Abstract
Objectives
To determine the hospitalization rates and outcomes of endocarditis among older adults.
Background
Endocarditis is the most serious cardiovascular infection and is especially common among older adults. Little is known about recent trends for endocarditis hospitalizations and outcomes.
Methods
Using Medicare inpatient Standard Analytic Files, we identified all Fee-For-Service beneficiaries aged ≥65 years with a principal or secondary diagnosis of endocarditis from 1999-2010. We used Medicare Denominator Files to report hospitalizations per 100,000 person-years. Rates of 30-day and 1-year mortality were calculated using Vital Status Files. We used mixed-effects models to calculate adjusted rates of hospitalization and mortality and to compare the results before and after 2007, when the American Heart Association revised recommendations for endocarditis prophylaxis.
Results
Overall, 262,658 beneficiaries were hospitalized with endocarditis. The adjusted hospitalization rate increased from 1999-2005, reaching 83.5 per 100,000 person-years in 2005, and declined during 2006-2007. After 2007, the decline continued, reaching 70.6 per 100,000 person-years in 2010. Adjusted 30-day and 1-year mortality rates ranged from 14.2% to 16.5% and from 32.6% to 36.2%, respectively. There were no consistent changes in adjusted rates of 30-day and 1-year mortality after 2007. Trends in rates of hospitalization and outcomes were consistent across demographic subgroups. Adjusted rates of hospitalization and mortality declined consistently in the subgroup with principal diagnosis of endocarditis.
Conclusions
Our study highlights the high burden of endocarditis among older adults. We did not observe an increase in adjusted rates of hospitalization or mortality associated with endocarditis after publication of the 2007 guidelines.
Keywords: Endocarditis, prophylaxis, guidelines, hospitalizations, mortality
Introduction
Infective endocarditis is the most serious infection of the cardiovascular system.(1-3) Studies from the United States have shown a marked rise in endocarditis hospitalization rates during the 1990s and early 2000s, (4,5) consistent with findings from Europe.(3,6) Recent advances in medical care could impact endocarditis hospitalizations and outcomes. Whereas historical risk factors such as rheumatic heart disease have declined,(2,7-9) increased use and longevity of recipients of devices such as prosthetic heart valves, permanent pacemakers, cardiac resynchronization therapy and implantable cardioverter defibrillators have potentially increased the number of patients at risk of endocarditis.(4,10-12) Furthermore, in May 2007, indications for antibiotic prophylaxis were markedly narrowed by the American Heart Association merely to certain high-risk subgroups undergoing dental procedures,(8) potentially increasing the risk of endocarditis in vulnerable patients.(13)
Little is known about more recent national trends in endocarditis hospitalizations and outcomes among older adults, a growing population who may be at disproportionately high risk of developing and dying from endocarditis.(2,14,15) Therefore, we sought to assess the annual rates of hospitalization with endocarditis from 1999 through 2010 among all Medicare Fee-For-Service beneficiaries aged 65 years or older in the United States. We also investigated trends in the rates of hospitalization for endocarditis and in outcomes across demographic subgroups.
Methods
Data Source
Using the Centers for Medicare & Medicaid Services Medicare inpatient Standard Analytic Files, we analyzed all inpatient admissions of Fee-For-Service beneficiaries aged 65 years or older from 1999 to 2010. We included patients who had participated for at least 1 month in Fee-For-Service and resided or were hospitalized in the United States. Medicare inpatient Standard Analytic Files contain patient demographics and procedural and diagnostic information for hospitalizations based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), as well as dates of hospital admission and discharge disposition. We used Denominator Files from 1999 to 2010 to determine beneficiaries’ eligibility and enrollment in Medicare and used a single beneficiary in a single year as the unit of observation. We determined death through the Centers for Medicare & Medicaid Services Vital Status File, which includes information on out-of-hospital mortality. This study was exempt from additional review by the Human Investigation Committee at Yale University, since all data were de-identified.
Patient Population
Patients with a principal or secondary ICD-9-CM discharge diagnosis of endocarditis were included using the following codes: 421.0 (acute and subacute infective endocarditis), 421.1 (endocarditis, valve unspecified, in diseases classified elsewhere), 421.9 (acute endocarditis, unspecified), and 424.9 (endocarditis, valve unspecified). These codes have been frequently used in previous studies of endocarditis.(3-5,13,16) We excluded patients with a principal or secondary discharge diagnosis of infection or inflammation of intracardiac devices (996.61), as the population of older adults who receive intracardiac devices has been increasing over time(17) and it was not possible to define a denominator of all patients who had a device in order to calculate a rate. Nevertheless, we repeated our analysis without excluding these patients to determine if the results changed substantively. In addition, we examined hospitalizations for endocarditis and outcomes in the subset of patients with only a principal discharge diagnosis of endocarditis. For patients who had multiple hospitalizations for endocarditis in a given year (18%), we selected a random hospitalization because we were interested in the patient as the unit of analysis.
Patient Characteristics and Comorbidities
We examined the demographic and clinical characteristics of patients hospitalized with endocarditis. We determined patient-reported race from the Medicare Denominator File. We used comorbidities in the models employed by the Centers for Medicare & Medicaid Services to profile hospital 30-day mortality measures for cardiovascular conditions.(18,19) They were identified from secondary diagnosis codes (which did not represent a potential complication) recorded at the time of discharge from the hospitalization for endocarditis, as well as the primary or secondary diagnosis codes of all inpatient stays up to 1 year before the index hospitalization.
Outcome Measures
For each year, we calculated the rate of hospitalization for endocarditis. The numerator included all patients discharged with endocarditis in a given year. We calculated the denominator using the total number of months that Fee-For-Service beneficiaries were enrolled or at risk during the year, to account for new enrollment, disenrollment, or death, converted to person-years. All rates are reported per 100,000 person-years.
Among patients hospitalized with endocarditis, we determined the annual rates of in-hospital, 30-day, 6-month, and 1-year all-cause mortality. We used the date of admission as the “time zero” for all mortality measures. In addition, we examined trends in hospital length of stay, defined as the difference between the discharge and admission dates.
