Abstract
The appropriate use of systemic antifungals is vital in the prevention and treatment of invasive fungal infection (IFI) in immunosuppressed children and neonates. This multicenter observational study describes the inpatient prescribing practice of antifungal drugs for children and neonates and identifies factors associated with prescribing variability. A single-day point prevalence study of antimicrobial use in hospitalized neonates and children was performed between October and December 2012. The data were entered through a study-specific Web-based portal using a standardized data entry protocol. Data were recorded from 17,693 patients from 226 centers. A total of 136 centers recorded data from 1,092 children and 380 neonates receiving at least one antifungal agent. The most frequently prescribed systemic antifungals were fluconazole (n = 355) and amphotericin B deoxycholate (n = 195). The most common indications for antifungal administration in children were medical prophylaxis (n = 325), empirical treatment of febrile neutropenia (n = 122), and treatment of confirmed or suspected IFI (n = 100 [14%]). The treatment of suspected IFI in low-birthweight neonates accounted for the majority of prescriptions in the neonatal units (n = 103). An analysis of variance (ANOVA) demonstrated no significant effect of clinical indication (prophylaxis or treatment of systemic or localized infection) on the total daily dose (TDD). Fewer than one-half of the patients (n = 371) received a TDD within the dosing range recommended in the current guidelines. Subtherapeutic doses were prescribed in 416 cases (47%). The predominance of fluconazole and high incidence of subtherapeutic doses in participating hospitals may contribute to suboptimal clinical outcomes and an increased predominance of resistant pathogenic fungi. A global consensus on antifungal dosing and coordinated stewardship programs are needed to promote the consistent and appropriate use of antifungal drugs in neonates and children.
INTRODUCTION
Antimicrobial agents are among the most commonly prescribed drugs in neonates and children. The widespread use of broad-spectrum antimicrobials is known to contribute to antimicrobial resistance, while the failure to initiate appropriate treatment is associated with significantly increased attributable mortality (1). The appropriate use of antifungals is of particular importance in the prevention and treatment of infection in the presence of severe intercurrent illness, prematurity, and immunosuppression. Invasive fungal infections (IFI) continue to be associated with an unacceptably high mortality rate in these vulnerable populations. In low- and extremely low-birthweight neonates, IFI is associated with an attributable mortality rate of 30 to 40% (2). In the setting of hematopoietic stem cell transplantation (HSCT), the attributable mortality rates from IFI are 30 to 40% for yeast infections and up to 70% for mold infections (3, 4).
The surveillance of antimicrobial use in hospitals is an important means of observing prescribing trends, linking results with antimicrobial resistance patterns, and identifying areas for improvement in safe and effective prescribing. Cross-sectional point prevalence surveys (PPS) have provided informative data on the patterns of antimicrobial prescribing in adults and, more recently, in children (5, 6). To our knowledge, PPS methodologies have until now not been used to describe antifungal use in children and neonates. Here, we present data on antifungal prescribing from the Antibiotic Resistance and Prescribing in European Children (ARPEC) study, a multicenter global observational study investigating the current variation in antimicrobial prescription practices in hospitals.
MATERIALS AND METHODS
A single-day point prevalence study (PPS) of antimicrobial use was carried out in 226 centers. The details of the ARPEC study design have been outlined elsewhere (5). Briefly, the ARPEC study group was a collaborative partnership between members of the European Surveillance of Antimicrobial Consumption, European Society of Pediatric Infectious Diseases, and Global Research in Pediatrics networks. Hospital-based physicians caring for neonates or children within these networks were invited to participate. The departments within the participating centers recorded data between October and December 2012. All inpatients <18 years of age present at 8 a.m. on the day of survey were included in the denominator. Data were recorded for patients who were prescribed antimicrobial agents on the day of the survey. Neonates and children receiving antifungal agents known to have negligible bioavailability administered via the oral route (amphotericin B, nystatin, and miconazole) were excluded from analysis. The patient exclusion criteria included emergency admission on the day of study, patients on psychiatric wards, and patients <18 years old admitted to an adult ward.
Anonymized patient data were collected through a study-specific online portal using a standardized data entry protocol. The project focused on European centers. In addition, centers outside Europe that collected and submitted data according to the same methodology during the study periods were included in the analysis. The data collected for all patients receiving antimicrobials included age, gender, current weight and birthweight, ventilation status, and the prescribed antimicrobial agents, single unit dose, number of doses per 24 h, route of administration, and drug indication (therapeutic or prophylaxis). To facilitate data collection on the underlying diagnosis and reason for treatment, a predefined list of grouped underlying conditions, acute diagnoses, and anatomical site of infection was used and is described elsewhere (5). The individual center and department type were recorded categorically. Neonates were defined based on postmenstrual age at the time of participation. The classification of the level of neonatal care was defined locally, according to previously described predetermined categories (7). Where dose frequencies were less than daily and an antifungal agent was consequently prescribed but not administered on the day of study, patients were included and doses decimalized to account for the frequency of administration.
Descriptive statistical analysis was carried out using Stata 10 and R (version 2.15.3) (8, 9). An analysis of antifungal dosing was performed using the total daily dose in 24 h (TDD). The TDDs were analyzed per unit of current weight or estimated surface area (kg or m2), according to the current guidance for each drug (Table 1). The following Anatomical Therapeutic Chemical classes (version 2011) were analyzed: antimycotics (ATC J02), antifungals for systemic use (ATC D01B), and intestinal anti-infectives (ATC A07) (10). The dosing regimens were analyzed by nation and macrogeographical regions using the United Nations geoscheme (11).
TABLE 1.
