Abstract
The aim of this study was to compare the prosthetic joint infection (PJI) rate after total joint arthroplasty in two consecutive periods of treatment with different antibiotic prophylaxes: cefuroxime versus cefuroxime plus teicoplanin. We retrospectively reviewed 1,896 patients who underwent total hip arthroplasty or total knee arthroplasty between March 2010 and February 2013. From March 2010 to August 2011, patients received 1.5 g of cefuroxime during induction of anesthesia and another 1.5 g 2 h later (the C group). From September 2011, 800 mg of teicoplanin was added to cefuroxime (the CT group). Throughout the period studied, there were no variations in pre- or postoperative protocols. Univariate and multivariate analyses were performed to evaluate independent predictors of PJI. There were 995 (55.7%) patients in the C group and 791 (44.3%) in the CT group. Patients in the CT group had a significantly lower PJI rate than patients in the C group (1.26% versus 3.51%, P = 0.002). There were no infections due to Staphylococcus aureus in the CT group (0% versus 1.6% in the C group, P < 0.001). A stepwise forward Cox regression model identified male sex (hazard ratio [HR], 3.85; 95% confidence interval [CI], 2.09 to 7.18), a body mass index of ≥35 kg/m2 (HR, 2.93; 95% CI, 1.37 to 6.27), the presence of lung disease (HR, 2.46; 95% CI, 1.17 to 5.15), and red blood cell transfusion (HR, 3.70; 95% CI, 1.89 to 7.23) to be independent variables associated with a higher risk of PJI. The addition of teicoplanin was associated with a lower risk of infection (HR, 0.35; 95% CI, 0.17 to 0.74). In conclusion, the addition of teicoplanin to cefuroxime during primary arthroplasty was associated with a significant reduction in the global PJI rate due to a reduction of infections caused by Gram-positive bacteria.
INTRODUCTION
Prosthetic joint infection (PJI) after total hip arthroplasty (THA) or total knee arthroplasty (TKA) is a devastating complication. According to a recent retrospective study performed in the United States from 2001 to 2009, the number of procedures for infected arthroplasty significantly increased over that interval (1). Several reasons could explain this finding, including the fact that candidates for surgery are progressively older and have more comorbidities or the increase in the rate of resistance to cephalosporins among common pathogens involved in orthopedic infections (2). The most recent guidelines for antimicrobial prophylaxis during surgery recommend the administration of cefazolin for total joint arthroplasty; however, the authors consider it to be logical to provide prophylaxis with an agent active against methicillin-resistant Staphylococcus aureus (MRSA) for any patient known to be colonized with this Gram-positive pathogen (3). A meta-analysis of clinical trials comparing beta-lactams to glycopeptides concluded that both regimens had similar levels of effectiveness for preventing surgical site infection (4–6), but the etiology of the infection varied. In the glycopeptide arms, infections due to MRSA were less frequent and were balanced by an increase in the incidence of infections due to methicillin-susceptible S. aureus (MSSA) (4, 7). In addition, prophylaxis with glycopeptides has been associated with a higher rate of infection due to Gram-negative bacilli (8).
In order to avoid the risks of switching the prophylaxis from a cephalosporin to a glycopeptide, some authors advocate for dual prophylaxis. In our institution in 2002, there was a progressive increase in the number of MRSA infections among patients who underwent surgery for femoral neck fracture. The addition of teicoplanin to cefuroxime was associated with a significant decrease in the global rate of infection, particularly infections due to MRSA but also infections due to other staphylococci (9). Recently, other authors have retrospectively reviewed their experience with dual prophylaxis (vancomycin plus cefazolin) versus prophylaxis with cefazolin alone in primary arthroplasty (10). Although the rate of MRSA infections was significantly reduced, the rate of MSSA infections increased and the global infection rate was not significantly different (1.1% for patients treated with vancomycin plus cefazolin versus 1.4% for patients treated with cefazolin alone).
In our institution, the standard antibiotic prophylaxis for primary arthroplasty consisted of 1.5 g of cefuroxime administered during the induction of anesthesia (30 min before incision) and a second dose of 1.5 g of cefuroxime administered 2 h after the first dose (11). The prevalence of PJI between 2009 and 2010 was 2.4%, and the main pathogens were S. aureus and coagulase-negative staphylococci (CoNS) (12). In order to reduce the infection rate, the prophylaxis was modified by the addition of 800 mg of teicoplanin to cefuroxime, and the infection rate was compared with that obtained during a previous period in which cefuroxime alone was used.
