Autoimmune encephalitis related to antibodies against neuronal cell surface and synaptic proteins is a new category of disorders in which the targets are well-known proteins and receptors involved in synaptic transmission and neuronal excitability. GABAA receptor is one of the latest identified antigens within this category.1 High-titer serum and CSF GABAA receptor antibodies were recently reported in 6 patients with autoimmune encephalitis associated with seizures or status epilepticus, 4 of them requiring pharmacologic-induced coma. Patients' brain MRIs showed characteristic multiple cortical and subcortical abnormalities with fluid-attenuated inversion recovery (FLAIR)/T2 hyperintensity. Antibodies to LGI1 are associated with limbic encephalitis previously attributed to voltage-gated potassium channels (VGKC).2 Coexistence of these antibodies is rare and intriguing.
We report here the presence of antibodies to the GABAA receptor and LGI1 in a patient with autoimmune encephalitis and thymoma.
Case.
A 45-year-old woman presented with subacute onset of memory loss, confabulation, and behavioral changes. Eight years earlier she was diagnosed with myasthenia gravis (MG) associated with type B2 thymoma, which was treated with surgery and radiation therapy. Four years later, she developed retroperitoneal and mediastinal metastases that were surgically removed. In addition, she had well-controlled epilepsy since childhood and had been asymptomatic on phenobarbital, pyridostigmine, prednisone, and azathioprine.
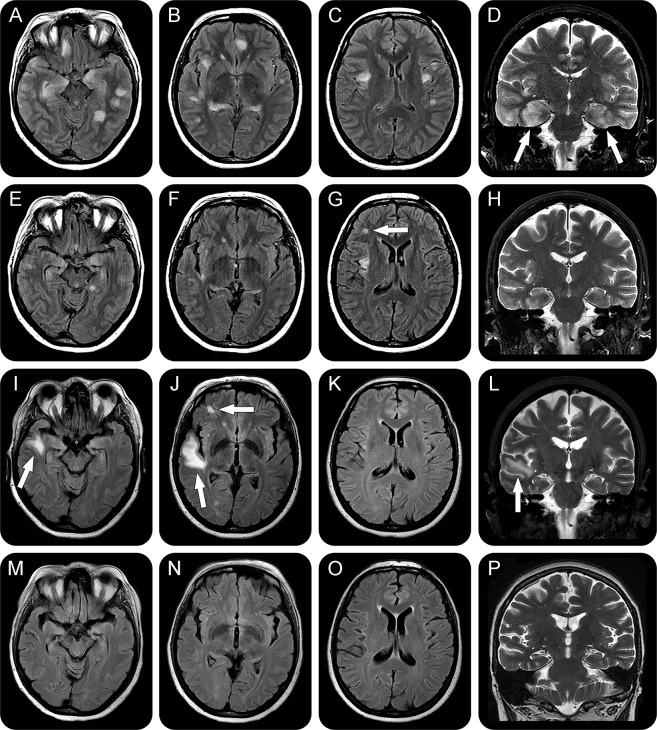
On examination, she was disoriented to time and space, showed impaired memory with confabulations, demonstrated mild executive dysfunction, and had a Mini-Mental State Examination (MMSE) score of 20. The remainder of the neurologic and physical examination was unremarkable. Brain MRI showed multiple cortical and subcortical T2/FLAIR hyperintense non-contrast-enhancing lesions with extensive mesial temporal lobe involvement that was worse on the right side (figure 1). CSF was normal. EEG showed periodic lateralized epileptiform discharges (PLEDs) in the right temporal region and left temporal onset electroencephalographic seizures without clinical manifestations. Brain fluorodeoxyglucose (FDG) PET showed uptake in the right insular and temporal regions. Whole-body FDG-PET disclosed a hypermetabolic pleural lesion. Additional laboratory tests showed moderately increased C-reactive protein (33.3 mg/dL) and erythrocyte sedimentation rate (28 mm), and positive acetylcholine receptor, antinuclear antibody, and double-stranded DNA antibodies. Thyroid and GAD65 antibodies were negative.
Figure 1. Follow-up of brain MRI.
Axial fluid-attenuated inversion recovery (A–C, E–G, I–K, and M–O) and coronal T2-weighted (D, H, L, P) images. Initial exam (A–D) shows multiple hyperintensities involving both hemispheres, without restriction on diffusion sequences. Note the prominent bilateral mesial temporal lobe involvement, mainly on the right side (arrows in D). A repeat MRI 40 days later (E–H) shows a substantial reduction in the number and size of the lesions; however, a new hyperintensity in the subcortical region of the right frontal lobe is evident (arrow in G). There is also an interval enlargement of the lateral ventricles and sulci. A third brain MRI, 108 days after the first one (I–L), demonstrates new predominantly subcortical lesions (arrows in I, J, and L), while the previous ones have disappeared. The last MRI (M–P), 143 days after the first one, shows that after immunotherapy and removal of the metastatic thymoma all abnormalities have disappeared.
