Abstract
We evaluated the in vitro and in vivo activities of the investigational arylamidine T-2307 against echinocandin-resistant Candida albicans. T-2307 demonstrated potent in vitro activity, and daily subcutaneous doses between 0.75 and 6 mg/kg of body weight significantly improved survival and reduced fungal burden compared to placebo control and caspofungin (10 mg/kg/day) in mice with invasive candidiasis caused by an echinocandin-resistant strain. Thus, T-2307 may have potential use in the treatment of echinocandin-resistant C. albicans infections.
TEXT
Invasive candidiasis remains a significant challenge to clinicians, as Candida species remain the fourth most common cause of nosocomial bloodstream infections in the United States (1–3). In most institutions in the United States and most countries worldwide, Candida albicans is the species most frequently associated with disease (4, 5). Invasive disease caused by echinocandin-resistant isolates may be especially difficult to treat due to limited treatment options as well as drug interactions and toxicities associated with the azoles and amphotericin B. T-2307 is a novel arylamidine (Fig. 1) that is structurally similar to a class of aromatic diamidines that includes pentamidine (6). This investigational agent has been shown to have potent in vitro activity against Candida species and Cryptococcus neoformans (6) and works by causing the collapse of fungal mitochondrial membrane potential (7). Our preliminary data also suggest that T-2307 maintains in vitro potency against echinocandin-resistant C. albicans and Candida glabrata isolates (8). In addition, previous studies have also demonstrated in vivo efficacy in murine models of invasive candidiasis, systemic cryptococcosis, and disseminated aspergillosis (6, 9). Our objective was to evaluate the in vitro activity of T-2307 against echinocandin-resistant C. albicans and determine its efficacy against experimental invasive candidiasis caused by a resistant and virulent strain.
FIG 1.
Chemical structure of the novel arylamidine T-2307.
In vitro susceptibility testing was performed according to the CLSI M27-A3 method with T-2307 and caspofungin against 37 C. albicans clinical isolates, including 18 echinocandin-resistant strains, of which 12 had known FKS1 hot spot mutations (10, 11). The MICs for T-2307 were read at 50% and 100% inhibition of growth compared to the growth controls after 24 h of incubation at 35°C, while the MICs for caspofungin were read at 50% inhibition. The MICs at which 50% and 90% of the isolates were inhibited (MIC50 and MIC90, respectively) and geometric mean (GM) MICs were determined. Differences in the GM MIC values were assessed for significance with the Student t test. For the in vivo model, immunocompetent outbred male ICR mice (Harlan), weighing between 22 and 25 g, were used in all the experiments (http://www.sacmm.org/pdf/SOP-murine-model-candida-albicans.pdf) (12, 13). On the day of infection (day 0), each mouse was inoculated through the lateral tail vein with C. albicans isolate 43001 (∼1 × 106 cells/mouse; T-2307 MIC50 and MIC100, ≤0.008 and >16 μg/ml, respectively; caspofungin MIC, 1 μg/ml; F641S amino acid change in Fks1p) (11, 13). Mice were then randomly assigned to one of six groups: placebo control (physiologic saline administered subcutaneously once daily), T-2307 at doses of 0.75, 1.5, 3, or 6 mg/kg of body weight administered subcutaneously once daily, or caspofungin at a dose of 10 mg/kg by intraperitoneal injection once daily. Treatment was initiated 1 day after inoculation and continued through day 7. In the survival arm, the mice were monitored off therapy until day 21. Any animal that appeared moribund was humanely euthanized, with death recorded as occurring the next day. In the fungal burden arm, kidneys were collected on day 8, 1 day after treatment stopped. Kidneys were weighed and homogenized in sterile saline. Serial dilutions were prepared and plated, and following 24 h of incubation at 37°C, fungal burdens (CFU/g) were determined. Each group in the survival and fungal burden arms consisted of 10 mice. With the exception of the 0.75-mg/kg T-2307 group, which was tested only once, each dose group within the survival and fungal burden arms was tested in duplicate to evaluate the reproducibility of the results (n = 20 mice per dosage group per study arm). Survival was plotted by Kaplan-Meier analysis, and differences in median survival time and the percent survival among the groups were analyzed by the log-rank test and Fischer's exact test, respectively. Differences in kidney fungal burden (reported as mean CFU/g ± standard deviation) among the groups were assessed for significance by analysis of variance (ANOVA) with Tukey's posttest for multiple comparisons. For mice in which no organisms were recovered from the kidney tissue, the fungal burden was set at 10 CFU/g for data analysis purposes. This study was approved by the Institutional Animal Care and Use Committee at the UT Health Science Center at San Antonio.