MATERIALS AND METHODS
All patients who underwent TKA or THA between March 2010 and February 2013 were prospectively registered in a database. For this study, only patients undergoing primary surgeries were selected. Patients with femoral neck fracture were excluded from the study. Relevant patient information was gathered: demographics (age and gender), comorbidities (having or not having one of the following entities: hypertension, diabetes mellitus, malignancy, liver disease, lung disease, or chronic renal failure), body mass index (BMI), drug allergies, preoperative performance status (measured by use of the American Association of Anesthesiology [ASA] classification, laterality, type of implant (TKA or THA), duration of surgery, duration (in days) of hospitalization, preoperative and postoperative (day +4) hemoglobin value, and the need for red blood cell transfusion.
From March 2010 to August 2011, antibiotic prophylaxis consisted of 1.5 g of cefuroxime administered during the induction of anesthesia (infused over 5 to 10 min starting 30 min before the surgical incision) and another 1.5 g of cefuroxime administered 2 h later. From September 2011 it was decided to add one dose of 800 mg of teicoplanin during the induction of anesthesia (infused over 15 min after the infusion of cefuroxime). Thus, we defined two groups of patients according to the type of antibiotic prophylaxis: one group of patients who received only cefuroxime (the C group) and one group of patients who received cefuroxime and teicoplanin (the CT group). Surgeries were performed in an operating room with a nonlaminar airflow, and throughout the study period there were no variations in the preoperative washing protocol, the method of skin preparation, the hand hygiene solutions used, the type of sterilization of surgical equipment, the surgical team, surgical techniques, or operating theaters. Screening for S. aureus carriers was not performed, and antibiotic-loaded cement was never used in these patients. The Ethical Committee of our institution approved the study.
After being discharged, the patients were followed up according to the protocol of our hospital, which includes a first visit 1 month after surgery and a second visit 3 months after surgery. One patient was excluded from the analysis because he died 10 days after surgery due to nonseptic postoperative complications. PJI was defined according to recent criteria (13). All patients with an early PJI were taken back to the operating room for debridement, and six deep samples of synovial fluid or periprosthetic tissue were submitted to the Microbiology Laboratory.
Continuous variables were expressed as median and interquartile range (IQR) and, according to the Kolmogorov-Smirnov test of normality, were compared by use of the Student t test or the Mann-Whitney U test. Continuous variables were also categorized as an age of <70 years and an age of ≥70 years; BMIs of <30 kg/m2, 30 kg/m2 to 35 kg/m2, and ≥35 kg/m2; and durations of surgery of <105 min and ≥105 min. Categorical variables were compared by the chi-square test or Fisher's exact test when necessary. The Kaplan-Meier survival method was used to estimate the cumulative probability of failure due to PJI within the first 100 days after surgery. A stepwise forward Cox regression model was performed to identify independent variables associated with infection within 100 days after surgery. All variables included in the univariate analysis were included in the multivariate analyses. The presence of an interaction and the role of confounding factors were evaluated. Statistical significance was defined as a two-tailed P value of <0.05. The analysis was performed using SPSS, version 19.0, software (SPSS, Inc., Chicago, IL, USA).
RESULTS
A total of 1,896 patients were included in the study, but 110 (5.8%) were excluded due to allergy to penicillin. The median age of the cohort was 71.5 years (IQR, 64 to 77 years), and 1,196 (66.9%) were female. There were 1,290 (72.7%) TKAs and 496 (27.8%) THAs. Forty-five (2.5%) patients had a PJI within the first 100 days after surgery. The baseline characteristics of the patients according to outcome are shown in Table 1. Male sex (4.4% for the C group versus 1.6% for the CT group, P < 0.001), the presence of lung disease (5.4% versus 2.2%, P = 0.031), the median duration of surgery (85 min versus 95 min, P = 0.010), and the need for a red blood cell transfusion (6.2% versus 2.1%, P = 0.002) were variables associated with a higher infection rate.
TABLE 1.
