Abstract
Azithromycin, administered with ceftriaxone, is recommended by the CDC for the treatment of gonorrhea. Many experts have expressed concern about the ease with which Neisseria gonorrhoeae can acquire macrolide resistance. We sought to describe gonococcal azithromycin susceptibility in the United States and to determine whether azithromycin susceptibility has changed over time. We analyzed data from 2005 to 2013 from the Gonococcal Isolate Surveillance Project, a CDC-supported sentinel surveillance network that monitors gonococcal antimicrobial susceptibility. A total of 44,144 N. gonorrhoeae isolates were tested for azithromycin susceptibility by agar dilution methods. The overall azithromycin MIC50 was 0.25 μg/ml, and the MIC90 was 0.5 μg/ml. There were no overall temporal trends in geometric means. Isolates from men who had sex with men had significantly higher geometric mean MICs than isolates from men who had sex exclusively with women. The overall prevalence of reduced azithromycin susceptibility (MIC, ≥2 μg/ml) was 0.4% and varied by year from 0.3% (2006 and 2009) to 0.6% (2013). We did not find a clear temporal trend in gonococcal azithromycin MICs in the United States, and the prevalence of reduced azithromycin susceptibility remains low. These findings support the continued use of azithromycin in a combination therapy regimen for gonorrhea.
INTRODUCTION
Gonorrhea is the second most commonly reported notifiable disease in the United States, second only to chlamydia. In 2012, 334,826 gonococcal infections were reported in the United States, representing a continuing gradual increase over the preceding 4 years (1). In women, Neisseria gonorrhoeae is a major cause of pelvic inflammatory disease, ectopic pregnancy, and infertility (2, 3). Public health control of gonorrhea relies on prompt detection and effective treatment. However, treatment has become more challenging because N. gonorrhoeae has successively developed resistance to each antimicrobial recommended for treatment. Penicillinase-producing N. gonorrhoeae and gonococcal fluoroquinolone resistance emerged first in the United States in Hawaii, California, and other western states, possibly due to their geographic proximity to East Asia and to travel, before spreading elsewhere in the United States (4–6). Fluoroquinolone-resistant gonorrhea initially also became prevalent among men who have sex with men (MSM) with gonorrhea before emerging among heterosexuals (7). Similar epidemiological patterns were observed more recently for reduced susceptibility to oral cephalosporins; cefixime MICs increased sharply in isolates from the western United States and from MSM during 2006 and 2011 (8, 9).
Currently, the Centers for Disease Control and Prevention (CDC) recommends only a single first-line regimen for gonorrhea treatment, i.e., the combination of 250 mg of ceftriaxone (an injectable cephalosporin) as a single intramuscular dose and either 100 mg of doxycycline orally twice daily or a single 1-g oral dose of azithromycin (9). Because of the high prevalence of tetracycline resistance, azithromycin, an azalide macrolide which binds to the bacterial 50S ribosomal subunit and inhibits protein synthesis, is preferred over doxycycline as the second agent. Observational outcome data of pharyngeal gonorrhea also suggest that compared to the addition of doxycycline, the addition of azithromycin to an oral cephalosporin improves the likelihood of cure (10).
Azithromycin has not been recommended for gonorrhea monotherapy because of the reported ease with which N. gonorrhoeae can acquire macrolide resistance (11). Two mechanisms have been commonly implicated in gonococcal reduced azithromycin susceptibility: overexpression of an efflux pump due to mtrR-coding region mutations (12–15) and decreased antimicrobial affinity due to mutations in genes encoding the 23S ribosomal subunit (15, 16). Because of the importance of azithromycin in the currently recommended treatment regimen and the risk of macrolide resistance, careful monitoring of azithromycin susceptibility trends is important. We sought to describe gonococcal azithromycin susceptibility in the United States and to determine whether gonococcal azithromycin susceptibility has changed between 2005 and 2013.
MATERIALS AND METHODS
The Gonococcal Isolate Surveillance Project.