Methylprednisolone 1 g per day for 5 days was started, followed by 6 plasma exchange sessions. After treatment, she scored 25 points on the MMSE, and her memory and anxiety improved. Follow-up brain MRI showed substantial reduction in the number and size of all abnormalities, mainly in the temporal lobes (figure 1), and the PLEDs resolved. Antibodies against cell surface or synaptic proteins were assessed in serum and CSF obtained before immunotherapy using rat brain immunohistochemistry and cell-based-assays, as reported1,2 These studies showed high levels of serum (1:320) and CSF (1:80) antibodies against the GABAA receptor and low levels of antibodies against LGI1 (serum 1:80, CSF 1:20). Antibodies against GABAB receptor, AMPA receptor, NMDA receptor, Caspr2, GlyR, mGLUR5, and mGLUR1 were negative.
Three months after discharge, the patient was having a good recovery, but the brain MRI showed a new subcortical lesion in the right frontal lobe. She underwent repeat methylprednisolone and plasma exchange and surgical removal of the pleural lesion, whose pathology was consistent with thymoma. Tumor antigen expression was examined in tissue obtained from the first thymoma resection (8 years earlier), which showed lack of GABAA receptor and LGI1 reactivity (not shown), and in tissue from the pleural lesion, which showed expression of both antigens (figure 2). After the indicated treatment, the patient's neurologic function returned close to baseline, and a repeat brain MRI showed resolution of all lesions.
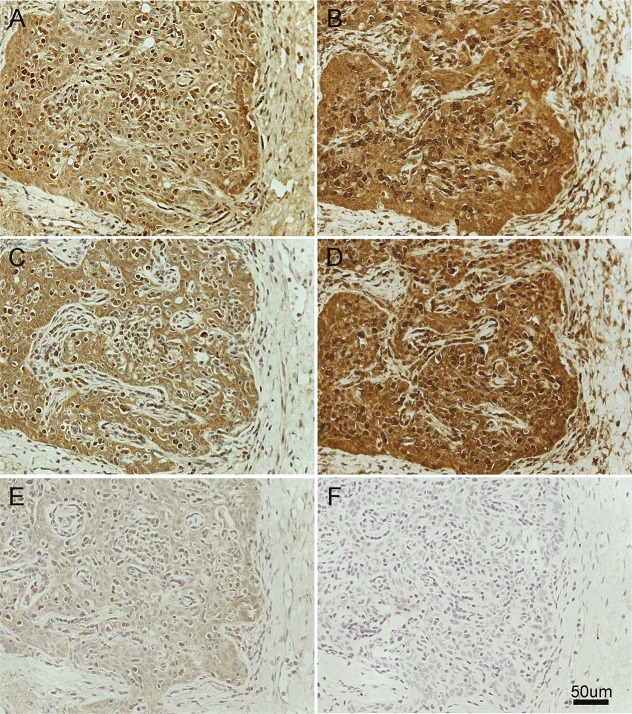
Figure 2. Expression of GABAA receptor and LGI1 by patient's thymoma.
Tissue sections of the patient's thymoma incubated with biotinylated immunoglobulin G (IgG) from a patient with only GABAA receptor antibodies (A), another patient with only LGI1 antibodies (C), and a control subject without antibodies (E) compared with the reactivity of a commercial antibody against GABAA receptor (B) (rabbit polyclonal anti-α1 GABAA receptor, abcam, ab33299, diluted 1:50), a commercial antibody against LGI1 (D) (rabbit polyclonal anti-LGI1, abcam, ab30868, diluted 1:50), and the secondary antibody alone (F). Note the similar pattern of reactivity of human antibodies and commercial antibodies, indicating that the tumor expresses epitopes of GABAA receptor and LGI1 proteins. The dilution of human IgG was 1:50. All sections were mildly counterstained with hematoxylin.
Discussion.
Although thymoma is frequently associated with autoimmune disorders, the most common being MG,3 encephalitis associated with thymoma is rare. A review of the literature demonstrates 30 previously reported cases (table e-1 at Neurology.org/nn). These patients often developed clinical features of limbic dysfunction and coexistence of other autoimmunities, similar to the case reported here, but the target antigens were largely unknown. After the initial description of anti-GABAA receptor encephalitis, Ohkawa et al.4 reported 2 cases with anti-GABAA receptor encephalitis and thymoma. These 2 patients had been previously reported as having limbic encephalitis associated with VGKC antibodies5,6 and shared similarities with our patient, including subacute onset of cognitive and memory deficits associated with thymoma recurrence or residual thymoma, coexistence of LGI1 or Caspr2 antibodies with GABAA receptor antibody, and remarkable brain MRI abnormalities, which are strikingly similar to those reported in other patients with GABAA receptor encephalitis.