Statistical Analysis
We used the Mantel-Haenszel Chi-squared test to determine the significance of temporal changes in the rates of hospitalization for endocarditis and mortality. In addition to overall results, we stratified trends in the rates of hospitalization and outcomes by age (65-74, 75-84, ≥85 years), sex, and race (white, black, other).
We fitted a linear mixed-effects model with a Poisson link function and state-specific random intercepts to assess annual rates of hospitalization for endocarditis adjusted for age, sex, and race. We considered the rate of hospitalization for endocarditis during 1999 as the referent and calculated the incidence rate ratio for each subsequent year by including indicator variables for the subsequent years in the mixed-effects model.
To obtain annual mortality rates adjusted for patient demographics and comorbidities, we fitted a linear mixed-effects model with a logit link function and state-specific random intercepts. Using data from 1999 as the referent and indicator variables for each subsequent year, we calculated the risk-adjusted odds ratio for mortality for subsequent years. Using the method described by Zhang and Yu, we converted the odds ratio values to risk ratio estimates.(20) We then multiplied the risk ratio for each year by the mortality rate in the baseline year (i.e., 1999) to calculate the adjusted mortality rates across years.
As a secondary objective, we compared the rates of hospitalization for endocarditis and mortality before and after 2007, when the American Heart Association narrowed the indications for antibiotic prophylaxis for endocarditis. A possible increase in endocarditis hospitalization rates or worsening of outcomes in recent years, if it exists, could be attributable to many factors including change in the clinical comorbidities and underlying conditions that predispose to endocarditis, in addition to the changes in the guidelines. However, we assumed that such an increase would be an important safety signal warranting further surveillance investigation. For comparing the hospitalization rates and outcomes before versus after 2007, we used separate mixed-effects models similar to those described above but with 2007 as the reference. We also calculated a 95% confidence interval for each point estimate from the models.
All analyses were performed with SAS version 9.3 64-bit (SAS Institute, Cary, North Carolina). A p-value <0.05 was considered significant and all tests were 2-sided. The funding source had no role in the study design, analysis, and interpretation, or submission of the results.
Results
Overall, 262,658 patients aged ≥65 years were hospitalized with endocarditis between 1999 and 2010 in the Fee-For-Service population (Table 1). The mean age of patients was similar over time (79.2-79.4 years). The proportion of female patients declined from 58.8% to 55.7% (P=0.002 for trend), while the proportion of black patients increased from 9.2% to 10.0% (P=0.02 for trend). Some comorbidities were more frequently coded over time, including history of hypertension (48.2% to 58.9%, P=0.003 for trend) and history of renal failure (11.4% to 29.2%, P<0.001 for trend).
Table 1.
Characteristics of Patients Hospitalized with Infective Endocarditis in Medicare Fee-for-Service, 1999 to 2010.
| 1999-2000 | 2001-2002 | 2003-2004 | 2005-2006 | 2007-2008 | 2009-2010 | |
|---|---|---|---|---|---|---|
| Patients with Endocarditis (no.) | 38,671 | 43,795 | 47,820 | 47,860 | 44,761 | 39,751 |
| Demographics | ||||||
| Age (mean, SD) (years) | 79.4 ( 8.0) | 79.3 ( 8.0) | 79.2 ( 8.2) | 79.2 ( 8.3) | 79.4 ( 8.6) | 79.2 ( 8.8) |
| Female (%) | 58.8 | 58.8 | 58.1 | 57.7 | 56.9 | 55.7 |
| Race | ||||||
| White (%) | 87.1 | 87.1 | 86.5 | 86.6 | 86.3 | 85.7 |
| Black (%) | 9.2 | 9.2 | 9.7 | 9.5 | 9.6 | 10.0 |
| Other (%) | 3.7 | 3.7 | 3.8 | 3.9 | 4.1 | 4.2 |
| CV Risk Factors and History | ||||||
| Hypertension (%) | 48.2 | 52.7 | 54.7 | 54.8 | 59.1 | 58.9 |
| Diabetes mellitus (%) | 25.8 | 27.3 | 28.6 | 29.1 | 28.8 | 29.7 |
| History of: | ||||||
| Atherosclerotic disease (%) | 41.6 | 43.2 | 43.6 | 42.8 | 41.8 | 40.3 |
| Myocardial infarction (%) | 4.5 | 5.1 | 5.3 | 5.2 | 5.5 | 5.8 |
| Unstable angina (%) | 4.9 | 4.6 | 3.9 | 3.3 | 2.9 | 2.7 |
| Peripheral vascular disease (%) | 10.2 | 10.8 | 11.6 | 11.7 | 12.5 | 12.4 |
| Heart failure (%) | 32.4 | 32.9 | 34.1 | 34.2 | 33.9 | 34.1 |
| Stroke (%) | 4.0 | 3.7 | 3.9 | 3.9 | 4.1 | 4.1 |
| CVD other than stroke (%) | 7.1 | 7.3 | 6.8 | 5.8 | 6.0 | 5.7 |
| History of Other Clinical | ||||||
| Conditions/Comorbidities | ||||||
| Cancer (%) | 11.6 | 11.7 | 11.8 | 11.3 | 11.8 | 11.8 |
| Chronic obstructive pulmonary disease (%) | 25.3 | 27.0 | 28.4 | 29.7 | 28.0 | 24.5 |
| Pneumonia (%) | 17.3 | 18.3 | 19.4 | 20.6 | 23.3 | 25.7 |
| Respiratory failure (%) | 5.6 | 6.0 | 6.7 | 8.1 | 11.3 | 12.3 |
| Renal failure (%) | 11.4 | 13.4 | 15.9 | 20.0 | 25.2 | 29.2 |
| Liver disease (%) | 2.1 | 2.1 | 2.4 | 2.2 | 2.2 | 2.3 |
| Malnutrition (%) | 7.4 | 7.6 | 8.4 | 8.8 | 11.3 | 14.1 |
| Depression (%) | 6.1 | 7.1 | 7.9 | 7.4 | 7.3 | 6.8 |
| Other psychiatric disorder | 2.7 | 2.8 | 2.7 | 2.4 | 2.9 | 3.2 |
| Dementia (%) | 10.9 | 11.7 | 11.7 | 11.9 | 12.8 | 13.2 |
| Functional disability (%) | 4.3 | 4.1 | 4.1 | 3.8 | 4.3 | 4.7 |
| Trauma (%) | 9.0 | 10.0 | 10.9 | 10.8 | 11.2 | 10.6 |
CVD, cerebrovascular disease; SD, standard deviation of the mean
Hospitalizations
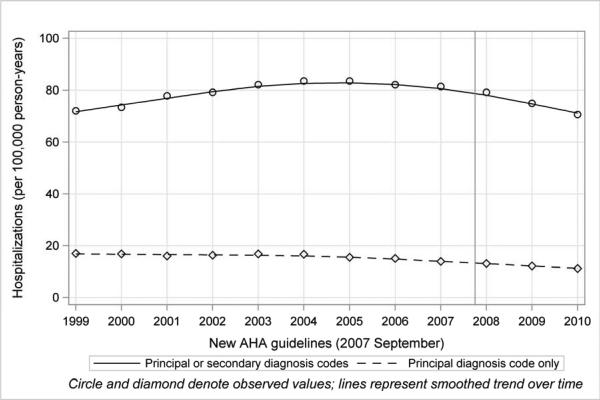
The adjusted rate of hospitalization for endocarditis was 72.0 per 100,000 person-years in 1999. It increased gradually to 83.5 per 100,000 person-years in 2005, and declined thereafter (Figure 1 and Table 2). In particular, the rate of hospitalizations in which endocarditis was the principal or secondary diagnosis declined consistently after 2007 (incidence rate ratio [95% confidence interval]: 0.97 [0.94-0.99], 0.91 [0.89-0.93], and 0.86 [0.84-0.88] for 2008, 2009, and 2010, respectively, compared with 2007).