The Gonococcal Isolate Surveillance Project (GISP) is a U.S.-based national sentinel surveillance system established in 1986 to monitor trends in antimicrobial susceptibility in N. gonorrhoeae and to establish a rational basis for the selection of gonococcal therapies in the United States (17). Sexually transmitted disease (STD) clinics in 25 to 30 cities throughout the United States participate in the GISP as sentinel sites. At each participating STD clinic, urethral N. gonorrhoeae isolates are collected from the first 25 men presenting with symptomatic gonococcal urethritis each month. Isolates are submitted to regional reference laboratories for antimicrobial susceptibility testing by agar dilution according to a common protocol (www.cdc.gov/std/gisp), and the results are sent to the CDC. The GISP also collects deidentified data on patient characteristics and clinical information from clinic medical records.
Laboratory methods.
Gonococcal isolates collected at each sentinel clinic are subcultured at the clinic's local public health laboratory on supplemented chocolate medium and frozen in Trypticase soy broth containing 20% glycerol. Isolates are shipped monthly to one of the regional reference laboratories, where they are tested for β-lactamase production and susceptibility to azithromycin, penicillin, tetracycline, ciprofloxacin, spectinomycin, cefixime, and ceftriaxone using the agar dilution method. Isolates were inoculated on Difco GC medium base supplemented with 1% IsoVitaleX enrichment (Becton-Dickinson Diagnostic Systems, Sparks, MD). During 2005 to 2007, the lowest azithromycin concentration tested was 0.008 μg/ml; this increased to 0.03 μg/ml in 2008. The routine testing range for azithromycin extended to 16.0 μg/ml during 2005 to 2013. Laboratories were asked to conduct agar dilution testing to identify an endpoint for isolates with an MIC of ≥16.0 μg/ml on the initial testing run. Testing to an endpoint was not conducted on three isolates collected during 2005 to 2013. In the absence of Clinical and Laboratory Standards Institute (CLSI) breakpoints for gonococcal azithromycin susceptibility or resistance (18), we defined reduced azithromycin susceptibility for this analysis as an MIC of ≥2.0 μg/ml. Quality assurance processes are described in detail in the GISP protocol (19).
To ensure accuracy and reproducibility of antimicrobial susceptibility results from the regional reference laboratories, a set of seven control N. gonorrhoeae strains with known MICs of various antimicrobials are included with each susceptibility run. In addition, reference laboratories test a CDC-provided panel of 15 unidentified strains twice yearly to compare results and ensure consistency among laboratories (19). The results obtained from the testing of control strains and CDC-provided panels are used for internal quality assurance.
Statistical analysis.
Data were limited to sites that continuously participated during the analysis period: Albuquerque, NM; Atlanta, GA; Baltimore, MD; Birmingham, AL; Chicago, IL; Cleveland, OH; Dallas, TX; Denver, CO; Greensboro, NC; Honolulu, HI; Las Vegas, NV; Los Angeles, Orange County, San Diego, and San Francisco, CA; Miami, FL; Minneapolis, MN; New Orleans, LA; Oklahoma City, OK; Philadelphia, PA; Phoenix, AZ; Portland, OR; and Seattle, WA. Clinical sites were grouped into U.S. census regions. The Northeast and South were combined because of the small number of sites in the Northeast.
Azithromycin MIC values of ≤0.03 μg/ml were considered to have MICs of 0.03 μg/ml for these analyses. Similarly, the four values reported as ≥16.0 μg/ml without reported endpoints were considered to have MICs of 16.0 μg/ml for the primary analysis. Sensitivity analyses were performed by considering the azithromycin MIC value for these three isolates to be 256.0 μg/ml (the highest azithromycin MIC detected thus far in the United States was ≥256.0 μg/ml [1]). MIC50s, geometric means with 95% confidence intervals (CIs), and the percentage of isolates with reduced susceptibility with 95% CIs were calculated. Proportions were compared using the chi-square test, and medians were compared using the Wilcoxon-Mann-Whitney test. Two-sided P values of <0.05 were considered statistically significant. Statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
During 2005 to 2013, 44,144 isolates were tested; 43% were collected in the West and 44% in the Northeast/South (Table 1). Most men who submitted isolates were black, and 69% of men reported sex with only female partners. The percentage of men with gonorrhea treated with azithromycin monotherapy (2 g) increased from 0.1% in 2005 to 3.0% in 2012 (P < 0.001) and then decreased to 1.4% in 2013 (P < 0.001). Overall, azithromycin monotherapy was prescribed to 0.6% (range by year, 0% to 1.8%) of men in the Midwest, 1.2% (0.1 to 1.8%) in the Northeast and South, and 1.3% (0.04% to 5.3%) in the West.