Currently published systemic antifungal dosing recommendations for neonates and children
| Drug (dose units)a and patient category | Summary of product characteristics | Manual of childhood infections: the blue book, 3rd ed (12) | Red book 2012: 2012 report of the Committee on Infectious Diseases (13) |
|---|---|---|---|
| Amphotericin B lipid complex (mg/kg) | |||
| Neonatal | No recommendation | No recommendation | No recommendation |
| Pediatric | 5 daily | 5 daily | 5 daily |
| Liposomal amphotericin B (mg/kg) | |||
| Neonatal | No recommendation | 3–5 daily | No recommendation |
| Pediatric | 1–3 daily | 3–5 daily | 3–5 daily |
| Amphotericin B deoxycholate (mg/kg) | |||
| Neonatal | No recommendation | 1 daily | No recommendation |
| Pediatric | 1 daily | 1–1.5 daily | 1–1.5 daily |
| Caspofungin (mg/m2) | |||
| Neonatal | 25 daily | 25 daily | No recommendation |
| Pediatric | 50 daily | 1–3 mo, 25 daily; 3–12 mo, 50 daily; 1–18 yr, 70 daily | 70 daily |
| Fluconazole (mg/kg) | |||
| Neonatal | 0–14 days, 3–12 every 72 h; 15–17 days, 3–12 every 48 h | 12 daily | No recommendation |
| Pediatric | Treatment, 6–12 daily; prophylaxis, 3–12 daily | 12 daily (max, 400 mg daily)b | 6–12 daily |
| Itraconazole (mg/kg) | |||
| Neonatal | No recommendation | No recommendation | No recommendation |
| Pediatric | 5 daily | Intravenous, 2.5 every 12 h for first 2 doses and then 2.5 daily; oral, 3–5 daily (max, 200 mg daily) | 5–10 daily |
| Micafungin (mg/kg) | |||
| Neonatal | Treatment, 2–4 daily; prophylaxis, 1 daily | Treatment, 8 daily; prophylaxis, 1 daily | No recommendation |
| Pediatric (mg/kg) | Body wt ≤40 kg: treatment, 2–4 daily; prophylaxis, 1 daily; body wt >40 kg: treatment, 100–200 mg daily; prophylaxis, 50 mg daily | Treatment, 2–8 daily (max, 100 mg daily); prophylaxis, 1 daily (max, 50 mg daily) | 4–12 daily |
| Posaconazole | |||
| Neonatal | No recommendation | No recommendation | No recommendation |
| Pediatric | No recommendation | >12 yr, 800 mg per day | No recommendation |
| Voriconazole (mg/kg) | |||
| Neonatal | No recommendation | No recommendation | No recommendation |
| Pediatric | Intravenous load, 9 every 12 h for first 2 doses and then 8 every 12 h; oral, 9 every 12 h (max, 700 mg daily) | Intravenous: 2–12 yr, 4–7 every 12 h; 2–12 yr, 4–7 every 12 h; 12–18 yr, 6 every 12 h for first 2 doses and then 3–4 mg every 12 h; oral: 10 daily (max, 400 mg) every 12 h for first 2 doses and then 7 every 12 h (max, 200 mg daily) | Intravenous: 2–12 yr, 9 every 12 h (max, 700 mg daily); 12–18 yr, 6 every 12 h for first 2 doses and then 4 every 12 h; oral: 9 every 12 h |
The doses given for each drug are in the units in parentheses, unless otherwise specified.
max, maximum.
The mean and variance of the TDDs for each antifungal agent were compared to currently recommended regimens. The recommended daily doses (RDD) were defined using collated guidance from the summary of product characteristics (SPC), the European Society of Pediatric Infectious Disease (ESPID), the Manual of childhood infections: the blue book, 3rd ed. (12), and Red book 2012: 2012 report of the Committee on Infectious Diseases (13). Where specific dosing recommendations for pediatric or neonatal populations could be derived from the respective SPC, these formed the basis of the total daily dose recommendations. The dosing recommendations are summarized in Table 1.
The upper and lower limits for recommended TDD were identified from the range produced from the collated dosing recommendations and used to define the maximum recommended daily doses (MaxRDD) and minimum recommended daily doses (MinRDD). Dosing errors were defined as prescription of medication at a dose meeting one of the following criteria: (i) TDD prescribed at ≥110% of the MaxRDD, or (ii) TDD prescribed at ≤90% of the MinRDD. For example, the MinRDD and MaxRDD for voriconazole in children age 2 to 12 years were determined as follows: the highest current recommended dosing regimen (excluding loading doses) was 8 mg/kg of body weight every 12 h, and the lowest dosing regimen was 4 mg/kg every 12 h (see Table 1) during the study period (14–22). The MaxRDD and MinRDD were therefore calculated to be 16 mg/kg and 8 mg/kg daily, respectively, and the supra- and subtherapeutic doses were calculated to be ≥17.6 mg/kg and ≤7.2 mg/kg, respectively. The doses were defined according to age categories and route of administration (where specified in the guidelines). A full description of this methodology is described in detail elsewhere (23). Doses exceeding values 10-fold above the MaxRDD or below the MinRDD were considered isolated prescribing or data entry errors and were excluded from analysis.
In accordance with European regulations, the anonymized data obtained during this observational study were gathered without additional therapy, monitoring procedures, or a change from existing clinical practices. Submission to the institutional review boards of participating centers was at the discretion of individual lead investigators.
RESULTS
Patient demographics.
Data were recorded from 17,693 neonatal and pediatric inpatients from 226 centers. The participating centers were from 19 European countries and 17 countries outside Europe (see Table 2). A total of 1,345 inpatients from 136 centers were prescribed at least one oral or parenteral antifungal. This included 203 neonates and 379 children receiving antifungal agents known to have negligible bioavailability administered via the oral route (amphotericin B, nystatin, and miconazole) and were therefore excluded. The data from five patients were excluded due to errors in data entry. In total, 885 patients were evaluable (Fig. 1), with 174 neonates and 711 children (1 month to 18 years old). The median ages of the neonates and children in the study were 13 days (interquartile range [IQR], 7 to 19 days) and 6.5 years (IQR, 1 to 13 years), respectively.