Baseline characteristics of patients according to outcomea
| Characteristic | Result for patients with: |
P valueb | |
|---|---|---|---|
| No PJI (n = 1,741) | PJI (n = 45) | ||
| Median (IQR) age (yr) | 72 (64–77) | 70 (62.5–76.5) | 0.320 |
| No. (%) of patients ≥70 yr of age | 1,008 (57.9) | 24 (53.3) | 0.540 |
| No. (%) of patients by gender | |||
| Female | 1,175 (67.5) | 19 (42.2) | <0.001 |
| Male | 566 (32.5) | 26 (57.8) | |
| Median (IQR) BMI (kg/m2) | 29.6 (26.6–32.8) | 30.5 (26.9–35.0) | 0.143 |
| No. (%) of patients with BMI of: | 0.151 | ||
| <30 kg/m2 | 939 (54.1) | 21 (46.7) | |
| 30–<35 kg/m2 | 552 (31.8) | 13 (2.3) | |
| ≥35 kg/m2 | 246 (14.2) | 11 (24.4) | |
| No. (%) of patients with preoperative ASA classification of: | |||
| I or II | 1,410 (81.1) | 34 (75.6) | 0.351 |
| III or IV | 329 (18.9) | 11 (24.4) | |
| No. (%) of patients with the following comorbidity: | |||
| Hypertension | 905 (52.0) | 25 (55.6) | 0.636 |
| Diabetes mellitus | 222 (12.8) | 7 (15.6) | 0.504 |
| Malignancy | 116 (6.7) | 1 (2.2) | 0.361 |
| Liver disease | 33 (1.9) | 2 (4.4) | 0.220 |
| Lung disease | 157 (9.0) | 9 (20.0) | 0.031 |
| Chronic renal failure | 14 (0.8) | 0 (0) | 1.000 |
| No. (%) of patients with the following laterality: | |||
| Left | 863 (50.5) | 22 (50.0) | 0.951 |
| Right | 847 (49.5) | 22 (50.0) | |
| Median (IQR) duration of surgery (min) | 85 (75–105) | 95 (85–122.5) | 0.010 |
| No. (%) of patients with duration of surgery of ≥105 min | 440 (25.3) | 16 (35.6) | 0.118 |
| No. (%) of patients with arthroplasty at the following site: | |||
| Knee | 1,260 (72.4) | 30 (66.7) | 0.399 |
| Hip | 481 (27.6) | 15 (33.3) | |
| Median (IQR) Hg value (g/dl) | |||
| Preoperative | 137 (128–145) | 143 (126–152.5) | 0.154 |
| Postoperative | 113 (104–121) | 114 (104–122.5) | 0.722 |
| No. (%) of patients with the following red blood cell transfusion amt: | 181 (10.4) | 12 (26.7) | 0.002 |
| 1 unit | 25 (1.4) | 0 (0) | 0.173 |
| 2 units | 126 (7.2) | 9 (20.0) | |
| ≥3 units | 30 (0.7) | 3 (6.7) | |
| No. (%) of patients in the following prophylaxis group: | |||
| Cefuroxime | 960 (55.1) | 35 (77.8) | 0.002 |
| Cefuroxime + teicoplanin | 781 (44.9) | 10 (22.2) | |
PJI, prosthetic joint infection; IQR, interquartile range; BMI, body mass index; Hg, hemoglobin.
Boldface values indicate statistically significant differences.
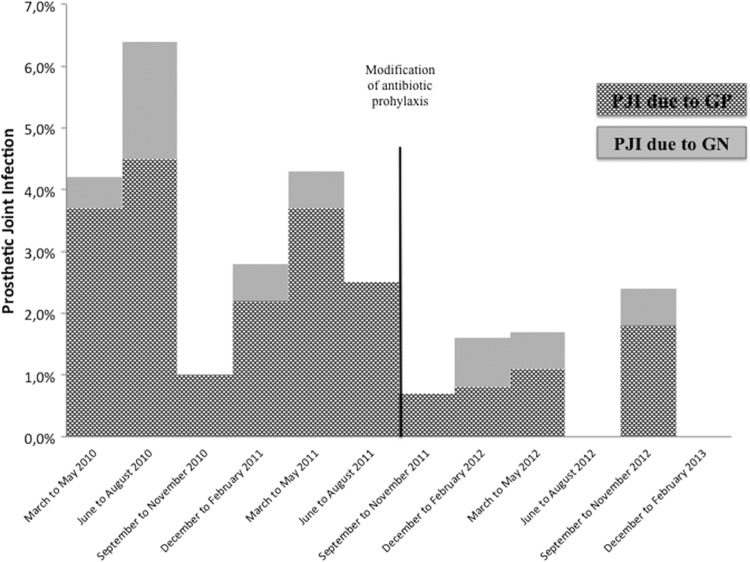
There were 995 (55.7%) patients who received only cefuroxime as antibiotic prophylaxis (the C group) and 791 (44.3%) patients who received cefuroxime and teicoplanin prophylaxis (the CT group). Patients in the CT group had a lower rate of PJI than patients who received only cefuroxime (1.26% versus 3.51%, P = 0.002). Figure 1 shows the cumulative probability of being free of PJI within the first 100 days of follow-up for each group (P = 0.003, log-rank test). The evolution of the PJI rate according to the microorganism isolated (a Gram-positive or a Gram-negative microorganism) is shown in Fig. 2.
FIG 1.