TABLE 1.
Characteristics of men who submitted urethral Neisseria gonorrhoeae isolates, Gonococcal Isolate Surveillance Project, United States, 2005 to 2013
| Characteristic | Value |
|---|---|
| Total no. | 44,144 |
| Regiona (no. [%]) | |
| West | 18,960 (43.0) |
| Midwest | 5,738 (13.0) |
| Northeast/South | 19,446 (44.1) |
| Age, median (IQR) (yr)b | 27 (22-35) |
| Race/ethnicity (no. [%]) | |
| Black (non-Hispanic) | 28,055 (63.6) |
| White (non-Hispanic) | 8,171 (18.5) |
| Hispanic or Latino | 5,268 (11.9) |
| Other | 1,952 (4.4) |
| Unknown | 698 (1.6) |
| Sex of sex partner (no. [%]) | |
| Female only | 30,712 (69.6) |
| Male only | 10,662 (24.2) |
| Male and female | 1,965 (4.5) |
| Unknown | 805 (1.8) |
Limited to sites that continuously participated during 2005 to 2013. West included Albuquerque, NM; Denver, CO; Honolulu, HI; Las Vegas, NV; Los Angeles, Orange County, San Diego, and San Francisco, CA; Phoenix, AZ; Portland, OR; and Seattle, WA. Midwest included Chicago, IL; Cleveland, OH; and Minneapolis, MN. Northeast/South included Atlanta, GA; Baltimore, MD; Birmingham, AL; Dallas, TX; Greensboro, NC; Miami, FL; New Orleans, LA; Oklahoma City, OK; and Philadelphia, PA.
Missing data from 425 patients. IQR, interquartile range.
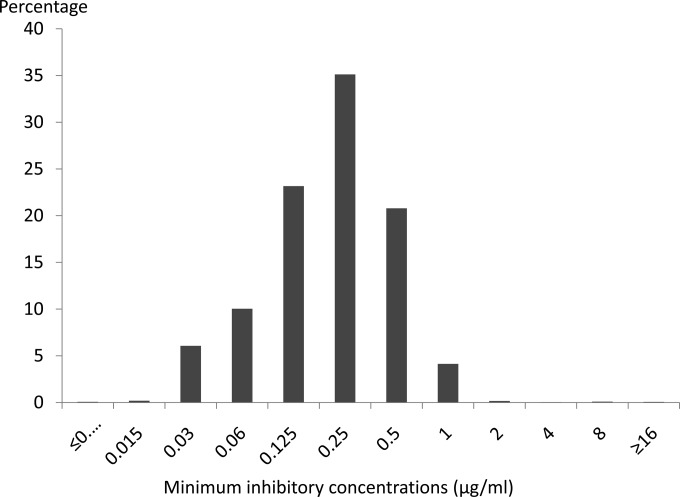
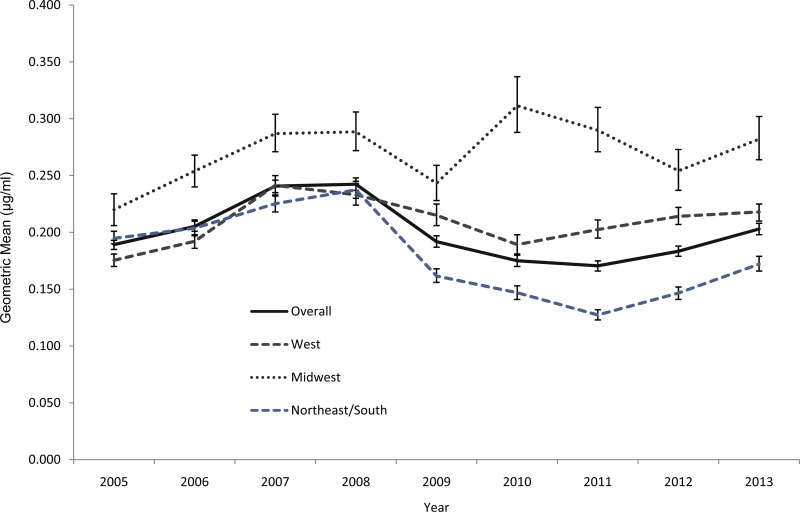
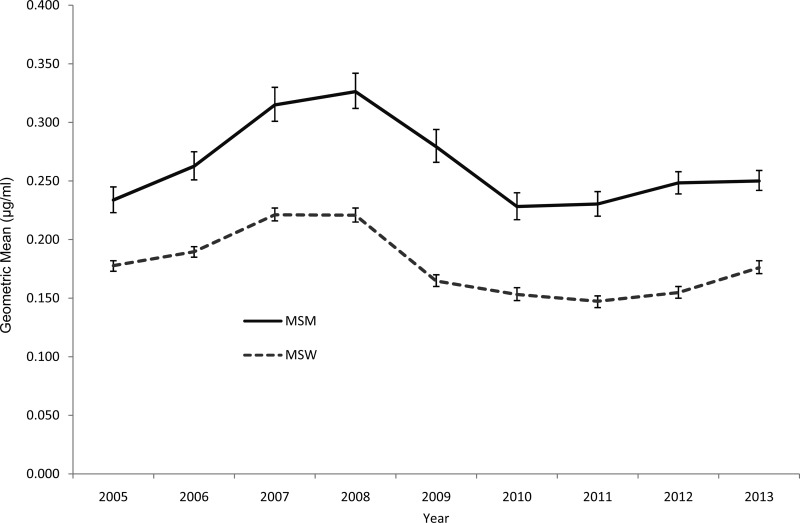
The distribution of azithromycin MICs is displayed in Fig. 1. Overall and each year, the azithromycin susceptibilities were stable; MIC50s were 0.25 μg/ml and MIC90s were 0.5 μg/ml. Among all the isolates, only 32 isolates (0.1%) had an MIC of 16 μg/ml, and 1 isolate had an MIC of ≥256 μg/ml. Geometric mean MICs increased between 2005 and 2008, decreased until 2011, and then increased slightly between 2011 and 2013 (Fig. 2). There was no overall increase in the geometric mean MIC during the entire analysis period. Isolates from the Midwest exhibited higher geometric means than isolates from elsewhere, overall and regardless of sex of sex partner (Fig. 2; see also Fig. S1 in the supplemental material). Isolates from MSM tended to have greater geometric mean MICs than isolates from men who report having sex exclusively with women (MSW) (Fig. 3); this pattern was consistent across geographic regions (see Fig. S2 through S4 in the supplemental material).