TABLE 2.
Number of children and neonates from participating centers by country
| Location | No. of centers (n = 136) | No. of pediatric patients (n = 711) | No. of neonatal patients (n = 174) |
|---|---|---|---|
| Western Europe | |||
| Belgium | 6 | 27 | 1 |
| France | 6 | 75 | 19 |
| Germany | 15 | 96 | 16 |
| Netherlands | 1 | 10 | |
| Switzerland | 1 | 19 | |
| Northern Europe | |||
| Estonia | 1 | 7 | 4 |
| Latvia | 2 | 14 | 1 |
| Lithuania | 1 | 1 | |
| United Kingdom | 37 | 94 | 33 |
| Eastern Europe | |||
| Hungary | 1 | 8 | |
| Romania | 1 | 9 | |
| Southern Europe | |||
| Croatia | 2 | 9 | |
| Greece | 5 | 35 | 9 |
| Italy | 6 | 67 | 17 |
| Malta | 1 | 2 | |
| Portugal | 3 | 12 | 1 |
| Slovenia | 1 | 8 | |
| Spain | 6 | 42 | 16 |
| Asia | |||
| Bahrain | 1 | 3 | 1 |
| India | 7 | 29 | 21 |
| Iran | 3 | 15 | 1 |
| Kuwait | 2 | 3 | 3 |
| Saudi Arabia | 3 | 16 | 6 |
| Oman | 1 | 6 | |
| Oceania | |||
| Australia | 6 | 53 | 2 |
| Africa | |||
| Gambia | 2 | 1 | |
| Ghana | 1 | 1 | |
| Malawi | 2 | 3 | |
| South Africa | 1 | 3 | 7 |
| Latin America | |||
| Argentina | 1 | 21 | 2 |
| Colombia | 3 | 3 | 2 |
| Mexico | 1 | 25 | 3 |
| North America | |||
| USA | 3 | 64 | 4 |
FIG 1.
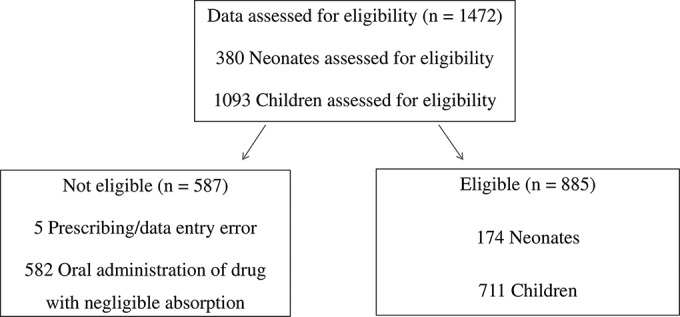
Study flowchart and reasons for patient data eligibility/exclusion.
Antifungal use.
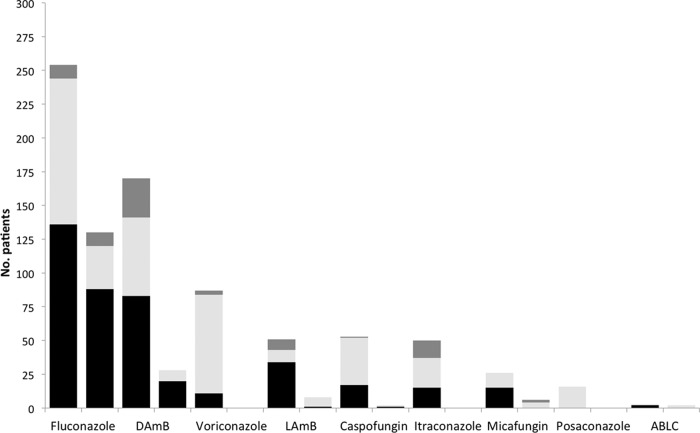
The most commonly prescribed agent for neonates and children during the study was fluconazole, accounting for 355 (40%) prescriptions (see Fig. 2). Second-generation triazoles were less commonly prescribed (voriconazole, n = 87 [10%]; posaconazole, n = 14 [2%]). Fifty children (6%) received oral itraconazole. Amphotericin B deoxycholate was the second most frequently prescribed drug. Of 262 patients prescribed amphotericin B, 197 (75%) were prescribed amphotericin B deoxycholate (DAmB). The majority of the DAmB prescriptions (n = 150 [76%]) were from European centers. Lipid amphotericin B preparations (liposomal, lipid complex, and colloid dispersion) were prescribed less frequently than was conventional amphotericin B (n = 65 [8%]), of which 51 prescriptions (78%) were within European centers. The use of echinocandins was less common than that of amphotericin B formulations and azoles (caspofungin, n = 55 [6%]; micafungin, n = 32 [4%]). One child received parenteral flucytosine for the treatment of a catheter-related bloodstream infection. Forty-two patients received combination antifungal therapy. The most commonly prescribed drug combinations used were amphotericin B-fluconazole (n = 7), amphotericin B-caspofungin (n = 6), and caspofungin-voriconazole (n = 6).
FIG 2.
Numbers of patients receiving individual antifungal agents. For each drug, the left bar is pediatric patients and the right bar is neonates. Supratherapeutic (TDD, ≥110% of published MaxRDD), therapeutic, and subtherapeutic (TDD, ≤90% of published MinRDD) doses are represented in dark gray, light gray, and black, respectively. DAmB, amphotericin B deoxycholate; LAmB, liposomal amphotericin B; ABLC, amphotericin B lipid complex.
Indication.