Figure 1. Hospitalization Rates for Infective Endocarditis in Medicare Fee-For-Service Beneficiaries, 1999-2010.
Circles and diamonds denote observed values; lines represent smoothed trends over time. (Straight line indicates principal or secondary diagnosis codes; dashed line indicates principal diagnosis code only.)
Table 2.
Hospitalizations and Outcomes of Infective Endocarditis in Medicare Fee-for-Service Beneficiaries, 1999 to 2010.
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Denominator (person-years) | 26,479,079 | 26,768,087 | 27,553,904 | 28,345,999 | 28,821,487 | 29,109,293 | 29,157,293 | 28,452,501 | 27,899,732 | 27,675,586 | 27,343,436 | 27,696,576 |
| Adjusted* endocarditis (principal or secondary diagnosis) hospitalization rate per 100,000 person-years | 72.0 | 73.4 (72-75.6) | 77.8 (75.6-79.2) | 79.2 (77.8-81.4) | 82.1 (79.9-84.2) | 83.5 (81.4-85.0) | 83.5 (82.1-85.7) | 82.1 (79.9-84.2) | 81.4 (79.9-83.5) | 79.2 (77-81.4) | 74.9 (72.7-76.3) | 70.6 (69.1-72.0) |
| Adjusted* endocarditis (principal diagnosis) hospitalization rate per 100,000 person-years Outcomes | 17.0 | 16.8 (16.2-17.5) | 16.0 (15.3-16.7) | 16.3 (15.6-17.0) | 16.8 (16.2-17.6) | 16.7 (16.0-17.3) | 15.5 (14.8-16.2) | 15.1 (14.5-15.8) | 13.9 (13.3-14.5) | 13.1 (12.6-13.8) | 12.2 (11.7-12.9) | 11.2 (10.7-11.7) |
| Length of stay (d) | 9.6 (11.5) | 9.3 (11.4) | 9.3 (11.0) | 9.3 (10.9) | 9.1 (10.5) | 8.8 (10.7) | 8.7 (10.4) | 8.5 ( 9.8) | 8.4 ( 9.2) | 8.7 (10.4) | 8.5 ( 9.9) | 8.4 ( 8.9) |
| In-hospital mortality (%) | 11.1 (10.6-11.5) | 10.6 (10.2-11.1) | 11.1 (10.7-11.5) | 11.4 (11.0-11.8) | 10.9 (10.5-11.3) | 10.3 (9.90-10.7) | 9.4 (9.0-9.8) | 9.0 (8.6-9.4) | 8.8 (8.4-9.2) | 9.7 (9.3-10.1) | 9.3 (8.9-9.7) | 9.1 (8.8-9.6) |
| Adjusted 30-day mortality (%)† | 15.8 (15.3-16.3) | 15.7 (15.0-16.5) | 16.1 (15.4-16.9) | 16.5 (15.8-17.2) | 16.1 (15.3-16.7) | 15.8 (15.1-16.6) | 15.0 (14.3-15.7) | 14.2 (13.5-14.9) | 14.6 (13.9-15.3) | 15.7 (15.0-16.3) | 15.1 (14.4-15.8) | 15.3 (14.6-16.1) |
| Adjusted 6-month mortality (%)† | 31.6 (30.9-32.2) | 31.4 (30.5-32.5) | 31.6 (30.7-32.7) | 31.8 (30.7-32.7) | 31.6 (30.7-32.7) | 31.2 (30.3-32.2) | 30.3 (29.4-31.4) | 28.4 (27.7-29.4) | 29.1 (28.2-30.1) | 30.3 (29.4-31.2) | 29.6 (28.5-30.5) | 29.6 (28.5-30.7) |
| Adjusted 1-year mortality (%)† | 35.7 (35.1-36.4) | 35.7 (34.8-36.6) | 36.2 (35.2-37.0) | 35.9 (35.0-36.8) | 35.5 (34.5-36.4) | 35.0 (34.1-36.2) | 33.8 (33.3-34.8) | 32.6 (31.5-33.3) | 33.1 (32.3-34.1) | 34.3 (33.3-35.2) | 33.3 (32.3-34.3) | 33.8 (32.8-34.8) |
| Discharge | ||||||||||||
| Disposition | ||||||||||||
| Home | 40.0 | 40.7 | 40.1 | 38.8 | 37.0 | 34.9 | 34.6 | 34.2 | 32.4 | 30.8 | 29.9 | 27.6 |
| Home health care | 11.7 | 11.3 | 11.3 | 11.5 | 12.4 | 14.4 | 14.3 | 15.2 | 16.1 | 15.5 | 16.2 | 16.7 |
| ICF/SNF | 26.0 | 25.8 | 25.0 | 25.1 | 24.7 | 25.5 | 26.2 | 26.1 | 26.2 | 26.6 | 26.9 | 28 |
| Hospice | 0.2 | 0.3 | 0.6 | 1.0 | 1.5 | 2.1 | 2.5 | 3.1 | 3.5 | 4.0 | 4.4 | 4.6 |
| Transferred out | 5.3 | 5.3 | 5.2 | 4.5 | 4.5 | 4.3 | 4.3 | 3.8 | 4.0 | 4.0 | 3.7 | 4.0 |
| Hospital death | 11.1 | 10.6 | 11.1 | 11.4 | 10.9 | 10.3 | 9.4 | 9.0 | 8.8 | 9.7 | 9.3 | 9.1 |
| Other‡ | 5.7 | 6.0 | 6.7 | 7.7 | 9.0 | 8.5 | 8.7 | 8.6 | 9.0 | 9.4 | 9.6 | 10.0 |
Numbers in parentheses reflect standard deviations for length of stay and 95% confidence intervals for hospitalization rates and outcomes.
