores from the BCM IDDRC Grant Number 5P30HD024064 from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development.
Footnotes
AUTHOR CONTRIBUTIONS
KW and JR designed research; JR, JB, EM, DN, AS, DB and KW performed research; VRS and IVdV contributed new reagents/analytic tools, KW and JR analyzed data and wrote the paper.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute Of Child Health & Human Development or the National Institutes of Health.
References
- Aoki K, Aoki M, Sugai M, Harada N, Miyoshi H, Tsukamoto T, Mizoshita T, Tatematsu M, Seno H, Chiba T, et al. Chromosomal instability by beta-catenin/TCF transcription in APC or beta-catenin mutant cells. Oncogene. 2007;26:3511–3520. doi: 10.1038/sj.onc.1210141. [DOI] [PubMed] [Google Scholar]
- Aulicino F, Theka I, Ombrato L, Lluis F, Cosma MP. Temporal Perturbation of the Wnt Signaling Pathway in the Control of Cell Reprogramming Is Modulated by TCF1. Stem cell reports. 2014;2:707–720. doi: 10.1016/j.stemcr.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakre MM, Hoi A, Mong JC, Koh YY, Wong KY, Stanton LW. Generation of multipotential mesendodermal progenitors from mouse embryonic stem cells via sustained Wnt pathway activation. The Journal of biological chemistry. 2007;282:31703–31712. doi: 10.1074/jbc.M704287200. [DOI] [PubMed] [Google Scholar]
- Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biechele S, Cox BJ, Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Developmental biology. 2011;355:275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Blauwkamp TA, Nigam S, Ardehali R, Weissman IL, Nusse R. Endogenous Wnt signalling in human embryonic stem cells generates an equilibrium of distinct lineage-specified progenitors. Nature communications. 2012;3:1070. doi: 10.1038/ncomms2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brafman DA, Phung C, Kumar N, Willert K. Regulation of endodermal differentiation of human embryonic stem cells through integrin-ECM interactions. Cell death and differentiation. 2013;20:369–381. doi: 10.1038/cdd.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature chemical biology. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, et al. Chemically defined conditions for human iPSC derivation and culture. Nature methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS medicine. 2007;4:e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua AW, Ma D, Gan SU, Fu Z, Han HC, Song C, Sabapathy K, Phan TT. The role of R-spondin2 in keratinocyte proliferation and epidermal thickening in keloid scarring. The Journal of investigative dermatology. 2011;131:644–654. doi: 10.1038/jid.2010.371. [DOI] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes & development. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nature biotechnology. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ, Moon RT. Wnt/beta-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- Dhamdhere GR, Fang MY, Jiang J, Lee K, Cheng D, Olveda RC, Liu B, Mulligan KA, Carlson JC, Ransom RC, et al. Drugging a stem cell compartment using wnt3a protein as a therapeutic. PloS one. 2014;9:e83650. doi: 10.1371/journal.pone.0083650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathke C, Wilson L, Shah K, Kim B, Hocking A, Moon R, Isik F. Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC cell biology. 2006;7:4. doi: 10.1186/1471-2121-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Huggins IJ, Perna L, Brafman D, Lu D, Yao S, Gaasterland T, Carson DA, Willert K. The WNT receptor FZD7 is required for maintenance of the pluripotent state in human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1409–1414. doi: 10.1073/pnas.1323697111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nature reviews Cancer. 2001;1:55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- Galli LM, Barnes TL, Secrest SS, Kadowaki T, Burrus LW. Porcupine-mediated lipid-modification regulates the activity and distribution of Wnt proteins in the chick neural tube. Development. 2007;134:3339–3348. doi: 10.1242/dev.02881. [DOI] [PubMed] [Google Scholar]
- Green JL, Bauer M, Yum KW, Li YC, Cox ML, Willert K, Wahl GM. Use of a molecular genetic platform technology to produce human Wnt proteins reveals distinct local and distal signaling abilities. PloS one. 2013;8:e58395. doi: 10.1371/journal.pone.0058395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeschik KH, Bornholdt D, Oeffner F, Konig A, del Carmen Boente M, Enders H, Fritz B, Hertl M, Grasshoff U, Hofling K, et al. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nature genetics. 2007;39:833–835. doi: 10.1038/ng2052. [DOI] [PubMed] [Google Scholar]
- Habib SJ, Chen BC, Tsai FC, Anastassiadis K, Meyer T, Betzig E, Nusse R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjihannas MV, Behrens J. CIN By WNT: growth pathways, mitotic control and chromosomal instability in cancer. Cell cycle. 2006;5:2077–2081. doi: 10.4161/cc.5.18.3282. [DOI] [PubMed] [Google Scholar]
- Hadjihannas MV, Bruckner M, Jerchow B, Birchmeier W, Dietmaier W, Behrens J. Aberrant Wnt/beta-catenin signaling can induce chromosomal instability in colon cancer. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10747–10752. doi: 10.1073/pnas.0604206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Herr P, Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Developmental biology. 2012;361:392–402. doi: 10.1016/j.ydbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Ho R, Papp B, Hoffman JA, Merrill BJ, Plath K. Stage-specific regulation of reprogramming to induced pluripotent stem cells by Wnt signaling and T cell factor proteins. Cell reports. 2013;3:2113–2126. doi: 10.1016/j.celrep.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Molecular and cellular biology. 2003;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Zhang D, Bursac N, Zhang Y. WNT3 Is a Biomarker Capable of Predicting the Definitive Endoderm Differentiation Potential of hESCs. Stem cell reports. 