Systemic antifungal treatment was reported in 174 neonates. Extremely low-birthweight neonates accounted for the majority of antifungal prescriptions (n = 103 [60%]). The most common indications for systemic antifungal treatment in neonates were medical prophylaxis in 80 cases (46%) and treatment of suspected IFI in 77 cases (44%). The treatment of localized infection was uncommonly reported (cardiac infection, n = 2; central nervous system [CNS] infection, n = 4; genitourinary tract infection, n = 3; lower respiratory tract infection, n = 2; other/infective source unknown, n = 4). An ANOVA demonstrated no significant effect of clinical indication (prophylaxis or treatment of systemic or localized infection) on TDD (F = 1.1, P = 0.342).
The most common indication for antifungal administration in children was medical prophylaxis (n = 325 [46%]), followed by empirical treatment of febrile neutropenia (n = 122 [17%]) and treatment of confirmed or suspected IFI (n = 100 [14%]). Antifungal treatment of a localized infection was less commonly reported (respiratory tract, n = 41; skin and soft tissue, n = 15; urinary tract, n = 14; gastrointestinal, n = 12; CNS, n = 4; joint and bone, n = 4; cardiac, n = 4). In 70 cases, the indication for treatment was not known or recorded.
Route of administration.
Systemic antifungals were administered via the oral route in 58% and the parenteral route in 42% of children. The majority of prescriptions in neonates were administered via the parenteral route (n = 154 [89%]). Twenty neonates received oral fluconazole. In children, oral administration accounted for 108/254 (43%) of the fluconazole prescriptions and 50/87 (57%) of the voriconazole prescriptions. The mean TDD for children receiving fluconazole via the oral route was significantly lower (X̄ = 1.8 mg−1 kg−1 day; standard deviation [SD], 8.3) than that via the intravenous route (X̄ = 3.5 mg−1 kg−1 day; SD, 6.2; P = <0.001). Similarly, the mean TDD for children receiving oral voriconazole was lower (X̄ = 6.1 mg−1 kg−1 day; SD, 4.9) than that for intravenous voriconazole (X̄ = 7.3 mg−1 kg−1 day; SD, 5.8; P = 0.257).
Dosing.
The proportions of patients receiving doses outside the MinRDD and MaxRDD for each antifungal agent in neonates and children are shown in Fig. 2. Overall, fewer than one-half of the patients (n = 371 [42%]) received a TDD within the dosing range recommended in current guidelines. Subtherapeutic doses (<90% of the MinRDD) were prescribed in 416 cases (47%). The most commonly prescribed drug, fluconazole, was prescribed at subtherapeutic doses in 242/384 (63%) cases (Fig. 3). Amphotericin B deoxycholate was prescribed at a subtherapeutic TDD in 83/200 (42%) cases. Two neonates received amphotericin B lipid complex at a dose of 5 mg−1 kg−1 day. Recently, the efficacy and population pharmacokinetics of amphotericin B lipid complex (ABLC) have been described in neonates, and a dose of 2.5 to 5 mg−1 kg−1 day was incorporated into European guidelines for the treatment of invasive candidiasis (24).
FIG 3.
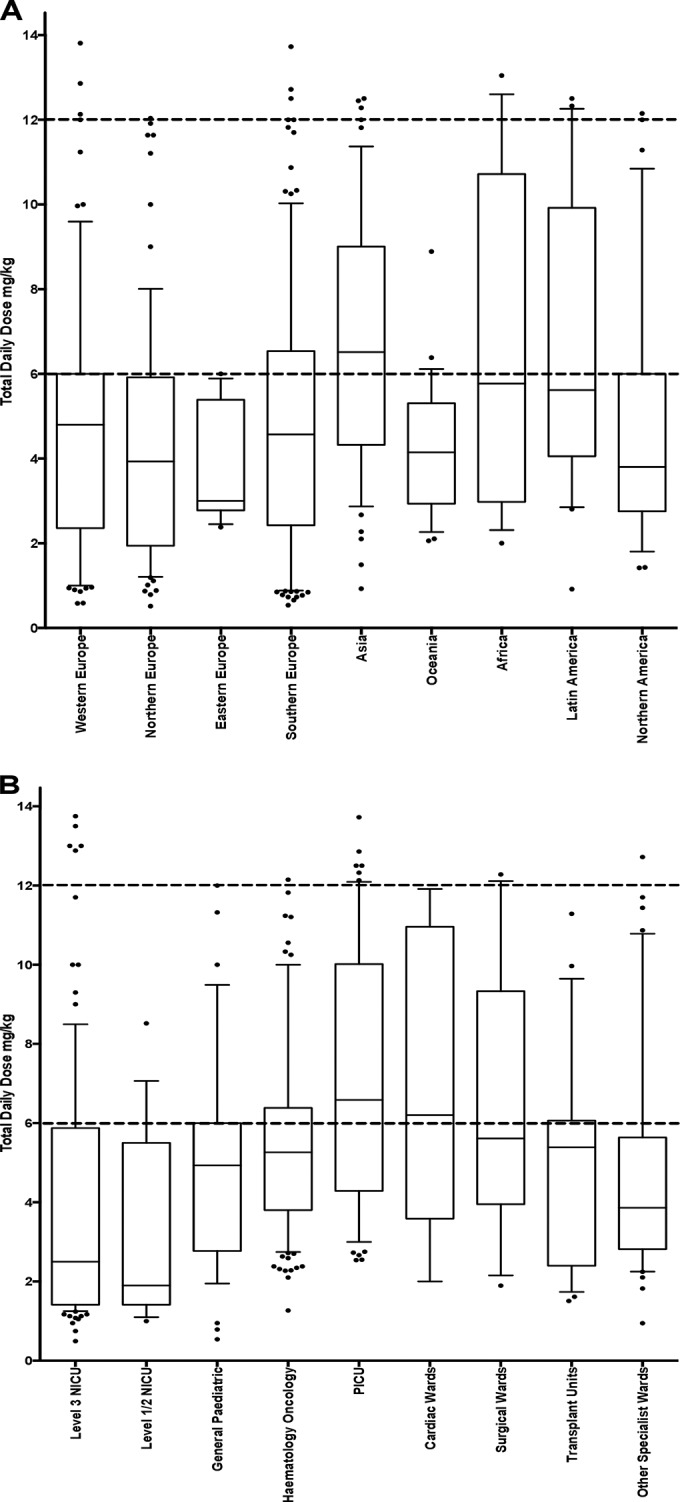
Total daily dosing of fluconazole prescribed as treatment for invasive fungal infection in pediatric patients by macrogeographical regions (A) and center type (B). Interquartile range (IQR), median, 1.5× the IQR, and outlying data points are represented by boxes, central lines, whiskers, and open circles, respectively. The minimum and maximum recommended daily doses are indicated by the dashed lines.