Adjusted for age, sex, and race (reference: 1999)
Adjusted for age, sex, race, and clinical comorbidities (reference: 1999)
Including discharge against medical advice
ICF/SNF, intermediate care facility/skilled nursing facility
Among all patients with infective endocarditis from 1999 to 2010, 52,145 had a principal discharge diagnosis of endocarditis. The adjusted hospitalization rate in this subgroup declined from 17.0 per 100,000 person-years in 1999 to 11.2 per 100,000 person-years in 2010 (P<0.001 for trend) (Figure 1 and Table 2). The rate of hospitalizations for endocarditis for these patients also consistently declined after 2007 (incidence rate ratio [95% confidence interval]: 0.94 [0.90-0.98], 0.89 [0.84-0.93], and 0.81 [0.77-0.85] for 2008, 2009, and 2010, respectively).
Mortality
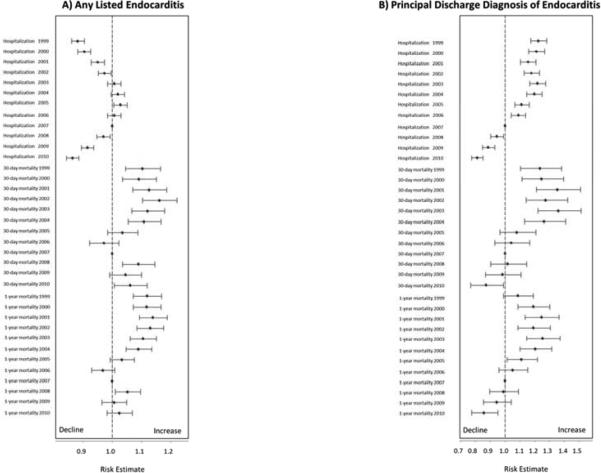
In-hospital mortality rates declined from 11.1% in 1999 to 9.1% in 2010 (P<0.001 for trend).From 1999 to 2010, adjusted 30-day mortality rates ranged from 14.2% to 16.5%. Adjusted 6-month mortality rates ranged from 28.4% to 31.8%. Across the years, 1-year mortality rates were fairly close to 6-month mortality rates, and ranged from 32.6% to 36.2% (Table 2). Except for 2006, adjusted rates of 30-day, 6-month, and 1-year mortality were lowest during 2007. Mortality at 30 days and 1 year appeared to increase slightly during 2008 compared with 2007 (odds ratio [95% confidence interval]: 1.08 [1.03-1.14] and 1.05 [1.009-1.09], respectively); however, the increased mortality was inconsistent and became non-significant during 2009 (odds ratio [95% confidence interval]: 1.04 [0.99-1.10] and 1.006 [0.96-1.04], respectively) (Figure 2, Panel A).
Figure 2. Comparison of Rates of Hospitalization for Endocarditis and Outcomes for Any Listed Endocarditis (Panel A) and Principal Discharge Diagnosis of Endocarditis (Panel B).
Data from 2007, the year of publication of the new American Heart Association guidelines, was the reference. Adjusted incidence rate ratios (for hospitalizations) and odds ratios (for outcomes) were calculated for each year before and after 2007.
Among the subgroup with a principal discharge diagnosis of endocarditis from 1999 to 2010, 30-day and 1-year mortality rates ranged from 14.3% to 20.6% and from 33.7% to 41.4%, respectively. There was a trend toward reduced adjusted 30-day and 1-year mortality rates after 2007 (Figure 2, Panel B).
Length of Stay and Discharge Disposition
From 1999 to 2010, the mean length of stay for hospitalizations for endocarditis consistently declined, from 9.6 (standard deviation: 11.5) days to 8.4 (standard deviation: 8.9) days (P<0.001 for trend). During that period, there was a reduction in the proportion of discharges to home (40.0% to 27.6%, P<0.001 for trend) and an increase in the proportion of patients discharged to home with home healthcare or discharged to hospice (11.7% to 16.7 and 0.2% to 4.6% respectively, P<0.001 for trend for both comparisons).
Hospitalization and Mortality Rates by Age, Sex, and Race
Across all age, sex, and race subgroups, rates of hospitalization for endocarditis increased from 1999 to 2005, declined slightly during 2006-2007, and continued to decline during 2008-2010 (Table 3). Stratified trends in the risk-adjusted rates of 30-day, 6-month, and 1-year mortality were consistent with those of the overall cohort. All results were substantively similar when device-related hospitalizations were included in the analyses (data not shown).
Table 3.
Hospitalizations for Endocarditis and Outcomes by Age, Sex, and Race.