Our patient provides 2 novelties: First, high levels of GABAA receptor antibodies were found both in serum and CSF; In the above-mentioned 2 cases with thymoma, the antibody testing was performed only in serum. Second, to our knowledge, this is the first case in which expression of the GABAA receptor is demonstrated in the tumor. It is interesting that this receptor and LGI1 were not detected in the initial sample of tumor obtained 8 years earlier but were present in the most recent sample. These findings raise the question of whether thymoma could express different antigens during its progression, leading to manifestations of different paraneoplastic diseases, as occurred in our and other cases of thymoma-associated encephalitis.5–7
This report emphasizes the importance of aggressive immunotherapy along with surgical removal of the tumor in the category of disorders associated with antibodies against relevant cell surface antigens (GABAA receptor, LGI1), which in our case resulted in clinical and radiologic improvement. This is in contrast with the previously reported patient, who was treated with immunotherapy but did not have tumor removal and had persistent severe cognitive deficits.5,6
Supplementary Material
Footnotes
Supplemental data at Neurology.org/nn
Author contributions: Dr. Simabukuro had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. He contributed to study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. M. Petit-Pedrol contributed to analysis and interpretation of data and critical revision of the manuscript for important intellectual content. Dr. Castro contributed to study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content. Dr. Nitrini contributed to study concept and design and critical revision of the manuscript for important intellectual content. Dr. Lucato contributed to analysis and interpretation of data and critical revision of the manuscript for important intellectual content. Dr. Zambon contributed to analysis and interpretation of data and critical revision of the manuscript for important intellectual content. Dr. Silva contributed to analysis and interpretation of data. Dr. Fortes contributed to analysis and interpretation of data. Dr. Soares Neto contributed to analysis and interpretation of data. Dr. Dalmau contributed to study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Study funding: This work was supported in part by NIH grant RO1NS077851, Fundació la Marató TV3, and Fondo de Investigaciones Sanitarias grant PI11/01780 (Dr. Dalmau). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure: M.M. Simabukuro and M. Petit-Pedrol report no disclosures. L.H. Castro has received research support from the State of Sao Paulo (Brazil) Research Council. R. Nitrini is on the advisory board for Janssen-Cilag and Brazilian Nutricia meetings; is editor for Dementia & Neuropsychologia; is on the editorial board for Alzheimer's Disease and Associated Disorders and International Journal of Alzheimer's Disease; has spoken at meetings sponsored by Novartis and Danone (Nutricia); and received research support from FAPESP, Federico Foundation. L. Lucato is on the editorial board for Aqruivos de Neuro-Psiquitria. A.A. Zambon, L.G. Silva, G.C.R. Fortes, and H.R. Soares Neto report no disclosures. J.O. Dalmau is the editor of Neurology: Neuroimmunology & Neuroinflammation; is on the editorial board for Neurology UpToDate; holds patents for and receives royalties from Ma2 autoantibody test, NMDA receptor autoantibody test, GABA(B) receptor autoantibody test, GABA(A) receptor autoantibody test, DPPX autoantibody test, and IgLON5 autoantibody test; and received research support from Euroimmun, NIH, Fondo de Investigaciones Sanitarias de la Seguridad Social (Spanish Government). Go to Neurology.org/nn for full disclosures. The Article Processing Charge was paid by the authors.
References
- 1.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014;13:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010;9:776–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evoli A, Lancaster E. Paraneoplastic disorders in thymoma patients. J Thorac Oncol 2014;9:S143–S147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohkawa T, Satake S, Yokoi N, et al. Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. J Neurosci 2014;34:8151–8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohshita T, Kawakami H, Maruyama H, Kohriyama T, Arimura K, Matsumoto M. Voltage-gated potassium channel antibodies associated limbic encephalitis in a patient with invasive thymoma. J Neurol Sci 2006;250:167–169. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki Y, Hirayama M, Watanabe H, et al. Paraneoplastic encephalitis associated with myasthenia gravis and malignant thymoma. J Clin Neurosci 2012;19:336–338. [DOI] [PubMed] [Google Scholar]
- 7.Evoli A, Lo Monaco M, Marra R, Lino MM, Batocchi AP, Tonali PA. Multiple paraneoplastic diseases associated with thymoma. Neuromuscul Disord 1999;9:601–603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.