Center and department categories.
The majority of neonatal antifungal prescriptions occurred in tertiary (level III) neonatal units (n = 146 [84%]). For the pediatric population, antifungals were prescribed most frequently in hematology-oncology wards (n = 352 [50%]) and pediatric intensive care units (n = 121 [17%]). Less frequently, antifungal prescriptions were recorded in surgical transplant units (n = 75), general pediatric wards (n = 73), surgical wards (n = 32), cardiac wards (n = 16), and other specialist pediatric wards (n = 42).
The TDDs for children receiving therapeutic dosing regimens of fluconazole varied substantially between centers and by department type; these are shown in Fig. 3. The mean TDDs were significantly lower in general pediatric wards (n = 18; X̄ = 3.9 mg−1 kg−1 day; SD, 4.9), hematology-oncology units (n = 50; X̄ = 3.6 mg−1 kg−1 day; SD, 5.4), and surgical transplant units (n = 12; X̄ = 6.1 mg−1 kg−1 day; SD, 3.7) than that in the pediatric intensive care unit (PICU) (n = 28; X̄ = 7.1 mg−1 kg−1 day; SD, 3.3), cardiac wards (n = 5; X̄ = 6.1 mg−1 kg−1 day; SD, 7.9), and surgical units (n = 15; X̄ = 6.3 mg−1 kg−1 day; SD, 7.5; P < 0.001) (Fig. 3). The mean therapeutic fluconazole TDDs were low in both level III (n = 67; X̄ = 2.2 mg−1 kg−1 day; SD, 6.3) and level I/II units (n = 6; X̄ = 3.5 mg−1 kg−1 day; SD, 3.9).
Amphotericin B was most frequently prescribed on hematology-oncology units and in the PICU. Overall, amphotericin B deoxycholate (DAmB) was prescribed more frequently than lipid formulations of amphotericin B (liposomal amphotericin B [LAmB]), accounting for 200 and 62 prescriptions, respectively. The mean TDDs for therapeutic amphotericin B were significantly lower in general pediatric wards (n = 14; X̄ = 0.6 mg−1 kg−1 day; SD, 2.0), hematology-oncology units (n = 78; X̄ = 0.7 mg−1 kg−1 day; SD, 2.8), and level I/II neonatal units (for DAmB, n = 10; X̄ = 0.4 mg−1 kg−1 day; SD, 3.2) than those in the PICU (for DAmB, n = 27; X̄ = 1.3 mg−1 kg−1 day; SD, 4.1), level III neonatal units (for DAmB, n = 20; X̄ = 1.2 mg−1 kg−1 day; SD, 0.8), surgical transplant units (for DAmB, n = 14; X̄ = 1.1 mg−1 kg−1 day; SD, 0.7), and other specialty medical wards (for DAmB, n = 11; X̄ = 1.2 mg−1 kg−1 day; SD, 2.0; P < 0.001).
DISCUSSION
This prospective observational study has provided a detailed description of the current prescribing practices of systemic antifungals for hospitalized neonates and children. Significant underdosing within the participating centers was identified, with fewer than one-half of the recorded prescriptions delivering a daily dose within the ranges recommended in current international guidelines and SPCs. The lowest mean daily therapeutic doses were reported in general pediatric and hematology-oncology units. The dosing varied across countries and regions, but no specific relationship was found between geographical distribution and the proportion of patients receiving subtherapeutic doses. A similar striking variability in dosing has been identified in the treatment of invasive fungal infections in pediatric cancer patients (25). Recent observational studies have also identified that suboptimal dosing of antibacterial and antiviral drugs in children is similarly prevalent. Saxena et al. (26), reporting prescribing surveillance data from the United Kingdom, identified that children and adolescents prescribed oral penicillin received doses below the national recommendations in 40% and 70% of cases, respectively. Menson et al. (27) described the widespread suboptimal dosing of antiretroviral drugs in children and identified several potential causes of error, including the inadequacy of pharmacokinetic-pharmacodynamic (PK-PD) data. Evidence-based dosing recommendations for the older and more commonly prescribed antifungal agents in this study are extremely limited for neonates and children. Optimal doses have never been specifically defined for fluconazole and amphotericin B deoxycholate in pediatric populations, despite their widespread use. Many of the more recently introduced antifungal agents, such as echinocandins and second-generation triazoles, have been specifically studied and differentially licensed for use in neonates and children (28–31). Well-designed prospective PK-PD studies in clinical settings, in conjunction with modeling and simulation based on preclinical data, are the best tools for establishing equivalent evidence-based optimal dosing regimens for these older agents (32).
As in previous observational studies, systemic antifungals were most frequently prescribed for the prevention and treatment of IFI in immunosuppressed children and preterm neonates (33–35). Indication-specific dosing was recently described for fluconazole use in neonates. In the treatment of suspected or confirmed IFI in neonates, a minimum daily dose of fluconazole of 12 mg−1 kg−1 day in the first 90 days of life results in comparable exposure to adults receiving 800 mg daily and achieves an area under the concentration-time curve (AUC)/MIC ratio of ≥50 (36). This study observed a large proportion of patients receiving prophylactic treatment and no significant differences between the mean daily doses of fluconazole prescribed for prophylaxis versus therapeutic use. This suggests a lack of awareness of indication-specific dosing in clinical practice and may reflect the difficulties associated with diagnosing IFI in children.