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted* hospitalization rate per 100,000 person-years | ||||||||||||
| Age | ||||||||||||
| 65-74 | 45.0 | 45.5 | 47.3 | 48.6 | 50.0 | 50.9 | 50.4 | 49.1 | 48.6 | 46.8 | 45.0 | 42.3 |
| 75-84 | 87.0 | 89.6 | 96.6 | 98.3 | 102.7 | 103.5 | 103.5 | 103.5 | 100.9 | 98.3 | 94.0 | 87.9 |
| ≥85 | 146.0 | 150.4 | 153.3 | 159.1 | 163.5 | 165.0 | 171.0 | 163.5 | 166.4 | 163.5 | 148.9 | 143.1 |
| Sex | ||||||||||||
| Female | 70.0 | 72.8 | 77.0 | 78.4 | 81.2 | 83.3 | 83.3 | 81.2 | 81.2 | 77.7 | 72.8 | 68.6 |
| Male | 73.0 | 73.0 | 75.9 | 78.8 | 81.8 | 81.8 | 82.5 | 81.0 | 80.3 | 78.8 | 75.2 | 70.8 |
| Race | ||||||||||||
| Black | 86.0 | 85.1 | 88.6 | 93.7 | 100.6 | 100.6 | 100.6 | 99.8 | 98.9 | 102.3 | 96.3 | 89.4 |
| White | 72.0 | 74.2 | 77.8 | 79.2 | 81.4 | 82.8 | 83.5 | 82.1 | 81.4 | 78.5 | 74.2 | 69.8 |
| Other | 47.0 | 51.2 | 46.5 | 52.6 | 49.8 | 52.6 | 52.2 | 48.9 | 50.8 | 48.9 | 45.6 | 42.3 |
| Adjusted 30-day mortality rate (%)† | ||||||||||||
| Age | ||||||||||||
| 65-74 | 15.4 (14.5-16.4) | 15.3 (13.9-16.7) | 16.4 (15.1-17.9) | 16.0 (14.7-17.4) | 15.3 (14.1-16.7) | 15.3 (14.1-16.6) | 14.2 (13.0-15.4) | 13.1 (12.0-14.3) | 13.4 (12.3-14.6) | 14.9 (13.7-16.2) | 13.5 (12.4-14.9) | 14.5 (13.3-15.8) |
| 75-84 | 14.1 (13.4-14.9) | 14.1 (13.1-15.2) | 13.7 (12.7-14.8) | 14.7 (13.7-15.8) | 14.3 (13.4-15.3) | 14.1 (13.1-15.2) | 13.0 (12.1-14.0) | 12.7 (11.9-13.6) | 13.0 (12.0-13.9) | 13.5 (12.5-14.5) | 13.2 (12.2-14.2) | 13.4 (12.5-14.5) |
| ≥85 | 18.7 (17.7-19.8) | 18.2 (16.8-19.9) | 19.2 (17.6-20.8) | 19.5 (17.9-21.1) | 19.2 (17.8-20.8) | 19.0 (17.5-20.5) | 18.7 (17.2-20.2) | 17.5 (16.0-19.0) | 18.1 (16.7-19.6) | 19.5 (17.9-21.1) | 19.3 (17.8-20.9) | 18.9 (17.3-20.5) |
| Sex | ||||||||||||
| Female | 15.3 (14.7-16.0) | 14.8 (13.9-15.7) | 15.3 (14..4-16.2) | 15.4 (14.5-16.3) | 15.2 (14.3-16.1) | 15.3 (14.4-16.2) | 14.5 (13.7-15.4) | 14.1 (13.3-15.0) | 13.9 (13.0-14.8) | 15.6 (14.6-16.5) | 14.5 (13.6-15.4) | 14.8 (13.9-15.8) |
| Male | 16.5 (15.7-17.3) | 16.9 (15.8-18.1) | 17.2 (16.1-18.4) | 18.0 (16.8-19.2) | 17.2 (16.1-18.4) | 16.6 (15.5-17.7) | 15.5 (14.5-16.6) | 14.2 (13.2-15.2) | 15.4 (14.4-16.5) | 15.8 (14.8-16.9) | 15.9 (14.8-17.0) | 15.9 (14.8-17.0) |
| Race | ||||||||||||
| Black | 17.0 (15.3-18.9) | 18.0 (15.4-20.6) | 17.6 (15.1-20.3) | 16.9 (14.7-19.5) | 16.7 (14.5-19.2) | 17.1 (14.8-19.6) | 15.7 (13.6-18.1) | 15.4 (13.3-17.7) | 14.4 (12.4-16.7) | 16.3 (14.2-18.8) | 16.0 (13.8-18.4) | 16.4 (14.2-18.9) |
| White | 15.7 (15.2-16.3) | 15.4 (14.6-16.2) | 15.8 (15.0-16.6) | 16.6 (15.8-17.4) | 16.0 (15.2-16.7) | 15.7 (14.9-16.5) | 14.8 (14.1-15.4) | 14.1 (13.4-14.8) | 14.5 (13.8-15.3) | 15.6 (14.8-16.4) | 14.9 (14.2-15.7) | 15.3 (14.5-16.1) |
| Other | 15.1 (12.5-18.0) | 15.0 (11.6-19.1) | 17.5 (13.7-21.9) | 14.3 (11.2-18.3) | 16.5 (13.0-20.7) | 16.7 (13.3-20.9) | 17.9 (14.3-22.3) | 14.7 (11.5-18.5) | 15.2 (11.9-19.1) | 15.1 (11.9-19.0) | 16.9 (13.3-21.2) | 13.3 (10.2-17.0) |
| Adjusted 1-year mortality rate (%)† | ||||||||||||
| Age | ||||||||||||
| 65-74 | 32.8 (31.6-34.1) | 33.0 (31.2-34.7) | 33.5 (31.7-35.5) | 32.1 (30.5-33.9) | 31.7 (30.0-33.5) | 31.7 (30.0-33.2) | 30.0 (28.6-31.8) | 28.6 (37.0-30.3) | 28.8 (27.1-30.3) | 30.3 (28.8-32.1) | 28.8 (27.3-30.5) | 29.8 (28.1-31.5) |
| 75-84 | 32.9 (31.9-34.0) | 33.1 (31.5-34.6) | 33.1 (31.5-34.4) | 33.6 (32.0-34.8) | 33.3 (32.0-34.8) | 32.2 (30.9-34.6) | 30.9 (29.4-32.2) | 30.1 (28.7-31.5) | 30.4 (28.9-31.8) | 31.1 (29.7-32.7) | 30.4 (29.4-32.5) | 31.1 (29.7-32.7) |
| ≥85 | 42.9 (41.6-44.3) | 42.4 (40.3-44.3) | 43.6 (41.6-45.7) | 43.4 (41.4-45.5) | 42.2 (41.1-44.1) | 43.1 (41.1-45.0) | 42.4 (40.3-44.3) | 40.1 (38.1-41.9) | 41.9 (39.8-43.9) | 42.7 (40.9-44.8) | 41.4 (39.3-43.4) | 41.4 (39.5-43.4) |
| Sex | ||||||||||||
| Female | 35.3 (34.4-36.2) | 34.4 (33.2-35.5) | 34.8 (33.7-36.2) | 34.6 (33.4-36.0) | 33.9 (32.7-35.3) | 34.1 (32.9-35.5) | 32.7 (31.7-33.9) | 31.9 (30.9-33.2) | 32.2 (30.9-33.4) | 33.7 (32.4-35.1) | 31.9 (30.6-35.4) | 32.7 (31.4-33.9) |
| Male | 36.3 (35.3-37.4) | 37.7 (36.1-39.2) | 37.9 (36.3-39.6) | 37.7 (36.3-39.4) | 37.4 (36.1-39.0) | 36.5 (34.9-37.9) | 35.6 (34.1-37.0) | 33.1 (31.8-34.6) | 34.6 (33.1-36.1) | 35.1 (33.7-36.5) | 35.1 (33.7-36.8) | 35.4 (33.9-36.8) |
| Race | ||||||||||||