T-2307 demonstrated potent in vitro activity against C. albicans, including isolates that were echinocandin resistant (Table 1), when the 50% growth inhibition endpoint was used. With this endpoint, the T-2307 MICs were low for all isolates (MICs, ≤0.008 μg/ml), and the GM MICs for T-2307 were significantly lower than those of caspofungin against all the isolates, including the caspofungin-susceptible (0.008 μg/ml versus 0.1197 μg/ml; P < 0.0001) and caspofungin-resistant isolates (0.008 μg/ml versus 1.587 μg/ml; P < 0.0001). However, T-2307 did not result in complete in vitro inhibition of growth, as light growth was observed at all concentrations tested against all but 4 isolates. Thus, when the 100% inhibition of growth endpoint was used, the MICs were >4 μg/ml. These results are consistent with an in vitro trailing effect previously reported with this agent (9). A significant survival advantage over placebo control and caspofungin was also observed with T-2307 (Fig. 2). Treatment with T-2307 significantly lengthened the median survival time (>21 days for each dose) over those of the placebo control and caspofungin (8 days each; P < 0.0001). Percent survival on day 21, 14 days after therapy had stopped, was also significantly greater in mice that received T-2307 (range 70 to 95%) than those that received the placebo or caspofungin (15% each; P ≤ 0.0011). Kidney fungal burdens on day 8 were also significantly reduced in the mice with each dose of T-2307 (mean ± standard deviation, 1.81 ± 0.47, 2.24 ± 1.00, 2.12 ± 0.95, and 1.50 ± 0.46 log10 CFU/g for the 0.75-, 1.5-, 3-, and 6-mg/kg dose groups, respectively) compared to those in the mice that received placebo (5.68 ± 0.96 log10 CFU/g; P < 0.0001) or caspofungin (5.92 ± 0.59 log10 CFU/g; P < 0.0001) (Fig. 3). In fact, for many mice treated with T-2307 at doses of 3 and 6 mg/kg, fungal burdens were undetectable on day 8. However, for analysis purposes, these values were set at 10 CFU/g. The survival results and the fungal burden results for the T-2307 doses that were tested in duplicate, as well as for the placebo control and caspofungin, were reproducible (data not shown).
TABLE 1.
MICs for T-2307 (0.008 to 4 μg/ml) and caspofungin (0.03 to 8 μg/ml) against 37 C. albicans isolates, including caspofungin-susceptible (n = 17), caspofungin-intermediate (n = 2), and caspofungin-resistant (n = 18) strains
| Isolate type (no. of isolates) | MIC data (μg/ml) for indicated antifungal agent |
||
|---|---|---|---|
| T-2307 (50% inhibition) | T-2307 (100% inhibition) | Caspofungin | |
| All Candida albicans (37) | |||
| MIC range | ≤0.008 | 0.008 to >4 | 0.06 to >8 |
| MIC50 | ≤0.008 | >4 | 0.5 |
| MIC90 | ≤0.008 | >4 | >8 |
| GM MICa | 0.008 | >4 | 0.4548 |
| Echinocandin susceptible (17) | |||
| MIC range | ≤0.008 | 0.008 to >4 | 0.06 to 0.125 |
| MIC50 | ≤0.008 | >4 | 0.125 |
| MIC90 | ≤0.008 | >4 | 0.125 |
| GM MIC | 0.008 | 3.836 | 0.1197 |
| Echinocandin resistant (18) | |||
| MIC range | ≤0.008 | 0.008 to >4 | 1 to >8 |
| MIC50 | ≤0.008 | >4 | 1 |
| MIC90 | ≤0.008 | >4 | 8 |
| GM MIC | 0.008 | 3.996 | 1.587 |
GM MIC, geometric mean MIC.