In conjunction with an improved evidence base to underpin antifungal dosing, a global consensus between key organizations issuing dosing recommendations (including those representing specialized high-risk populations) is needed for the treatment and prevention of IFI in pediatrics. An excellent example of such a consensus is the unification of pediatric antiretroviral guidelines published by the WHO, CDC, and Pediatric European Network for Treatment of AIDS (PENTA), which have resulted in consolidated international guidelines for the treatment of HIV (37, 38). The harmonization of existing international pediatric antifungal guidelines (for example, the European Conference on Infections in Leukaemia [ECIL] and the European Society of Clinical Microbiology and Infectious Diseases [ESCMID]) should similarly aim to select pragmatic dosing and monitoring schedules while accounting for differences in PK across children and neonates of different sizes and developmental stages (25, 39).
Antimicrobial stewardship programs (ASP) have been shown to increase appropriate antimicrobial prescribing practices, improve individual patient outcomes, and reduce health care costs (40). Recently, ASPs incorporating antifungal stewardship have been described in adults. These programs have identified key components of antifungal stewardship, which include (i) the utilization of appropriate antifungal drugs, including consideration and monitoring of local resistance patterns, (ii) appropriate antifungal doses based on published guidelines, with considerations of patient-specific PK-PD, (iii) clinical considerations, including the removal of intravenous catheters, adequate diagnostics (serial blood culture, antigen testing, and imaging techniques), and the performance of examinations to investigate disseminated disease, and (iv) a clear distinction between prophylactic and therapeutic antifungal use, with appropriate use and duration of therapy based on explicit clinical criteria in these settings (41–44).
Antibacterial ASPs in pediatric centers worldwide have recently been reported (45–47). However, as in the adult population, the relative infrequency of antifungal use compared with the use of antibacterial drugs has led to the development of antifungal stewardship being less forthcoming. The integration of antifungal stewardship within existing ASPs that currently focus predominantly on antibacterial use seems to be a pragmatic way forward to stimulate and improve appropriate antifungal prescribing practices.
PPS studies provide a large volume of information from a wide range of clinical settings at one time, and the participation rates in this study were excellent compared with those in equivalent studies. A great strength of such a PPS study is the opportunity to collect data on dosing and specific indications without relying on subjective questionnaire-based studies. The opportunistic sampling technique does, however, mean that the prescribing practices reported in this study may not be representative of centers and/or countries that did not take part. Additionally, dosing episodes for rare indications or of antifungal agents that are infrequently prescribed may be omitted on the day of study. Further systematic observational studies should aim to increase the number of participating centers in a variety of global settings (including high- and low-income countries) in order to further describe geographical variations, center-specific outcomes, and patient-related trends in antimicrobial use. A significant proportion of centers reported therapeutic antifungal use. Further data about infective organism and resistance patterns will yield important information to guide antifungal stewardship programs. Furthermore, this study did not gather qualitative information, such as clinician rationale (for example, a consideration of renal and/or hepatic impairment) and team member involvement in clinical decisions regarding drug and dose selection. Repeated and/or longitudinal surveys within participating institutions may gather such data and identify prescribing trends over time.
Overall, variability in antifungal prescriptions and widespread systematic suboptimal prescription of antifungals appears to be significant problems in neonates and children. Well-designed clinical studies in conjunction with PK modeling and simulation to inform guidelines, as well as the incorporation of antifungal stewardship into current and future ASPs, are urgently needed to improve prescribing practices and clinical outcomes.
ACKNOWLEDGMENTS
We thank the ARPEC Project Group members for their assistance in the collection, management, and analysis of clinical data.
This study was funded by the European Commission Health and Consumer Protection Directorate General (DG SANCO) through the Executive Agency for Health and Consumers (EAHC).
J.M.L. has received grant support from Gilead. E.R. has received research grant support from Pfizer, Gilead, Enzon, Schering, and Wyeth, has served as a consultant to Schering, Gilead, Astellas Gilead, Cephalon, and Pfizer, and has been in the speakers' bureaus of Wyeth, Schering, Merck, and Astellas. A.W. has received unrestricted research grants from Pfizer and Gilead. H.G. has received research grants from bioMérieux.