| Black | 41.2 (38.9-43.6) | 43.5 (40.2-47.1) | 42.4 (39.2-45.9) | 41.9 (38.7-45.3) | 40.7 (37.6-44.0) | 39.5 (36.2-42.6) | 37.9 (34.7-41.0) | 37.3 (34.1-40.5) | 36.2 (33.2-39.5) | 38.1 (35.1-41.4) | 37.6 (34.4-41.0) | 37.3 (34.1-40.7) |
| White | 35.2 (34.4-35.9) | 35 (33.8-35.9) | 35.4 (34.5-36.5) | 35.2 (34.3-36.3) | 35.0 (33.8-35.9) | 34.5 (33.6-35.7) | 33.3 (32.3-34.5) | 32.1 (31.1-33.1) | 32.8 (31.8-34.0) | 34.0 (32.8-35.0) | 32.8 (31.8-33.8) | 33.3 (32.3-34.5) |
| Other | 35.2 (31.7-39.0) | 37.8 (32.6-43.2) | 38.4 (33.3-44.1) | 36.5 (31.6-41.8) | 36.1 (31.1-41.2) | 38.9 (33.8-44.1) | 36.8 (31.8-41.9) | 32.3 (27.5-37.2) | 32.3 (27.5-37.2) | 34.0 (29.2-39.3) | 35.4 (30.5-40.4) | 33.8 (28.9-38.9) |
Adjusted for age, sex, and race
Adjusted for age, sex, race, and clinical comorbidities
Discussion
Our study demonstrates the high burden of endocarditis among older adult Fee-For-Service Medicare beneficiaries. The rates of 30-day, 6-month, and 1-year mortality remained consistently high (approximately 15%, 30%, and 35%, respectively), similar to those found in previous studies.(2,3,21) We did not observe an increase in rates of hospitalization or mortality with endocarditis in more recent years. In fact, rates of hospitalization for endocarditis declined across all age, sex, and race subgroups from 2006-2007 and continued to do so from 2008-2010. Among the subgroup with a principal discharge diagnosis of endocarditis, rates of hospitalizations and mortality showed a declining trend throughout the study period.
The annual endocarditis hospitalization rates that we observed among older adults aged ≥65 years with Fee-For-Service coverage from 1999 to 2010 were higher than the rates found in previous studies.(22,23) A combination of younger age, lower risk profile, under-reporting in the previous studies, and over-coding in our study may have contributed to the differences. Over time, we observed slight declines in hospital length of stay and rates of in-hospital mortality, along with an increase in the proportion of patients discharged to hospice or to home with healthcare assistance, a pattern that has been recognized.(24) Patients aged ≥85 years had higher rates of hospitalization and mortality compared with those in other age groups, likely related to their greater comorbidity burden and immunosenescence.(25) We also observed consistently higher rates of hospitalization and mortality among black patients. Although some reports suggest ethnic differences in the incidence of endocarditis,(26-28) this topic has been largely understudied in the United States.(4,29)
We did not observe an increase in rates of hospitalization for endocarditis or of mortality following the 2007 release of more restrictive recommendations for prophylaxis by the American Heart Association. Our analysis, however, was not meant to be a comparative effectiveness study to prove the non-inferiority of more restrictive use of antibiotics for endocarditis prophylaxis. National data regarding antibiotic utilization after the publication of the 2007 American Heart Association guidelines are not available. However, it is likely that the guidelines have caused a decline in prophylactic use of antibiotics. For example, a study from the United Kingdom showed marked reduction in the use of prophylactic antibiotics following the release of the guidelines for endocarditis from the National Institute for Health and Clinical Excellence.(6) Similarly, results of recent surveys in the United States suggest a decline in the use of antibiotic prophylaxis for patients with non-high risk cardiac conditions.(30,31)
The intent of our secondary analysis was to determine whether the possible reduced use of antibiotics has been associated with temporal changes in trends for hospitalizations or outcomes of endocarditis among older adults. If an increase in endocarditis hospitalizations had occurred after 2007, it could have been due to a combination of more widespread use of intracardiac and intravascular devices (such as mechanical valves, pacemakers, and dialysis catheters) among older adults, a change in the pattern of comorbidities, or restrictive antibiotic use, and would warrant further investigation. However, we did not observe an increase in hospitalization rates after 2007, but rather found a significant decline in endocarditis hospitalization rates that continued through 2007-2010.