FIG 2.
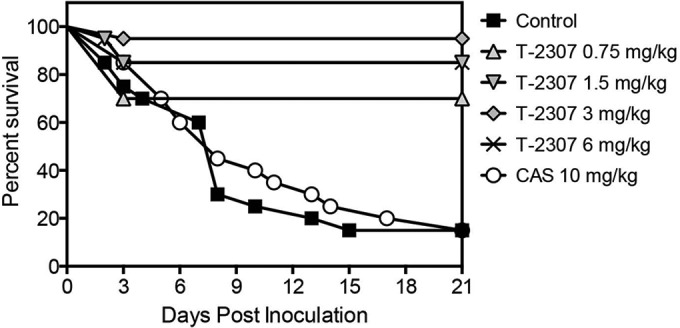
Survival curves in mice infected with C. albicans isolate 43001 and treated with placebo by subcutaneous injection once daily (physiologic saline); T-2307 at doses of 0.75 mg/kg, 1.5 mg/kg, 3 mg/kg, or 6 mg/kg by subcutaneous injection once daily; or caspofungin (CAS) at a dose of 10 mg/kg by intraperitoneal injection once daily. Treatment began 1 day postinoculation and continued for 7 days. Mice were then monitored off therapy until day 21. Twenty mice were used per group for all groups except the 0.75-mg/kg T-2307 group, in which 10 mice were used.
FIG 3.
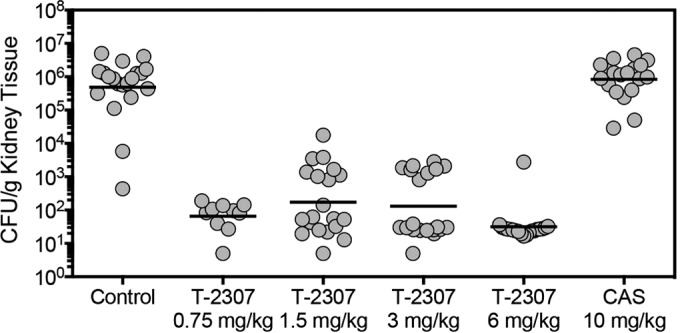
Kidney fungal burden (CFU/g of tissue) on day 8 in mice infected with C. albicans isolate 43001 and treated with placebo by subcutaneous injection once daily (physiologic saline); T-2307 at doses of 0.75 mg/kg, 1.5 mg/kg, 3 mg/kg, or 6 mg/kg by subcutaneous injection once daily; or caspofungin (CAS) at a dose of 10 mg/kg by intraperitoneal injection once daily. Treatment began 1 day postinoculation and continued for 7 days. Twenty mice per group were used for all groups except the 0.75-mg/kg T-2307 group, in which 10 mice were used. Lines represent mean values.
These results demonstrate the potential use of T-2307 in the treatment of invasive candidiasis caused by echinocandin-resistant C. albicans. Potent in vitro activity against C. albicans using the 50% inhibition endpoint was observed with this investigational arylamidine, and this activity was maintained against isolates with elevated caspofungin MICs. The in vitro potency with the 50% inhibition endpoint also translated into in vivo efficacy in the murine model of invasive candidiasis caused by a resistant isolate despite not observing complete inhibition of growth with T-2307 against this strain with susceptibility testing. Survival and fungal burdens were significantly improved compared to those of placebo controls and caspofungin. These results are consistent with previous work that demonstrated in vitro potency and in vivo efficacy for T-2307 against C. albicans and C. glabrata (6, 9). Although further work is warranted, including pharmacokinetics/pharmacodynamics studies and clinical trials, our results suggest that T-2307 may be useful for the treatment of invasive infections caused by echinocandin-resistant C. albicans isolates.