FIG 1.
Azithromycin MIC distribution of urethral Neisseria gonorrhoeae isolates, 2005 to 2013.
FIG 2.
Geometric means of azithromycin MICs by geographic region and year among continuously participating sites, Gonococcal Isolate Surveillance Project, United States, 2005 to 2013. West included Albuquerque, NM; Denver, CO; Honolulu, HI; Las Vegas, NV; Los Angeles, Orange County, San Diego, and San Francisco, CA; Phoenix, AZ; Portland, OR; and Seattle, WA. Midwest included Chicago, IL; Cleveland, OH; and Minneapolis, MN. Northeast/South included Atlanta, GA; Baltimore, MD; Birmingham, AL; Dallas, TX; Greensboro, NC; Miami, FL; New Orleans, LA; Oklahoma City, OK; and Philadelphia, PA.
FIG 3.
Geometric means of azithromycin MICs by sex of sex partner and year among continuously participating sites, Gonococcal Isolate Surveillance Project, United States, 2005 to 2013. MSM, men who have sex with men; MSW, men who report having sex exclusively with women.
We conducted sensitivity analyses by replacing values with isolates of ≥16.0 μg/ml (and with no endpoints) with assigned MICs of 256 μg/ml. After replacement, the geometric means in 2007 and 2013 increased by only 0.003 μg/ml, without changes in the relative position of the geometric mean by year, compared to the values in the primary analysis.
During 2005 to 2013, 0.4% of isolates exhibited MICs of ≥2 μg/ml (see Table S1 in the supplemental material). No overall increase in the prevalence of reduced azithromycin susceptibility was observed. The prevalence of reduced susceptibility was highest among isolates from the West and Midwest. At individual geographic sites, isolates with reduced susceptibility were detected sporadically (see Table S2 in the supplemental material). For example, no isolates with a reduced susceptibility were detected in Denver in 2005 or 2006, six were detected in 2007, and then none were detected during the subsequent 3 years. Men whose isolates exhibited reduced susceptibility were significantly more likely to be white and to report having only male sexual partners (see Table S3 in the supplemental material).