Compared with 2007, the upper bound of the confidence interval for adjusted hospitalization rates consistently remained below 1.0 for years 2008, 2009, and 2010. This lack of increased hospitalization rates after 2007 could be due to several reasons, including lack of a substantial effect for widespread endocarditis prophylaxis, lack of penetrance of guideline recommendations into widespread clinical practice change, or pronounced change in endocarditis hospitalizations for reasons that are not related to antibiotic prophylaxis. Irrespective of the magnitude of change in prophylactic antibiotic use and other risk factors, our current surveillance investigation does not show a safety concern in terms of increased hospitalizations. The decline that we observed in endocarditis hospitalization rates warrants further investigation and might be in part due to concerted strategies that have been used to reduce the rates of catheter-associated bloodstream infections.(32)
Our findings are consistent with three other studies that evaluated the incidence of endocarditis after publication of the 2007 guidelines. In a single-center study, Rogers and Schiller(13) did not find an increase in hospitalizations for endocarditis during the first 9 months after publication of the guidelines. Similarly, Pasquali and colleagues(33) did not find an increase in admissions for endocarditis in their study of 37 children's hospitals in the United States. Desimone and colleagues(30) did not observe an increase in hospitalization rates for patients with diagnosed or suspected endocarditis caused by viridans streptococci among residents of Olmsted County, Minnesota, after the 2007 guidelines were published. Using records from the Nationwide Inpatient Sample, they also reported little change after 2007.(30) That report, however, only reflected endocarditis caused by viridans streptococci, did not include hospitalization rates that accounted for changes in the denominator, did not analyze demographic subgroups, and extended only until 2009.
The impact of reduced antibiotic prophylaxis on the rates of hospitalization for endocarditis has also been the topic of international investigation. A study from the United Kingdom found that although overall incidence of endocarditis rose from 2000 to 2010, the increase did not accelerate after publication of the 2008 guidelines on cessation of antibiotic prophylaxis from the National Institute for Health and Clinical Excellence.(6) A study by Duval and colleagues, conducted over 3 cross-sectional time periods in France, did not find major changes in the incidence of endocarditis related to the change in the prophylaxis recommendations.(22) Neither of these studies reported outcomes such as 30-day or 1-year mortality.
Our study had several strengths. It represents national data for endocarditis hospitalizations in the entire population of Medicare Fee-For-Service older adults. Adjusted short-term and 1-year mortality rates are also important and were not available from previous studies that reported trends in hospitalizations with endocarditis. Consistent results across several subgroups and in sensitivity analyses support the validity of the findings.
Our study had several limitations. First, we studied the hospitalizations and outcomes in older adults. However, we expect trends in this high-risk group to be indicative of overall trends. Second, we did not have access to data from beneficiaries in the Medicare Advantage plans (approximately 10 million beneficiaries in February 2009, or a quarter of all Medicare users).(34) Given that Medicare Advantage patients are generally considered healthier (35), the growing migration of (healthier) patients to Medicare Advantage could have biased the trends toward showing increased endocarditis hospitalization rates in recent years. Third, although we analyzed data for 100% of Fee-For-Service beneficiaries rather than a select cohort and thus eliminated referral bias,(36) the use of administrative discharge data may have overestimated the number of cases of endocarditis among older adults.(22) However, results from two previous reports suggested good accuracy for detection of endocarditis cases using the ICD-9 codes, with reference to the revised Duke criteria.(3,37) Fourth, the most prominent change in the recent American Heart Association guidelines was about the restriction of indications for antibiotic prophylaxis following dental procedures, which would most possibly affect streptococcal endocarditis. Therefore, analysis of the rates of all-cause endocarditis might be unable to clearly detect a signal for change of streptococcal endocarditis trends, if one existed. However, the 2007 guidelines also eliminated endocarditis prophylaxis recommendations for patients undergoing gastrointestinal, hepatobiliary, and genitourinary procedures. Although bacteremia following such procedures is not frequent, there have been recent reports of non-streptococcal bacteremia or endocarditis following these procedures, suggesting some risk.(38,39) Therefore, we believe that lack of an increase in hospitalization rates or mortality from all-cause endocarditis is an important finding for surveillance investigation. Fifth, our study determined the rates of hospitalization for endocarditis, rather than its incidence. However, because infective endocarditis requires initial hospitalization for virtually all patients, particularly older adults, this limitation is unlikely to fundamentally change our interpretation of the observed trends.
Conclusion
Endocarditis continues to carry a high burden and mortality rate among older adults. We did not detect an increase in the rates of hospitalization for endocarditis or of adjusted mortality after publication of the 2007 American Heart Association guidelines, which recommended a restriction of antibiotic prophylaxis.
Acknowledgment
The authors thank Deirdre Lombardi for her editorial assistance and Karl Minges for his administrative support.
Funding Sources: This study was supported by grant number U01HL105270-03 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. Dr. Quagliarello is supported by grant number K07AG030093. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: Dr. Krumholz reports that he is the recipient of a research grant from Medtronic through Yale University and chairs a cardiac scientific advisory board for United Healthcare. The other authors report no potential conflicts of interest.
