Abstract
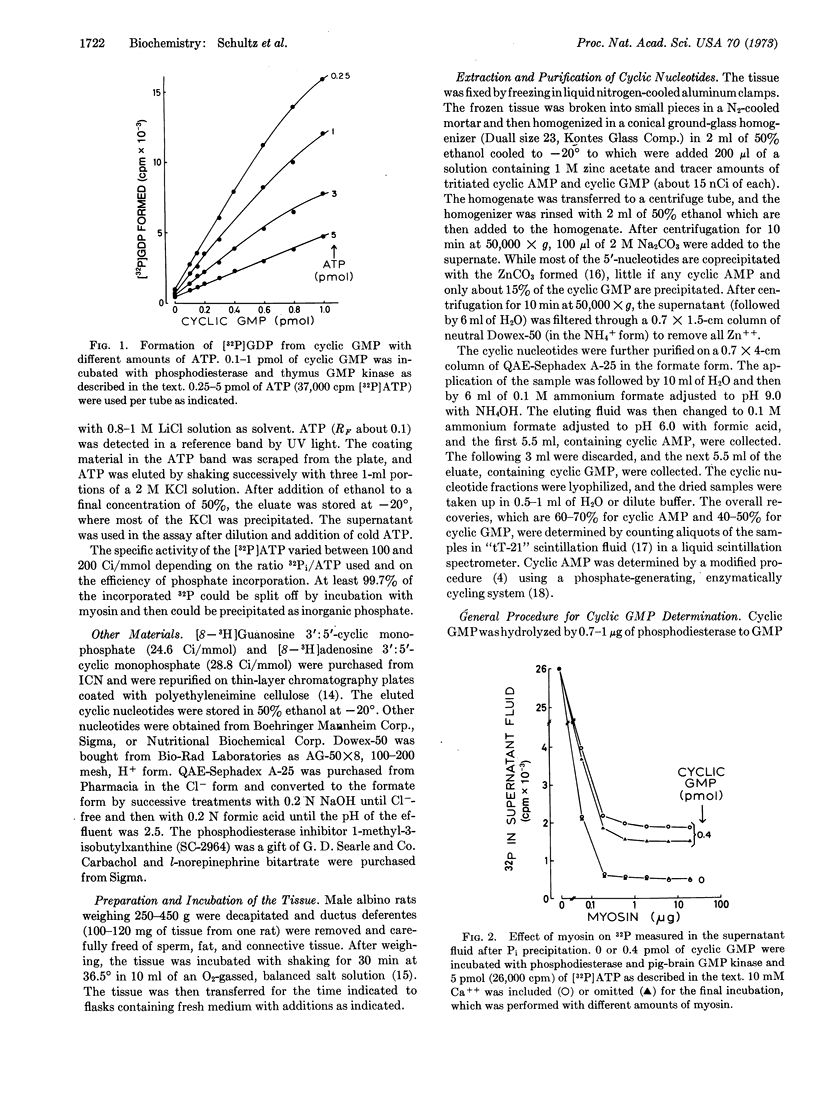
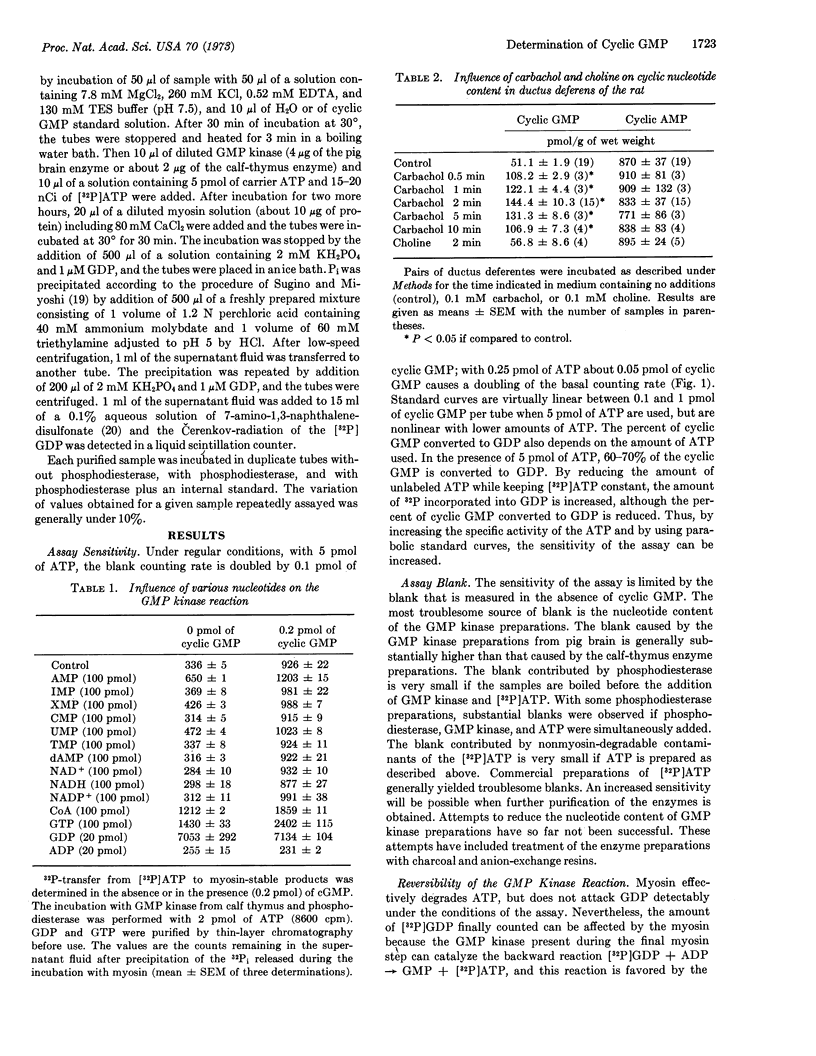
A sensitive enzymatic procedure has been developed for the determination of guanosine 3′:5′-cyclic monophosphate (cyclic GMP). It is based on the conversion of cyclic GMP to GMP by cyclic nucleotide phosphodiesterase and on the transfer of 32P from [γ-32P]ATP to GMP by the action of a specific ATP:GMP phosphotransferase (EC 2.7.4.8). The [32P]GDP is separated from the remaining [32P]ATP by enzymatic degradation of ATP by myosin and by precipitation of the 32Pi formed. The reaction blank, which is mostly caused by the nucleotide content of the enzymes, is doubled by about 0.1 pmol of cyclic GMP. The procedure has advantages in speed and/or accuracy over other methods in current use.