A total of 175 isolates had reduced azithromycin susceptibility (MICs of ≥2 μg/ml). Isolates with reduced susceptibility were more likely to exhibit resistance to penicillin, tetracycline, and ciprofloxacin than azithromycin-susceptible isolates (see Table S3 in the supplemental material). Among 33 isolates with azithromycin MICs of ≥16 μg/ml, 66.7% were collected in the West and 24.2% in the Midwest; 69.7% were collected from MSM, and 27.3% exhibited resistance to penicillin, 30.3% to tetracycline, and 21.2% to ciprofloxacin.
DISCUSSION
Gonococcal azithromycin MICs changed little in the United States between 2005 and 2013. Isolates collected in the Midwest demonstrated slightly higher azithromycin geometric means than isolates from other regions, but even in the Midwest, MICs do not appear to be increasing. Isolates from MSM exhibited higher azithromycin MICs than isolates from MSW, but MICs do not appear to be increasing in either group. The prevalence of reduced susceptibility remains low.
Azithromycin is the most commonly prescribed antimicrobial in the United States (20), and azithromycin prescribing rates increased during the 1990s and 2000s, including among individuals 18 to 49 years of age (21, 22). It is widely believed by experts that N. gonorrhoeae can readily acquire azithromycin resistance (11). Thus, it may be somewhat surprising that gonococcal azithromycin MICs have not increased in recent years. It is possible that we overestimated the capacity of N. gonorrhoeae to acquire azithromycin resistance. Apart from three case reports that demonstrated azithromycin MIC increases after treatment with azithromycin monotherapy (15, 23, 24) and studies that selected for macrolide resistance using erythromycin (25, 26), we are not aware of published data on mutational frequency with in vitro azithromycin exposure or on the stability of the mutants. Related to this, reduced azithromycin susceptibility may be acquired at a fitness cost that limits transmissibility, as appears to be the case with Streptococcus pneumoniae and Campylobacter jejuni (27–29). This may explain the sporadicity of the detection of isolates with reduced azithromycin susceptibility observed at individual geographic sites in the GISP. One mutation contributing to N. gonorrhoeae reduced azithromycin susceptibility, that in the mtrR-coding region, may confer a fitness advantage (30), but other mutations might not. Research into whether mutations conferring reduced susceptibility are associated with fitness costs would be helpful. Investigation of factors contributing to the development and transmission of azithromycin resistance in N. gonorrhoeae is needed.
Although multiple isolates with high-level azithromycin resistance have been detected in different countries (26, 30–35), international susceptibility data do not clearly indicate emerging gonococcal azithromycin resistance. The pooled prevalence of reduced azithromycin susceptibility (defined as MICs of ≥2.0 μg/ml) increased in Latin America from 0% in 1999 to 25.8% in 2008 and then fell to 1.0% by 2010 (36). However, these trends should be interpreted with caution because the number of participating countries and the number of submitted isolates varied over this period. In East Asia, where fluoroquinolone and oral cephalosporin resistance appeared to initially emerge, the prevalence of reduced azithromycin susceptibility (defined as MIC of ≥1 μg/ml) seems to be relatively low, ranging from <1% in Bhutan and Thailand (2011 and 2012) to 3.9% in Hong Kong (2011) (37). In the United Kingdom, the prevalence of azithromycin MICs of ≥1 μg/ml increased from 0.3% in 2001 to 4.1% by 2007, then fell to 0.7% by 2012 (38, 39).
In the United States, antimicrobial resistance phenotypes and increasing MICs were historically identified first in Hawaii and the western region, possibly due to the geographic proximity to East Asia, where penicillin-, fluoroquinolone-, and cephalosporin-resistant strains seemed to initially emerge. The epidemiological pattern of reduced azithromycin susceptibility appears to differ from this historical pattern: azithromycin MICs appear to be higher in isolates collected in the Midwest than in isolates collected from other regions. It is possible that factors other than travel or geographic proximity to Asia, such as antimicrobial consumption, contribute to introductions of strains with reduced azithromycin susceptibility. However, the epidemiological factors contributing to azithromycin resistance are unclear.