References
- 1.Thuny F, Grisoli D, Collart F, Habib G, Raoult D. Management of infective endocarditis: challenges and perspectives. Lancet. 2012;379:965–75. doi: 10.1016/S0140-6736(11)60755-1. [DOI] [PubMed] [Google Scholar]
- 2.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463–73. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fedeli U, Schievano E, Buonfrate D, Pellizzer G, Spolaore P. Increasing incidence and mortality of infective endocarditis: a population-based study through a record-linkage system. BMC Infect Dis. 2011;11:48. doi: 10.1186/1471-2334-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabell CH, Heidenreich PA, Chu VH, et al. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990-1999. Am Heart J. 2004;147:582–6. doi: 10.1016/j.ahj.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Mendiratta P, Tilford JM, Prodhan P, Cleves MA, Wei JY. Trends in hospital discharge disposition for elderly patients with infective endocarditis: 1993 to 2003. J Am Geriatr Soc. 2009;57:877–81. doi: 10.1111/j.1532-5415.2009.02224.x. [DOI] [PubMed] [Google Scholar]
- 6.Thornhill MH, Dayer MJ, Forde JM, et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. BMJ. 2011;342:d2392. doi: 10.1136/bmj.d2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yiu KH, Siu CW, Lee KL, et al. Emerging trends of community acquired infective endocarditis. Int J Cardiol. 2007;121:119–22. doi: 10.1016/j.ijcard.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 8.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736–54. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 9.Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest. 2007;132:1025–35. doi: 10.1378/chest.06-2048. [DOI] [PubMed] [Google Scholar]
- 10.Quagliarello V. Infective endocarditis: global, regional, and future perspectives. JAMA. 2005;293:3061–2. doi: 10.1001/jama.293.24.3061. [DOI] [PubMed] [Google Scholar]
- 11.Karchmer AW, Longworth DL. Infections of intracardiac devices. Cardiol Clin. 2003;21:253–71, vii. doi: 10.1016/s0733-8651(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 12.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers AM, Schiller NB. Impact of the first nine months of revised infective endocarditis prophylaxis guidelines at a university hospital: so far so good. J Am Soc Echocardiogr. 2008;21:775. doi: 10.1016/j.echo.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Vahanian A. The growing burden of infective endocarditis in the elderly. Eur Heart J. 2003;24:1539–40. doi: 10.1016/s0195-668x(03)00344-0. [DOI] [PubMed] [Google Scholar]
- 15.Di Salvo G, Thuny F, Rosenberg V, et al. Endocarditis in the elderly: clinical, echocardiographic, and prognostic features. Eur Heart J. 2003;24:1576–83. doi: 10.1016/s0195-668x(03)00309-9. [DOI] [PubMed] [Google Scholar]
- 16.Day MD, Gauvreau K, Shulman S, Newburger JW. Characteristics of children hospitalized with infective endocarditis. Circulation. 2009;119:865–70. doi: 10.1161/CIRCULATIONAHA.108.798751. [DOI] [PubMed] [Google Scholar]
- 17.Dodson JA, Maurer MS. Changing nature of cardiac interventions in older adults. Aging health. 2011;7:283–295. doi: 10.2217/ahe.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113:1683–92. doi: 10.1161/CIRCULATIONAHA.105.611186. [DOI] [PubMed] [Google Scholar]
- 19.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 21.Vikram HR, Buenconsejo J, Hasbun R, Quagliarello VJ. Impact of valve surgery on 6-month mortality in adults with complicated, left-sided native valve endocarditis: a propensity analysis. JAMA. 2003;290:3207–14. doi: 10.1001/jama.290.24.3207. [DOI] [PubMed] [Google Scholar]
- 22.Duval X, Delahaye F, Alla F, et al. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. J Am Coll Cardiol. 2012;59:1968–76. doi: 10.1016/j.jacc.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 23.van der Meer JT, Thompson J, Valkenburg HA, Michel MF. Epidemiology of bacterial endocarditis in The Netherlands. I. Patient characteristics. Arch Intern Med. 1992;152:1863–8. doi: 10.1001/archinte.152.9.1863. [DOI] [PubMed] [Google Scholar]
- 24.Drye EE, Normand SL, Wang Y, et al. Comparison of hospital risk-standardized mortality rates calculated by using in-hospital and 30-day models: an observational study with implications for hospital profiling. Ann Intern Med. 2012;156:19–26. doi: 10.1059/0003-4819-156-1-201201030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 26.Allison MJ, Gerszten E, Dalton HP. Bacterial endocarditis: pathogenesis and racial susceptibility. South Med J. 1967;60:129–34. doi: 10.1097/00007611-196702000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Chang FY, MacDonald BB, Peacock JE, Jr., et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003;82:322–32. doi: 10.1097/01.md.0000091185.93122.40. [DOI] [PubMed] [Google Scholar]
- 28.Ivert TS, Dismukes WE, Cobbs CG, Blackstone EH, Kirklin JW, Bergdahl LA. Prosthetic valve endocarditis. Circulation. 1984;69:223–32. doi: 10.1161/01.cir.69.2.223. [DOI] [PubMed] [Google Scholar]
- 29.Tleyjeh IM, Steckelberg JM, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA. 2005;293:3022–8. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 30.Desimone DC, Tleyjeh IM, Correa de Sa DD, et al. Incidence of Infective Endocarditis due to Viridans Group Streptococci Before and After Publication of the 2007 American Heart Association's Endocarditis Prevention Guidelines. Circulation. 2012;126:60–4. doi: 10.1161/CIRCULATIONAHA.112.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pharis CS, Conway J, Warren AE, Bullock A, Mackie AS. The impact of 2007 infective endocarditis prophylaxis guidelines on the practice of congenital heart disease specialists. Am Heart J. 2011;161:123–9. doi: 10.1016/j.ahj.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Santana S, Saavedra P, Leon C. “Near zero” catheter-related bloodstream infections: turning dreams into reality*. Crit Care Med. 2012;40:3083–4. doi: 10.1097/CCM.0b013e3182632748. [DOI] [PubMed] [Google Scholar]
- 33.Pasquali SK, He X, Mohamad Z, et al. Trends in endocarditis hospitalizations at US children's hospitals: Impact of the 2007 American Heart Association Antibiotic Prophylaxis Guidelines. Am Heart J. 2012;163:894–9. doi: 10.1016/j.ahj.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biles B, Arnold G, Guterman S. Medicare Advantage in the era of health reform: progress in leveling the playing field. Issue Brief (Commonw Fund) 2011;5:1–14. [PubMed] [Google Scholar]
- 35.Morgan RO, Virnig BA, DeVito CA, Persily NA. The Medicare-HMO revolving door--the healthy go in and the sick go out. N Engl J Med. 1997;337:169–75. doi: 10.1056/NEJM199707173370306. [DOI] [PubMed] [Google Scholar]
- 36.Sy RW, Kritharides L. Health care exposure and age in infective endocarditis: results of a contemporary population-based profile of 1536 patients in Australia. Eur Heart J. 2010;31:1890–7. doi: 10.1093/eurheartj/ehq110. [DOI] [PubMed] [Google Scholar]
- 37.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veteran's affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60:397–409. doi: 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Siegman-Igra Y. Infective endocarditis following gastrointestinal and genitourinary procedures: an argument in favour of prophylaxis. Scand J Infect Dis. 2010;42:208–14. doi: 10.3109/00365540903443140. [DOI] [PubMed] [Google Scholar]
- 39.Karvaj M, Krcmery V, Kisac P. Infective endocarditis after endoscopy. Scand J Infect Dis. 2010;42:639–40. doi: 10.3109/00365541003796783. [DOI] [PubMed] [Google Scholar]