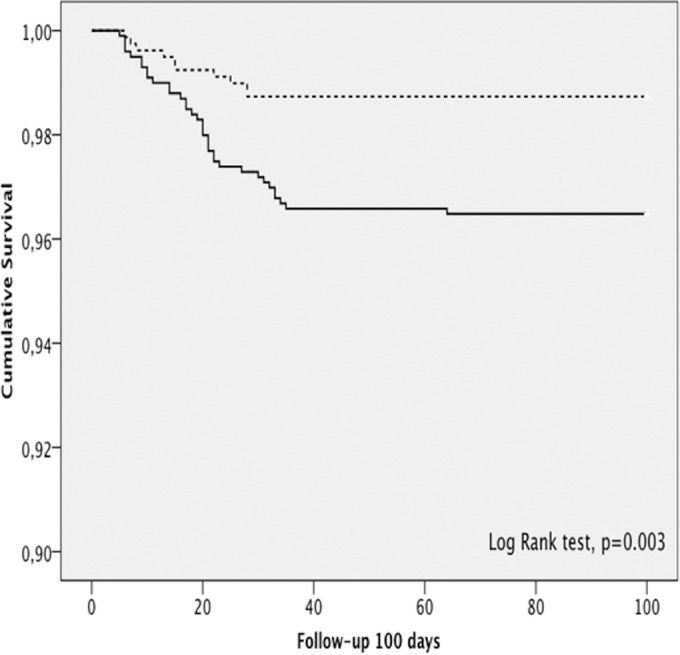
Cumulative probability of being free of PJI within the first 100 days of follow-up for each antibiotic prophylaxis group. —, cefuroxime prophylaxis; ┈, cefuroxime and teicoplanin prophylaxis.
FIG 2.
Evolution of the PJI rate according to the type of microorganism, a Gram-positive organism (GP) or a Gram-negative organism (GN).
Patients in the CT group had a lower PJI rate due to Gram-positive microorganisms (0.9%) than patients in the C group (2.9%) (P = 0.002), whereas no differences in the PJI rate due to Gram-negative microorganisms were found between the two groups (0.6% for the C group versus 0.4% for the CT group, P = 0.739). The microorganisms isolated from both groups are shown in Table 2. There were no infections due to S. aureus in the CT group (0% versus 1.6% in the C group, P < 0.001), and there was a nonsignificant reduction in the prevalence of infection due to coagulase-negative staphylococci (1.5% in the C group versus 0.76% in the CT group, P = 0.145).
TABLE 2.
PJI rate and microorganisms isolated according to prophylaxis group
| Variable | No. (%) of patients |
P valuea | |
|---|---|---|---|
| C group (n = 995) | CT group (n = 791) | ||
| Presence of PJI | 35 (3.5) | 10 (1.3) | 0.002 |
| Gram-positive microorganismb | 29 (2.9) | 7 (0.9) | 0.002 |
| Gram-negative microorganism | 6 (0.6) | 3 (0.4) | 0.739 |
| Polymicrobial infection | 8 (0.8) | 6 (0.8) | 0.914 |
| Microorganism | |||
| Staphylococcus aureus | 16 (1.6) | 0 (0) | <0.001 |
| Methicillin-resistant S. aureus | 5 (0.5) | 0 (0) | 0.046 |
| CoNS | 15 (1.5) | 6 (0.8) | 0.145 |
| Methicillin-resistant CoNS | 10 (1.0) | 3 (0.4) | 0.122 |
| Enterococcus faecalis | 2 (0.2) | 1 (0.1) | 0.842 |
| Escherichia coli | 3 (0.3) | 2 (0.3) | 0.847 |
| Pseudomonas aeruginosa | 2 (0.2) | 3 (0.4) | 0.847 |
| Klebsiella pneumoniae | 0 (0) | 1 (0.1) | 0.908 |
| Proteus mirabilis | 1 (0.1) | 0 (0) | 0.908 |
| Enterobacter cloacae | 1 (0.1) | 1 (0.1) | 0.871 |
| Morganella morganii | 0 (0) | 1 (0.1) | 0.908 |
| Citrobacter koseri | 1 (0.1) | 0 (0) | 1.000 |
Boldface values indicate statistically significant differences.
Data for polymicrobial infections with Gram-positive microorganisms were included in the Gram-positive microorganism group.
The baseline characteristics of patients according to prophylaxis group are shown in Table 3. The median BMI value (29.9 kg/m2 in the C group versus 29.3 kg/m2 in the CT group, P = 0.004) and the median duration of surgery (90 min in the C group versus 85 min in the CT group, P = 0.002) were significantly higher in the C group than in the CT group. Because these variables were also associated with a higher risk of infection, they were analyzed separately, and the results are shown in Table 4. The rate of PJI was lower in all subgroups, but among patients with a BMI of <30 kg/m2, the difference was not statistically significant.
TABLE 3.