As we reported previously (40), azithromycin MICs were higher in isolates collected from MSM than in isolates from MSW. This is consistent with findings from the United Kingdom (39) and with previously described associations between male-to-male sexual behavior and isolates with fluoroquinolone resistance or elevated cefixime MICs in the United States (6, 7). The reasons for the differences in susceptibility between isolates from MSM and MSW are unclear, although data suggest that isolates with the mtr mutation mentioned above can better survive in the rectum (41). Alternatively, the observed differences may be related to differences in sexual network structures and geographic scope, differences in antimicrobial usage, or differing anatomical sites of infection between MSM and MSW (40).
We defined azithromycin reduced susceptibility as MICs of ≥2 μg/ml, but CLSI has not established N. gonorrhoeae susceptibility or resistance breakpoints for azithromycin (18). Establishment of azithromycin breakpoints may facilitate N. gonorrhoeae azithromycin antimicrobial susceptibility testing in clinical laboratories. Although the correlation between N. gonorrhoeae azithromycin MIC and clinical outcome after treatment has not been well defined, the establishment of azithromycin epidemiological cutoff values (ECVs) may be a worthwhile first step. It is worth noting that the European Committee on Antimicrobial Susceptibility Testing (EUCAST) defines azithromycin resistance as ≥1.0 μg/ml (http://www.eucast.org).
This study is subject to at least two limitations. The reported treatment data reflect prescribing practices in specialty STD clinics participating in the GISP and are not expected to reflect prescribing practices in private clinical settings and other non-STD clinic health care settings (which reported >80% of gonorrhea cases in the United States in 2012) (1). Thus, the treatment data presented here likely represent an overestimate of the percentage of patients with gonorrhea who are treated with ceftriaxone-based therapy in the United States. One regional laboratory tested the isolates from all three Midwestern sites; it is possible that conditions or test result interpretation by laboratory personnel differed between this laboratory and others and might have contributed to the elevated MICs observed in Midwest isolates. Continued surveillance in the GISP, especially with recent changes in the laboratories participating in the GISP, is likely to shed light on the likelihood of this possibility.
Isolates with high azithromycin MICs have been sporadically detected in the United States, and resistant strains have been detected following azithromycin monotherapy in the United States and elsewhere, suggesting the possibility of within-host selection for azithromycin resistance (15, 23, 24, 33). Thus, gonorrhea treatment with azithromycin monotherapy is discouraged. However, N. gonorrhoeae strains with azithromycin resistance have not demonstrated sustained transmission and population-level emergence in the United States, and the prevalence of reduced azithromycin susceptibility remains low. These findings support the current use of azithromycin as part of combination therapy for gonorrhea.
Supplementary Material
ACKNOWLEDGMENTS
The Gonococcal Isolate Surveillance Project is funded by the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services.
We thank King K. Holmes, Tamara Baldwin, Elizabeth Delamater, Paula Dixon, Alesia Harvey, Connie Lenderman, Baderinwa Offut, Kevin Pettus, Tremeka Sanders, and Samera Sharpe.
The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04337-14.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2014. 2012 STD Surveillance Report. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 2.Landman GA, Phillips LV, Friend L. 1958. Treatment of acute gonorrheal pelvic inflammatory disease: the use of benzathine penicillin G in the ambulatory patient. South Med J 51:899–902. doi: 10.1097/00007611-195807000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Falk V. 1965. Treatment of acute non-tuberculous salpingitis with antibiotics alone and in combination with glucocorticoids: a prospective double blind controlled study of the clinical course and prognosis. Acta Obstet Gynecol Scand 44(Suppl 6):S3–S118. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2002. Increases in fluoroquinolone-resistant Neisseria gonorrhoeae—Hawaii and California, 2001. MMWR Morb Mortal Wkly Rep 51:1041–1044. [PubMed] [Google Scholar]