Cyclic nucleotide concentrations were studied in the ductus deferens of the rat; two agents were used, carbachol and norepinephrine, which cause contraction. Incubation with 0.1 mM carbachol caused a 3-fold increase in cyclic GMP content, which was maximal about 2 min after carbachol addition. Cyclic AMP concentrations were not significantly changed. Addition of 0.01 mM norepinephrine increased cyclic GMP content by about 25% within 1 min and by 40% within 3 min; cyclic AMP concentrations were only slightly increased. A 3-min incubation with the phosphodiesterase inhibitor 1-methyl-3-isobutylxanthine (0.1 mM) doubled the cyclic GMP content and increased cyclic AMP concentration by 50%.
Keywords: [32P]GDP formation, cholinergic agents, adrenergic agents, phosphodiesterase inhibitors, smooth muscle
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHMAN D. F., LIPTON R., MELICOW M. M., PRICE T. D. Isolation of adenosine 3', 5'-monophosphate and guanosine 3', 5'-monophosphate from rat urine. Biochem Biophys Res Commun. 1963 May 22;11:330–334. doi: 10.1016/0006-291x(63)90566-7. [DOI] [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Beavo J. A., Hardman J. G., Sutherland E. W. Hydrolysis of cyclic guanosine and adenosine 3',5'-monophosphates by rat and bovine tissues. J Biol Chem. 1970 Nov 10;245(21):5649–5655. [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Dietz S. B., O'Toole A. G. Cyclic guanosine 3',5'-monophosphate in mammalian tissues and urine. J Biol Chem. 1969 Aug 25;244(16):4458–4466. [PubMed] [Google Scholar]
- Hardman J. G., Davis J. W., Sutherland E. W. Effects of some hormonal and other factors on the excretion of guanosine 3',5'-monophosphate and adenosine 3',5'-monophosphate in rat urine. J Biol Chem. 1969 Dec 10;244(23):6354–6362. [PubMed] [Google Scholar]
- Hardman J. G., Davis J. W., Sutherland E. W. Measurement of guanosine 3',5'-monophosphate and other cyclic nucleotides. Variations in urinary excretion with hormonal state of the rat. J Biol Chem. 1966 Oct 25;241(20):4812–4815. [PubMed] [Google Scholar]
- Hurwitz L., Joiner P. D. Mobilization of cellular calcium for contraction in intestinal smooth muscle. Am J Physiol. 1970 Jan;218(1):12–19. doi: 10.1152/ajplegacy.1970.218.1.12. [DOI] [PubMed] [Google Scholar]
- Ishikawa E., Ishikawa S., Davis J. W., Sutherland E. W. Determination of guanosine 3',5'-monophosphate in tissues and of guanyl cyclase in rat intestine. J Biol Chem. 1969 Dec 10;244(23):6371–6376. [PubMed] [Google Scholar]
- Kuo J. F., Lee T. P., Reyes P. L., Walton K. G., Donnelly T. E., Jr, Greengard P. Cyclic nucleotide-dependent protein kinases. X. An assay method for the measurement of quanosine 3',5'-monophosphate in various biological materials and a study of agents regulating its levels in heart and brain. J Biol Chem. 1972 Jan 10;247(1):16–22. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee T. P., Kuo J. F., Greengard P. Role of muscarinic cholinergic receptors in regulation of guanosine 3':5'-cyclic monophosphate content in mammalian brain, heart muscle, and intestinal smooth muscle. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3287–3291. doi: 10.1073/pnas.69.11.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuchli A. Radioassay for beta-emitters in biological materials using Cerenkov radiation. Int J Appl Radiat Isot. 1969 Apr;20(4):265–270. doi: 10.1016/0020-708x(69)90054-4. [DOI] [PubMed] [Google Scholar]
- Murad F., Manganiello V., Vaughan M. A simple, sensitive protein-binding assay for guanosine 3':5'-monophosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):736–739. doi: 10.1073/pnas.68.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- SUGINO Y., MIYOSHI Y. THE SPECIFIC PRECIPITATION OF ORTHOPHOSPHATE AND SOME BIOCHEMICAL APPLICATIONS. J Biol Chem. 1964 Jul;239:2360–2364. [PubMed] [Google Scholar]
- Shimono H., Sugino Y. Metabolism of deoxyribonucleotides. Purification and properties of deoxyguanosine monophosphokinase of calf thymus. Eur J Biochem. 1971 Mar 11;19(2):256–263. doi: 10.1111/j.1432-1033.1971.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Turtle J. R., Kipnis D. M. A new assay for adenosine 3',5'-cyclic monophosphate in tissue. Biochemistry. 1967 Dec;6(12):3970–3976. doi: 10.1021/bi00864a044. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Field J. B. Elevation of cyclic guanosine 3',5'-monophosphate levels in dog thyroid slices caused by acetylcholine and sodium fluoride. J Biol Chem. 1972 Nov 10;247(21):7062–7066. [PubMed] [Google Scholar]