Characteristics of patients according to type of antibiotic prophylaxisa
| Characteristic | Result for the following group: |
P valueb | |
|---|---|---|---|
| C group (n = 995) | CT group (n = 791) | ||
| Median (IQR) age (yr) | 71 (64–77) | 72 (64–78) | 0.236 |
| No. (%) of patients by gender | |||
| Female | 669 (67.2) | 525 (66.4) | 0.700 |
| Male | 326 (32.8) | 266 (33.6) | |
| Median (IQR) BMI (kg/m2) | 29.9 (26.8–33.3) | 29.3 (26.4–32.3) | 0.003 |
| No. (%) of patients with preoperative ASA classification of III or higher | 185 (18.6) | 155 (19.6) | 0.590 |
| No. (%) of patients with the following comorbidity: | |||
| Hypertension | 424 (42.6) | 432 (54.6) | <0.001 |
| Diabetes mellitus | 131 (13.2) | 98 (12.4) | 0.626 |
| Malignancy | 64 (6.4) | 53 (6.7) | 0.820 |
| Liver disease | 21 (2.1) | 14 (1.8) | 0.606 |
| Lung disease | 96 (9.6) | 70 (8.8) | 0.564 |
| Chronic renal failure | 8 (0.8) | 6 (0.8) | 0.914 |
| Median (IQR) duration of surgery (min) | 90 (75–105) | 85 (75–100) | 0.002 |
| No. (%) of patients with arthroplasty at the following site: | |||
| Knee | 736 (74.0) | 554 (70.0) | 0.065 |
| Hip | 259 (26.0) | 237 (30.0) | |
| Median (IQR) Hg value (g/dl) | |||
| Preoperative | 136 (128–145) | 138 (128–147) | 0.185 |
| Postoperative | 113 (104–121) | 112 (104–122) | 0.884 |
| No. (%) of patients receiving a red blood cell transfusion | 110 (11.1) | 83 (10.5) | 0.704 |
IQR, interquartile range; BMI, body mass index; Hg, hemoglobin.
Boldface values indicate statistically significant differences.
TABLE 4.
PJI rate according to different antibiotic prophylaxis and different subgroups of patients
| Patient subgroup (no. of patients) and PJI subgroup | No. (%) of patients with PJI |
P valuea | |
|---|---|---|---|
| C group (n = 995) | CT group (n = 791) | ||
| BMI of <30 kg/m2 (n = 960)b | |||
| PJI | 13 (2.6) | 8 (1.8) | 0.383 |
| PJI due to GPc | 9 (1.8) | 6 (1.3) | 0.558 |
| BMI of ≥30 kg/m2 (n = 822)b | |||
| PJI | 22 (4.5) | 2 (0.6) | 0.001 |
| PJI due to GP | 20 (4.1) | 1 (0.3) | 0.001 |
| Duration of surgery of <105 min (n = 1,330) | |||
| PJI | 21 (3.0) | 8 (1.3) | 0.037 |
| PJI due to GP | 20 (2.8) | 5 (0.8) | 0.007 |
| Duration of surgery of ≥105 min (n = 456) | |||
| PJI | 14 (4.9) | 2 (1.2) | 0.037 |
| PJI due to GP | 9 (3.1) | 2 (1.2) | 0.223 |
Boldface values indicate statistically significant differences.
In 4 cases the BMI was not available.
GP, Gram-positive microorganism.
All variables studied in the univariate analysis (age, male sex, BMI, ASA classification of III or IV, hypertension, diabetes mellitus, malignancy, liver disease, lung disease, chronic renal failure, left side, duration of surgery, site, red blood cell transfusion, and prophylaxis group) were included in a multivariate analysis (hemoglobin values were excluded due to colinearity with the need for red blood cell transfusion). A stepwise forward Cox regression model identified male sex (hazard ratio [HR], 3.875; 95% confidence interval [CI], 2.091 to 7.183), a BMI of ≥35 kg/m2 (HR, 2.932; 95% CI, 1.370 to 6.275), the presence of lung disease (HR, 2.463; 95% CI, 1.178 to 5.151), and red blood cell transfusion (HR, 3.703; 95% CI, 1.896 to 7.231) to be independent variables associated with a higher risk of PJI. Addition of teicoplanin to the antibiotic prophylaxis rather than the use of cefuroxime alone was associated with a lower risk of infection (HR, 0.355; 95% CI, 0.170 to 0.740) (Table 5).
TABLE 5.
Independent predictors of PJI
| Variable | P value | HR (95% CI) |
|---|---|---|
| Gender (male) | <0.001 | 3.875 (2.091–7.183) |
| BMI | ||
| <30 kg/m2 (reference) | 1 | |
| 30–35 kg/m2 | 0.510 | 1.266 (0.628–2.554) |
| ≥35 kg/m2 | 0.006 | 2.932 (1.370–6.275) |
| Lung disease | 0.017 | 2.463 (1.178–5.151) |
| Red blood cell transfusion | <0.001 | 3.703 (1.896–7.231) |
| CT group vs C group | 0.006 | 0.355 (0.170–0.740) |
DISCUSSION
Reducing the PJI rate is of utmost importance to avoid severe consequences for patients, including additional surgeries, prolonged antibiotic therapy, and a poor functional outcome, which also lead to increased economic costs (1, 14). Antibiotic prophylaxis has been demonstrated to be efficacious when the following basic principles are fulfilled: selection of an antibiotic that covers the majority of potential contaminant microorganisms, administration of the antibiotic 10 to 30 min before incision, and readministration of the dose when surgery lasts more than 2 times the half-life of the antibiotic (15).