- 5.Jaffe HW, Biddle JW, Johnson SR, Wiesner PJ. 1981. Infections due to penicillinase-producing Neisseria gonorrhoeae in the United States: 1976-1980. J Infect Dis 144:191–197. doi: 10.1093/infdis/144.2.191. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2007. Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 56:332–336. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2004. Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men—United States, 2003, and revised recommendations for gonorrhea treatment, 2004. MMWR Morb Mortal Wkly Rep 53:335–338. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2011. Cephalosporin susceptibility among Neisseria gonorrhoeae isolates—United States, 2000–2010. MMWR Morb Mortal Wkly Rep 60:873–877. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2012. Update to CDC's sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep 61:590–594. [PubMed] [Google Scholar]
- 10.Barbee L, Kerani RP, Dombrowski JC, Soge OO, Golden MR. 2013. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhoea. Clin Infect Dis 56:1539–1545. doi: 10.1093/cid/cit084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recommend Rep 59(RR-12):1–110. [PubMed] [Google Scholar]
- 12.McLean CA, Wang SA, Hoff GL, Dennis LY, Trees DL, Knapp JS, Markowitz LE, Levine WC. 2004. The emergence of Neisseria gonorrhoeae with decreased susceptibility to azithromycin in Kansas City, Missouri, 1999 to 2000. Sex Transm Dis 31:73–78. doi: 10.1097/01.OLQ.0000109514.91508.FC. [DOI] [PubMed] [Google Scholar]
- 13.Zarantonelli L, Borthagaray G, Lee E-H, Shafer WM. 1999. Decreased azithromycin susceptibility of Neisseria gonorrhoeae due to mtrR mutations. Antimicrob Agents Chemother 43:2468–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson SR, Sandul AL, Parekh M, Wang SA, Knapp JS, Trees DL. 2003. Mutations causing in vitro resistance to azithromycin in Neisseria gonorrhoeae. Int J Antimicrob Agents 21:414–419. doi: 10.1016/S0924-8579(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 15.Soge OO, Harger D, Schafer S, Toevs K, Raisler KA, Venator K, Holmes KK, Kirkcaldy RD. 2012. Emergence of increased azithromycin resistance during unsuccessful treatment with Neisseria gonorrhoeae infection with azithromycin (Portland, OR, 2011). Sex Transm Dis 39:877–879. doi: 10.1097/OLQ.0b013e3182685d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. 2011. Neisseria gonorrhoeae with reduced susceptibility to azithromycin—San Diego County, California, 2009. MMWR Morb Mortal Wkly Rep 60:579–581. [PubMed] [Google Scholar]
- 17.Schwarcz SK, Zenilman JM, Schnell D, Knapp JS, Hook EW III, Thompson S, Judson FN, Holmes KK. 1990. National surveillance of antimicrobial resistance in Neisseria gonorrhoeae. JAMA 264:1413–1417. doi: 10.1001/jama.1990.03450110059027. [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. CLSI document M100-S21 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Centers for Disease Control and Prevention. 2014. Gonococcal Isolate Surveillance Project (GISP): protocol. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/std/gisp/GISP-Protocol-May-2014.pdf. [Google Scholar]
- 20.Hicks LA, Taylor TH, Hunter RJ. 2013. U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 368:1461–1462. doi: 10.1056/NEJMc1212055. [DOI] [PubMed] [Google Scholar]
- 21.Grijalva GC, Nuorti JP, Griffin MR. 2009. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 302:758–766. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCaig LF, Besser RE, Hughes JM. 2003. Antimicrobial-drug prescription in ambulatory care settings, United States, 1992–2000. Emerg Infect Dis 9:432–437. doi: 10.3201/eid0904.020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young H, Moyes A, McMillan A. 1997. Azithromycin and erythromycin resistant Neisseria gonorrhoeae following treatment with azithromycin. Int J STD AIDS 8:299–302. doi: 10.1258/0956462971920127. [DOI] [PubMed] [Google Scholar]
- 24.Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. 2011. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill 16:pii=19833 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19833. [PubMed] [Google Scholar]