REFERENCES
- 1.Shane AL, Stoll BJ. 2014. Neonatal sepsis: progress towards improved outcomes. J Infect 68(Suppl 1):S24–S32. doi: 10.1016/j.jinf.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Zaoutis TE, Heydon K, Localio R, Walsh TJ, Feudtner C. 2007. Outcomes attributable to neonatal candidiasis. Clin Infect Dis 44:1187–1193. doi: 10.1086/513196. [DOI] [PubMed] [Google Scholar]
- 3.Brissaud O, Guichoux J, Harambat J, Tandonnet O, Zaoutis T. 2012. Invasive fungal disease in PICU: epidemiology and risk factors. Ann Intensive Care 2:6. doi: 10.1186/2110-5820-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 5.Versporten A, Sharland M, Bielicki J, Drapier N, Vankerckhoven V, Goossens H, ARPEC Project Group Members. 2013. The antibiotic resistance and prescribing in European Children project: a neonatal and pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr Infect Dis J 32:e242–e253. doi: 10.1097/INF.0b013e318286c612. [DOI] [PubMed] [Google Scholar]
- 6.Zarb P, Amadeo B, Muller A, Drapier N, Vankerckhoven V, Davey P, Goossens H, ESAC-3 Hospital Care Subproject Group. 2011. Identification of targets for quality improvement in antimicrobial prescribing: the Web-based ESAC Point Prevalence Survey 2009. J Antimicrob Chemother 66:443–449. doi: 10.1093/jac/dkq430. [DOI] [PubMed] [Google Scholar]
- 7.BAPM. 2001. British Association of Perinatal Medicine standards for hospitals providing neonatal intensive and high dependency care (second edition). British Association of Perinatal Medicine, London, United Kingdom: http://www.bapm.org/publications/documents/guidelines/hosp_standards.pdf. [Google Scholar]
- 8.R Development Core Team. 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 9.Stata Corp. 2007. Stata statistical software: release 10. StataCorp, College Station, TX. [Google Scholar]
- 10.WHO. 2013. Anatomical Therapeutic Chemical (ATC) classification system: guidelines for ATC classification and DDD assignment. WHO Collaborating Centre for Drug Statistics Methodology, Oslo, Norway. [Google Scholar]
- 11.United Nations. 2011. Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. United Nations, New York, NY: http://unstats.un.org/unsd/methods/m49/m49regin.htm. [Google Scholar]
- 12.Sharland M, Cant A, Davies EG, Elliman DAC, Esposito S, Finn A, Gray J, Heath PT, Lyall H, Pollard AJ, Ramsay ME, Riordan A, Shingadia D (ed). 2011. Manual of childhood infections: the blue book, 3rd ed. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 13.Pickering LK, Baker, Kimberlin DW, Long SS (ed). 2012. Red book 2012: 2012 report of the Committee on Infectious Diseases. American Academy of Pediatrics, Elk Grove Village, IL. [Google Scholar]
- 14.Electronic Medicines Compendium. 2013. Fluconazole 50mg capsules: summary of product characteristics. Electronic Medicines Compendium, Surrey, United Kingdom: http://www.medicines.org.uk/emc/medicine/25882. [Google Scholar]
- 15.EMA. 2014. (Voriconazole) Annex I: summary of product characteristics. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002669/WC500144015.pdf. [Google Scholar]
- 16.EMA. 2014. (Caspofungin) Annex I: summary of product characteristics. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000379/WC500021033.pdf. [Google Scholar]
- 17.MHRA. 2013. (Itraconazole) Summary of product characteristics. Medicines and Healthcare Products Regulatory Agency, London, United Kingdom: http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1411711063531.pdf. [Google Scholar]
- 18.EMA. 2013. (Posaconazole) Annex I: summary of product characteristics. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000610/WC500037784.pdf. [Google Scholar]
- 19.MHRA. 2014. (Fungizone) Summary of product characteristics. Medicines and Healthcare Products Regulatory Agency, London, United Kingdom: http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1404458209656.pdf. [Google Scholar]
- 20.MHRA. 2014. (AmBisome) Summary of product characteristics. Medicines and Healthcare Products Regulatory Agency, London, United Kingdom: http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1410502129659.pdf. [Google Scholar]
- 21.MHRA. 2014. (Abelcet) Summary of product characteristics. Medicines and Healthcare Products Regulatory Agency, London, United Kingdom. [Google Scholar]
- 22.EMA. 2014. (Mycamine) Annex I: summary of product characteristics. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000734/WC500031075.pdf. [Google Scholar]
- 23.McPhillips HA, Stille CJ, Smith D, Hecht J, Pearson J, Stull J, Debellis K, Andrade S, Miller M, Kaushal R, Gurwitz J, Davis RL. 2005. Potential medication dosing errors in outpatient pediatrics. J Pediatr 147:761–767. doi: 10.1016/j.jpeds.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 24.Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Cornely OA, Cuenca-Estrella M, Donnelly JP, Garbino J, Herbrecht R, Jensen HE, Kullberg BJ, Lass-Flörl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect 18(Suppl 7):S38–S52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 25.Groll AH, Castagnola E, Cesaro S, Dalle JH, Engelhard D, Hope W, Roilides E, Styczynski J, Warris A, Lehrnbecher T, Fourth European Conference on Infections in Leukaemia, Infectious Diseases Working Party of the European Group for Blood Marrow Transplantation (EMBT-IDWP), Infectious Diseases Group of the European Organisation for Research and Treatment of Cancer (EORTC-IDG), International Immunocompromised Host Society (ICHS), European Leukaemia Net (ELN) 2014. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol 15:e327–e340. doi: 10.1016/S1470-2045(14)70017-8. [DOI] [PubMed] [Google Scholar]