2013;1:46–52. doi: 10.1016/j.stemcr.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes & development. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nature cell biology. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Rodriguez Esteban C, Raya M, Kawakami H, Marti M, Dubova I, Izpisua Belmonte JC. Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes & development. 2006;20:3232–3237. doi: 10.1101/gad.1475106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature biotechnology. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou H, Abujarour R, Zhu S, Young Joo J, Lin T, Hao E, Scholer HR, Hayek A, Ding S. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells. 2009;27:2992–3000. doi: 10.1002/stem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Q, Yin X, Yang W, Du Y, Hou P, Ge J, Liu C, Zhang W, Zhang X, et al. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell research. 2011;21:196–204. doi: 10.1038/cr.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, van Amerongen R, Klein AM, Nusse R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 2013;342:1226–1230. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nature genetics. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Liu W, Shaver TM, Balasa A, Ljungberg MC, Wang X, Wen S, Nguyen H, Van den Veyver IB. Deletion of Porcn in mice leads to multiple developmental defects and models human focal dermal hypoplasia (Goltz syndrome) PloS one. 2012;7:e32331. doi: 10.1371/journal.pone.0032331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyashenko N, Winter M, Migliorini D, Biechele T, Moon RT, Hartmann C. Differential requirement for the dual functions of beta-catenin in embryonic stem cell selfrenewal and germ layer formation. Nature cell biology. 2011;13:753–761. doi: 10.1038/ncb2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young RA, Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell stem cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior K, Weiss J, Zaehres H, Kim YM, Lutzko C, Roosta N, Hescheler J, Muschen M. The WNT receptor FZD7 contributes to self-renewal signaling of human embryonic stem cells. Biological chemistry. 2008;389:897–903. doi: 10.1515/BC.2008.108. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS biology. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proffitt KD, Virshup DM. Precise regulation of porcupine activity is required for physiological Wnt signaling. The Journal of biological chemistry. 2012;287:34167–34178. doi: 10.1074/jbc.M112.381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffner H, Sprunger J, Charlat O, Leighton-Davies J, Grosshans B, Salathe A, Zietzling S, Beck V, Therier M, Isken A, et al. R-Spondin potentiates Wnt/beta-catenin signaling through orphan receptors LGR4 and LGR5. PloS one. 2012;7:e40976. doi: 10.1371/journal.pone.0040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature medicine. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Schulz TC, Young HY, Agulnick AD, Babin MJ, Baetge EE, Bang AG, Bhoumik A, Cepa I, Cesario RM, Haakmeester C, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PloS one. 2012;7:e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS biology. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Temple IK, MacDowall P, Baraitser M, Atherton DJ. Focal dermal hypoplasia (Goltz syndrome) Journal of medical genetics. 1990;27:180–187. doi: 10.1136/jmg.27.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge D, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, Siu RK, Nusse R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nature cell biology. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe A, Ray-Sinha A, Staples OD, Taylor SS. GSK-3 inhibitors induce chromosome instability. BMC cell biology. 2007;8:34. doi: 10.1186/1471-2121-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Harryman-Samos C, Klingensmith J, Perrimon N, Nusse R. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. The EMBO journal. 1993;12:5293–5302. doi: 10.1002/j.1460-2075.1993.tb06225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Reid Sutton V, Omar Peraza-Llanes J, Yu Z, Rosetta R, Kou YC, Eble TN, Patel A, Thaller C, Fang P, et al. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nature genetics. 2007;39:836–838. doi: 10.1038/ng2057. [DOI] [PubMed] [Google Scholar]
- Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. The Journal of biological chemistry. 2007;282:15903–15911. doi: 10.1074/jbc.M701927200. [DOI] [PubMed] [Google Scholar]
- Whyte JL, Smith AA, Liu B, Manzano WR, Evans ND, Dhamdhere GR, Fang MY, Chang HY, Oro AE, Helms JA. Augmenting endogenous Wnt signaling improves skin wound healing. PloS one. 2013;8:e76883. doi: 10.1371/journal.pone.0076883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert KH. Isolation and application of bioactive Wnt proteins. Methods in molecular biology. 2008;468:17–29. doi: 10.1007/978-1-59745-249-6_2. [DOI] [PubMed] [Google Scholar]
- Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, Smith A. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nature cell biology. 2011;13:838–845. doi: 10.1038/ncb2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nature cell biology. 2011;13:762–770. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L, Chaturvedi D, Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. The Journal of biological chemistry. 2004;279:33220–33227. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- Zhang P, Chang WH, Fong B, Gao F, Liu C, Al Alam D, Bellusci S, Lu W. Regulation of iPS Cell Induction by Wnt/beta-catenin Signaling. The Journal of biological chemistry. 2014 doi: 10.1074/jbc.M113.542845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li Y, Wu Y, Shi K, Bing L, Hao J. Wnt/beta-catenin signaling pathway upregulates c-Myc expression to promote cell proliferation of P19 teratocarcinoma cells. Anatomical record. 2012;295:2104–2113. doi: 10.1002/ar.22592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.