- 25.Ng L-K, Martin I, Liu G, Bryden L. 2002. Mutation in 23S rRNA associated with macrolide resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother 46:3020–3025. doi: 10.1128/AAC.46.9.3020-3025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chisholm SA, Dave J, Ison CA. 2010. High-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23S rRNA genes. Antimicrob Agents Chemother 54:3812–3816. doi: 10.1128/AAC.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao H, Dai M, Wang Y, Peng D, Liu Z, Yuan Z. 2009. 23S rRNA mutation A2074C conferring high-level macrolide resistance and fitness cost in Campylobacter jenuni. Microb Drug Resist 15:239–243. doi: 10.1089/mdr.2009.0008. [DOI] [PubMed] [Google Scholar]
- 28.Zeitouni S, Collin O, Andraud M, Ermel G, Kempf I. 2012. Fitness of macrolide resistant Campylobacter coli and Campylobacter jenuni. Microb Drug Resist 18:101–108. doi: 10.1089/mdr.2011.0188. [DOI] [PubMed] [Google Scholar]
- 29.Maher MC, Alemayehu W, Lakew T, Gaynor BD, Haug S, Cevallos V, Keenan JD, Lietman TM, Porco TC. 2012. The fitness cost of antibiotic resistance in Streptococcus pneumoniae: insight from the field. PLoS One 7:e29407. doi: 10.1371/journal.pone.0029407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warner DM, Folster JP, Shafer WM, Jerse AE. 2007. Regulation of the MtrC-MtrD-MtrE efflux-pump system modulates the in vivo fitness of Neisseria gonorrhoeae. J Infect Dis 196:1804–1812. doi: 10.1086/522964. [DOI] [PubMed] [Google Scholar]
- 31.Galarza PG, Alcalá B, Salcedo C, Fernández L, Buscemi L, Pagano I, Oviedo C, Vázquez JA. 2009. Emergence of high level azithromycin-resistant Neisseria gonorrhoeae strain located in the Argentina. Sex Transm Dis 36:787–788. doi: 10.1097/OLQ.0b013e3181b61bb1. [DOI] [PubMed] [Google Scholar]
- 32.Palmer HM, Young H, Winter A, Dave J. 2008. Emergence and spread of azithromycin-resistant Neisseria gonorrhoeae in Scotland. J Antimicrob Chemother 62:490–494. doi: 10.1093/jac/dkn235. [DOI] [PubMed] [Google Scholar]
- 33.Katz AR, Komeya AY, Soge OO, Kiaha MI, Lee MVC, Wasserman GM, Maningas EV, Whelan AC, Kirkcaldy RD, Shapiro SJ, Bolan GA, Holmes KK. 2012. Neisseria gonorrhoeae with high-level resistance to azithromycin: case report of the first isolate identified in the United States. Clin Infect Dis 54:841–843. doi: 10.1093/cid/cir929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unemo M, Golparian D, Hellmark B. 2014. First three Neisseria gonorrhoeae isolates with high-level resistance to azithromycin in Sweden: a threat to currently available dual-antimicrobial regimens for treatment of gonorrhea? Antimicrob Agents Chemother 58:624–625. doi: 10.1128/AAC.02093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen VG, Seah C, Martin I, Melano RG. 2014. Azithromycin resistance is coevolving with reduced susceptibility to cephalosporins in Neisseria gonorrhoeae in Ontario, Canada. Antimicrob Agents Chemother 58:2528–2534. doi: 10.1128/AAC.02608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dillon JA, Trecker MA, Thakur SD, Gonococcal Antimicrobial Surveillance Program Network in Latin America and Caribbean 1990–2011. 2013. Two decades of the gonococcal antimicrobial surveillance program in South America and the Caribbean: challenges and opportunities. Sex Transm Infect 89(Suppl 4):iv36–41. doi: 10.1136/sextrans-2012-050905. [DOI] [PubMed] [Google Scholar]
- 37.Lahra MM, Lo YR, Whiley DM. 2013. Gonococcal antimicrobial resistance in the Western Pacific region. Sex Transm Infect 89(Suppl 4):iv19–23. doi: 10.1136/sextrans-2012-050906. [DOI] [PubMed] [Google Scholar]
- 38.Health Protection Agency. 2008. GRASP: The Gonococcal Resistance to Antimicrobials Surveillance Programme: annual report. Health Protection Agency, London, United Kingdom: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1221117895841. [Google Scholar]
- 39.Public Health England. 2012. GRASP 2012 report: The Gonococcal Resistance to Antimicrobials Surveillance Programme. Public Health England, London, United Kingdom: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317140152190. [Google Scholar]
- 40.Kirkcaldy RD, Zaidi A, Hook EW III, Holmes KK, Soge OO, del Rio C, Hall G, Papp JR, Bolan G, Weinstock HS. 2013. Neisseria gonorrhoeae antimicrobial resistance among men who have sex with men and men who have sex exclusively with women: the Gonococcal Isolate Surveillance Project, 2005–2010. Ann Intern Med 158:321–328. doi: 10.7326/0003-4819-158-5-201303050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shafer WM, Balthazar JT, Hagman KE, Morse SA. 1995. Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology 141:907–911. doi: 10.1099/13500872-141-4-907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.