- 26.Saxena S, Ismael Z, Murray ML, Barker C, Wong IC, Sharland M, Long PF. 2014. Oral penicillin prescribing for children in the UK: a comparison with BNF for children age-band recommendations. Br J Gen Pract 64:e217–e222. doi: 10.3399/bjgp14X677842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menson EN, Walker AS, Sharland M, Wells C, Tudor-Williams G, Riordan FA, Lyall EGH, Gibb DM, Collaborative HIV Paediatric Study Steering Committee. 2006. Underdosing of antiretrovirals in UK and Irish children with HIV as an example of problems in prescribing medicines to children, 1997–2005: cohort study. BMJ 332:1183–1187. doi: 10.1136/bmj.332.7551.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamin DK Jr, Driscoll T, Seibel NL, Gonzalez CE, Roden MM, Kilaru R, Clark K, Dowell JA, Schranz J, Walsh TJ. 2006. Safety and pharmacokinetics of intravenous anidulafungin in children with neutropenia at high risk for invasive fungal infections. Antimicrob Agents Chemother 50:632–638. doi: 10.1128/AAC.50.2.632-638.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hope WW, Smith PB, Arrieta A, Buell DN, Roy M, Kaibara A, Walsh TJ, Cohen-Wolkowiez M, Benjamin DK Jr. 2010. Population pharmacokinetics of micafungin in neonates and young infants. Antimicrob Agents Chemother 54:2633–2637. doi: 10.1128/AAC.01679-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsson MO, Lutsar I, Milligan PA. 2009. Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrob Agents Chemother 53:935–944. doi: 10.1128/AAC.00751-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh TJ, Adamson PC, Seibel NL, Flynn PM, Neely MN, Schwartz C, Shad A, Kaplan SL, Roden MM, Stone JA, Miller A, Bradshaw SK, Li SX, Sable CA, Kartsonis NA. 2005. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob Agents Chemother 49:4536–4545. doi: 10.1128/AAC.49.11.4536-4545.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker CI, Germovsek E, Hoare RL, Lestner JM, Lewis J, Standing JF. 2014. Pharmacokinetic/pharmacodynamic modelling approaches in paediatric infectious diseases and immunology. Adv Drug Deliv Rev 73:127–139. doi: 10.1016/j.addr.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castagnola E, Cesaro S, Giacchino M, Livadiotti S, Tucci F, Zanazzo G, Caselli D, Caviglia I, Parodi S, Rondelli R, Cornelli PE, Mura R, Santoro N, Russo G, De Santis R, Buffardi S, Viscoli C, Haupt R, Rossi MR. 2006. Fungal infections in children with cancer: a prospective, multicenter surveillance study. Pediatr Infect Dis J 25:634–639. doi: 10.1097/01.inf.0000220256.69385.2e. [DOI] [PubMed] [Google Scholar]
- 34.Hale KA, Shaw PJ, Dalla-Pozza L, MacIntyre CR, Isaacs D, Sorrell TC. 2010. Epidemiology of paediatric invasive fungal infections and a case-control study of risk factors in acute leukaemia or post stem cell transplant. Br J Haematol 149:263–272. doi: 10.1111/j.1365-2141.2009.08072.x. [DOI] [PubMed] [Google Scholar]
- 35.Steinbach WJ, Roilides E, Berman D, Hoffman JA, Groll AH, Bin-Hussain I, Palazzi DL, Castagnola E, Halasa N, Velegraki A, Dvorak CC, Charkabarti A, Sung L, Danziger-Isakov L, Lachenauer C, Arrieta A, Knapp K, Abzug MJ, Ziebold C, Lehrnbecher T, Klingspor L, Warris A, Leckerman K, Martling T, Walsh TJ, Benjamin DK Jr, Zaoutis TE, International Pediatric Fungal Network. 2012. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr Infect Dis J 31:1252–1257. doi: 10.1097/INF.0b013e3182737427. [DOI] [PubMed] [Google Scholar]
- 36.Wade KC, Benjamin DK Jr, Kaufman DA, Ward RM, Smith PB, Jayaraman B, Adamson PC, Gastonguay MR, Barrett JS. 2009. Fluconazole dosing for the prevention or treatment of invasive candidiasis in young infants. Pediatr Infect Dis J 28:717–723. doi: 10.1097/INF.0b013e31819f1f50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Department of Health and Human Services. 2000. AIDSinfo: guidelines for the use of antiretroviral agents in pediatric HIV infection. U.S. Department of Health and Human Services, Washington, DC: http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf [Google Scholar]
- 38.World Health Organization. 2010. Antiretroviral therapy for HIV infection in infants and children: towards universal access. Recommendations for a public health approach, 2010 revision. HIV/AIDS Programme, World Health Organization, Geneva, Switzerland: http://apps.who.int/medicinedocs/documents/s18809en/s18809en.pdf. [PubMed] [Google Scholar]
- 39.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Flörl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESMID Fungal Infection Study Group. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):S19–S37. [DOI] [PubMed] [Google Scholar]
- 40.Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, Ramsay CR, Wiffen PJ, Wilcox M. 2013. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev (4):CD003543. doi: 10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- 41.Ruhnke M. 2014. Antifungal stewardship in invasive Candida infections. Clin Microbiol Infect 20(Suppl 6):S11–S18. doi: 10.1111/1469-0691.12622. [DOI] [PubMed] [Google Scholar]
- 42.Caliz B. 2014. Efficiency of an antifungal stewardship program (PROMULGA Project). Eur J Hosp Pharm 21:A210–A211. doi: 10.1136/ejhpharm-2013-000436.515. [DOI] [Google Scholar]
- 43.Mondain V, Lieutier F, Dumas S, Gaudart A, Fosse T, Roger PM, Bernard E, Farhad R, Pulcini C. 2013. An antibiotic stewardship program in a French teaching hospital. Med Mal Infect 43:17–21. doi: 10.1016/j.medmal.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Valerio M, Rodriguez-Gonzalez CG, Muñoz P, Caliz B, Sanjurjo M, Bouza E, COMIC Study Group (Collaborative Group on Mycoses). 2014. Evaluation of antifungal use in a tertiary care institution: antifungal stewardship urgently needed. J Antimicrob Chemother 69:1993–1999. doi: 10.1093/jac/dku053. [DOI] [PubMed] [Google Scholar]
- 45.Hyun DY, Hersh AL, Namtu K, Palazzi DL, Maples HD, Newland JG, Saiman L. 2013. Antimicrobial stewardship in pediatrics: how every pediatrician can be a steward. JAMA Pediatr 167:859–866. doi: 10.1001/jamapediatrics.2013.2241. [DOI] [PubMed] [Google Scholar]
- 46.Agwu AL, Lee CK, Jain SK, Murray KL, Topolski J, Miller RE, Townsend T, Lehmann CU. 2008. A World Wide Web-based antimicrobial stewardship program improves efficiency, communication, and user satisfaction and reduces cost in a tertiary care pediatric medical center. Clin Infect Dis 47:747–753. doi: 10.1086/591133. [DOI] [PubMed] [Google Scholar]
- 47.Aston J, Nusgen U. 2012. Antimicrobial stewardship in a tertiary paediatric hospital. Arch Dis Child 97:e2. doi: 10.1136/archdischild-2012-301728.4. [DOI] [Google Scholar]