Although in our institution these rules are followed in 99% of cases, according to internal audits, S. aureus was the most common microorganism isolated during the first period of the study, when cefuroxime alone was used (Table 2). During this period, patients with a BMI of ≥30 kg/m2 had a PJI rate of 4.5% (Table 4). Potential explanations for this finding are (i) the low level of blood flow into fat (25 to 30 ml/min/100 g of tissue, <5% of cardiac output), (ii) the higher glomerular filtration in obese patients (16), and (iii) the high MIC90 of cefuroxime (2 mg/liter) for S. aureus and coagulase-negative staphylococci (CoNS; 32 mg/liter) (www.eucast.org/mic_distributions/). The first two explanations are potentially responsible for the low concentration of cephalosporins in adipose tissue of obese patients (17). Although cefazolin has a lower MIC90 (1 mg/liter), cefuroxime was preferred in our institution because it has better stability against class A and C beta-lactamases (18, 19), and use of this antibiotic resulted in a lower infection rate than the use of cefazolin in a randomized trial in patients undergoing cardiac surgery (20).
The addition of a high dose of teicoplanin (800 mg) to cefuroxime was associated with a significant reduction in the incidence of PJIs due to Gram-positive microorganisms, but, interestingly, the reduction was particularly important in infections due to S. aureus (strains susceptible and resistant to methicillin) and among obese patients (Table 4). Recent guidelines for antimicrobial prophylaxis in surgery (3) concluded that recommendations for weight-based dosing for antimicrobial prophylaxis in obese patients cannot be made because data demonstrating clinically relevant decreases in the rates of surgical site infections from the use of such dosing strategies instead of standard doses in obese patients are not available in the published literature. Our result supports the concept that cefuroxime, even when it is used at 3 g (1.5 g before surgery and 1.5 g 2 h later), does not achieve concentrations in obese patients high enough to prevent infections caused by MSSA and CoNS. The addition of 800 mg of teicoplanin probably provides effective antibiotic concentrations at the periprosthetic and in adipose tissue (21), but also we cannot eliminate the possibility of a synergistic effect between beta-lactams and glycopeptides, which has been demonstrated in vitro against MRSA and glycopeptide-intermediate S. aureus strains (22, 23). On the other hand, it is evident that narrow- or expanded-spectrum cephalosporins are not adequate for preventing infections caused by CoNS strains with a high rate of methicillin resistance (24). The incidence of these infections was also reduced in our study when teicoplanin was used, although the difference was not statistically significant.
Teicoplanin was selected instead of vancomycin because teicoplanin can be infused over 20 min without the risk of red man syndrome and it has a better safety profile than vancomycin even at high doses (25). The use of a high dose of teicoplanin was based on a previously reported experience in cardiac surgery, whereby 400 mg of teicoplanin showed a lower efficacy than cloxacillin plus tobramycin in preventing infections caused by Gram-positive microorganisms (26). The explanation for these findings could be related to the high protein binding of teicoplanin (>90%), since only the free fraction of an antibiotic is microbiologically active (27).
The main drawback of our study is the retrospective nature of the analysis and the fact that patients were not randomized to receive teicoplanin or not. However, throughout the study period there were no changes in the hygiene protocol, the surgeons, or the operating theaters used. Furthermore, information on the most important variables potentially associated with PJI was collected, and a multivariate analysis was performed to avoid bias. In addition, the infection rate in other types of surgery, like colon surgery, remained stable during the study period, supporting the value of adding teicoplanin. Another limitation was the fact that the MIC of teicoplanin for those CoNS organisms isolated during the period of use of teicoplanin was not determined. This information would have been interesting, since the MIC90 of teicoplanin for CoNS is 8 mg/liter (www.eucast.org/mic_distributions/) and could explain the lower efficacy of teicoplanin in preventing CoNS infection. Finally, the follow-up period of the study was 100 days, whereas the majority of authors recommend 365 days; however, recent studies (28) confirm that the majority of infections after arthroplasty occur within the first 3 months and only a minor number are detected afterwards, supporting the change made by the National Healthcare Safety Network (NHSN) in January 2013 to use a 90-day surveillance period for these procedures.