ACKNOWLEDGMENTS
We thank Arlene Farias for her assistance with the animal model.
This project utilized preclinical services funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and Department of Health and Human Services under contract no. HHS272201000018I and HHSN272201000038I, Task Orders A03 and A13, respectively.
The T-2307 powder was provided by Toyama Chemical Co, Ltd.
N.P.W. has received research support from Astellas, Dow, F2G, Merck, Merz, and Viamet and has served on advisory boards for Merck, Astellas, Toyama, and Viamet. T.F.P. has received research grants to UT Health Science Center San Antonio from Astellas and Merck and has served as a consultant for Astellas, Merck, Toyama, Viamet, and SCYNEXIS. L.K.N. has received travel support from Viamet. Y.F. and J.M. are employees of Toyama Chemical Co., Ltd.
We declare no conflicts of interest.
REFERENCES
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, National Healthcare Safety Network Team and Participating NHSN Facilities . 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 3.Yapar N. 2014. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag 10:95–105. doi: 10.2147/TCRM.S40160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfaller MA, Messer SA, Moet GJ, Jones RN, Castanheira M. 2011. Candida bloodstream infections: comparison of species distribution and resistance to echinocandin and azole antifungal agents in intensive care unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008–2009). Int J Antimicrob Agents 38:65–69. doi: 10.1016/j.ijantimicag.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Quindos G. 2014. Epidemiology of candidaemia and invasive candidiasis. A changing face. Rev Iberoam Micol 31:42–48. doi: 10.1016/j.riam.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Mitsuyama J, Nomura N, Hashimoto K, Yamada E, Nishikawa H, Kaeriyama M, Kimura A, Todo Y, Narita H. 2008. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob Agents Chemother 52:1318–1324. doi: 10.1128/AAC.01159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata T, Takahashi T, Yamada E, Kimura A, Nishikawa H, Hayakawa H, Nomura N, Mitsuyama J. 2012. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob Agents Chemother 56:5892–5897. doi: 10.1128/AAC.05954-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fothergill AW, Cushion MT, Collins MS, Kirkpatrick WR, Najvar LK, Patterson TF, Wiederhold NP. 2014. Antifungal and anti-Pneumocystis activity of the novel arylamidine T-2307, abstr F-1583 Abstr 54th Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 9.Yamada E, Nishikawa H, Nomura N, Mitsuyama J. 2010. T-2307 shows efficacy in a murine model of Candida glabrata infection despite in vitro trailing growth phenomena. Antimicrob Agents Chemother 54:3630–3634. doi: 10.1128/AAC.00355-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed. Approved standard M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Wiederhold NP, Grabinski JL, Garcia-Effron G, Perlin DS, Lee SA. 2008. Pyrosequencing to detect mutations in FKS1 that confer reduced echinocandin susceptibility in Candida albicans. Antimicrob Agents Chemother 52:4145–4148. doi: 10.1128/AAC.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najvar LK, Bocanegra R, Wiederhold NP, Lambros C, Najarian N, Patterson TF, Graybill JR. 2008. Therapeutic and prophylactic efficacy of aminocandin (IP960) against disseminated candidiasis in mice. Clin Microbiol Infect 14:595–600. doi: 10.1111/j.1469-0691.2008.01994.x. [DOI] [PubMed] [Google Scholar]
- 13.Wiederhold NP, Najvar LK, Bocanegra RA, Kirkpatrick WR, Patterson TF. 2011. Caspofungin dose escalation for invasive candidiasis due to resistant Candida albicans. Antimicrob Agents Chemother 55:3254–3260. doi: 10.1128/AAC.01750-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