The incidence of vancomycin-resistant Enterococcus faecium (VRE) in our institution is low, and there was no change in the incidence of this microorganism during the study period. However, the impact of adding a glycopeptide as antibiotic prophylaxis in hospitals with a high incidence of VRE needs to be further evaluated.
In conclusion, the addition of 800 mg of teicoplanin to cefuroxime during primary arthroplasty was associated with a significant reduction in the global PJI rate due to a reduction in the incidence of infections caused by Gram-positive microorganisms. The dual prophylaxis was particularly effective against S. aureus in a population with a BMI of ≥30 kg/m2. According to these results, the addition of teicoplanin could be restricted to S. aureus carriers. In the future, it will be necessary to compare the efficacy of nasal and skin decontamination of S. aureus carriers (29), the use of dual prophylaxis with a beta-lactam plus a glycopeptide, or both measures integrated in a bundle of measures, as suggested in a recent meta-analysis (30) considering not only the rate of PJI due to S. aureus but also the rate of PJI due to CoNS and other important epidemiological outcomes, such as the prevalence of VRE and mupirocin or chlorhexidine resistance.
ACKNOWLEDGMENTS
Potential conflicts of interest are as follows. A.S. has received honoraria for public speaking and from the advisory boards of Pfizer and Novartis. J.M. has received honoraria for public speaking from Pfizer, Novartis, and Gilead.
This work was supported by the Fundación Privada Máximo Soriano Jiménez.
REFERENCES
- 1.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. 2012. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 27:61–65. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Peel TN, Cheng AC, Buising KL, Choong PFM. 2012. Microbiological etiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother 56:2386–2391. doi: 10.1128/AAC.06246-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA. 2013. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 70:195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 4.Bolon MK, Morlote M, Weber SG, Koplan B, Carmeli Y, Wright SB. 2004. Glycopeptides are no more effective than beta-lactam agents for prevention of surgical site infection after cardiac surgery: a meta-analysis. Clin Infect Dis 38:1357–1363. doi: 10.1086/383318. [DOI] [PubMed] [Google Scholar]
- 5.Chambers D, Worthy G, Myers L, Weatherly H, Elliott R, Hawkins N, Sculpher M, Eastwood A. 2010. Glycopeptide vs. non-glycopeptide antibiotics for prophylaxis of surgical site infections: a systematic review. Surgery 11:455–462. doi: 10.1089/sur.2009.055. [DOI] [PubMed] [Google Scholar]
- 6.Crawford T, Rodvold KA, Solomkin JS. 2012. Vancomycin for surgical prophylaxis? Clin Infect Dis 54:1474–1479. doi: 10.1093/cid/cis027. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein R, Rabino G, Mashiah T, Bar-El Y, Adler Z, Kertzman V, Cohen O, Milo S. 2002. Vancomycin versus cefazolin prophylaxis for cardiac surgery in the setting of a high prevalence of methicillin-resistant staphylococcal infections. J Thorac Cardiovasc Surg 123:326–332. doi: 10.1067/mtc.2002.119698. [DOI] [PubMed] [Google Scholar]
- 8.Periti P, Mini E, Mosconi G. 1998. Antimicrobial prophylaxis in orthopaedic surgery: the role of teicoplanin. J Antimicrob Chemother 41:329–340. doi: 10.1093/jac/41.3.329. [DOI] [PubMed] [Google Scholar]
- 9.Soriano A, Popescu D, Garcia S, Bori G, Martínez JA, Balasso V, Marco F, Almela M, Mensa J. 2006. Usefulness of teicoplanin for preventing methicillin-resistant Staphylococcus aureus infections in orthopedic surgery. Eur J Clin Microbiol Infect Dis 25:35–38. doi: 10.1007/s10096-005-0073-z. [DOI] [PubMed] [Google Scholar]
- 10.Sewick A, Makani A, Wu C, O'Donnell J, Baldwin KD, Lee G-C. 2012. Does dual antibiotic prophylaxis better prevent surgical site infections in total joint arthroplasty? Clin Orthop Relat Res 470:2702–2707. doi: 10.1007/s11999-012-2255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soriano A, Bori G, García-Ramiro S, Martinez-Pastor JC, Miana T, Codina C, Maculé F, Basora M, Martínez JA, Riba J, Suso S, Mensa J. 2008. Timing of antibiotic prophylaxis for primary total knee arthroplasty performed during ischemia. Clin Infect Dis 46:1009–1014. doi: 10.1086/529145. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Lesmes SP, Tornero E, Martinez-Pastor JC, Pereira A, Marcos M, Soriano A. 2014. Length of storage of transfused red blood cells and risk of prosthetic joint infection after primary knee arthroplasty. J Arthroplasty 29:2016–2020. doi: 10.1016/j.arth.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Valle Della CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. 2011. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peel TN, Dowsey MM, Buising KL, Liew D, Choong PFM. 2013. Cost analysis of debridement and retention for management of prosthetic joint infection. Clin Microbiol Infect 19:181–186. doi: 10.1111/j.1469-0691.2011.03758.x. [DOI] [PubMed] [Google Scholar]
- 15.Mangram AJ, Pearson ML, Silver LC, Jarvis WR. 1999. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 27:97–132. [PubMed] [Google Scholar]
- 16.Pai MP, Bearden DT. 2007. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy 27:1081–1091. doi: 10.1592/phco.27.8.1081. [DOI] [PubMed] [Google Scholar]
- 17.Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. 2011. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol 117:877–882. doi: 10.1097/AOG.0b013e31820b95e4. [DOI] [PubMed] [Google Scholar]
- 18.Kernodle DS, Classen DC, Burke JP, Kaiser AB. 1990. Failure of cephalosporins to prevent Staphylococcus aureus surgical wound infections. JAMA 263:961–966. doi: 10.1001/jama.263.7.961. [DOI] [PubMed] [Google Scholar]
- 19.Nannini EC, Stryjewski ME, Singh KV, Rude TH, Corey GR, Fowler VG, Murray BE. 2010. Determination of an inoculum effect with various cephalosporins among clinical isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 54:2206–2208. doi: 10.1128/AAC.01325-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slama TG, Sklar SJ, Misinski J, Fess SW. 1986. Randomized comparison of cefamandole, cefazolin, and cefuroxime prophylaxis in open-heart surgery. Antimicrob Agents Chemother 29:744–747. doi: 10.1128/AAC.29.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergeron MG, Saginur R, Desaulniers D, Trottier S, Goldstein W, Foucault P, Lessard C. 1990. Concentrations of teicoplanin in serum and atrial appendages of patients undergoing cardiac surgery. Antimicrob Agents Chemother 34:1699–1702. doi: 10.1128/AAC.34.9.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagihara M, Wiskirchen DE, Kuti JL, Nicolau DP. 2012. In vitro pharmacodynamics of vancomycin and cefazolin alone and in combination against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 56:202–207. doi: 10.1128/AAC.05473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein FW, Atoui R, Ben Ali A, Nguyen JC, Ly A, Kitzis MD. 2004. False synergy between vancomycin and beta-lactams against glycopeptide-intermediate Staphylococcus aureus (GISA) caused by inappropriate testing methods. Clin Microbiol Infect 10:342–345. doi: 10.1111/j.1198-743X.2004.00856.x. [DOI] [PubMed] [Google Scholar]
- 24.Yourassowsky E, van der Linden MP, Crokaert F. 1990. Inoculum effect on growth-delay time of oxacillin-resistant strains of Staphylococcus aureus and Staphylococcus epidermidis exposed to cefamandole, cefazolin, and cefuroxime. Antimicrob Agents Chemother 34:505–509. doi: 10.1128/AAC.34.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svetitsky S, Leibovici L, Paul M. 2009. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother 53:4069–4079. doi: 10.1128/AAC.00341-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson AP, Treasure T, Grüneberg RN, Sturridge MF, Ross DN. 1988. Antibiotic prophylaxis in cardiac surgery: a prospective comparison of two dosage regimens of teicoplanin with a combination of flucloxacillin and tobramycin. J Antimicrob Chemother 21:213–223. doi: 10.1093/jac/21.2.213. [DOI] [PubMed] [Google Scholar]
- 27.Bailey EM, Rybak MJ, Kaatz GW. 1991. Comparative effect of protein binding on the killing activities of teicoplanin and vancomycin. Antimicrob Agents Chemother 35:1089–1092. doi: 10.1128/AAC.35.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokoe DS, Avery TR, Platt R, Huang SS. 2013. Reporting surgical site infections following total hip and knee arthroplasty: impact of limiting surveillance to the operative hospital. Clin Infect Dis 57:1282–1288. doi: 10.1093/cid/cit516. [DOI] [PubMed] [Google Scholar]
- 29.Bode LGM, Kluytmans JAJW, Wertheim HFL, Bogaers D, Vandenbroucke-Grauls CMJE, Roosendaal R, Troelstra A, Box ATA, Voss A, van der Tweel I, van Belkum A, Verbrugh HA, Vos MC. 2010. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 362:9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 30.Schweizer M, Perencevich E, McDanel J, Carson J, Formanek M, Hafner J, Braun B, Herwaldt L. 2013. Effectiveness of a bundled intervention of decolonization and prophylaxis to decrease Gram-positive surgical site infections after cardiac or orthopedic surgery: systematic review and meta-analysis. BMJ 346:f2743. doi: 10.1136/bmj.f2743. [DOI] [PMC free article] [PubMed] [Google Scholar]