Abstract
Chloroquine-primaquine (CQ-PQ) continues to be the frontline therapy for radical cure of Plasmodium vivax malaria. Emergence of CQ-resistant (CQR) P. vivax parasites requires a shift to artemisinin combination therapies (ACTs), which imposes a significant financial, logistical, and safety burden. Monitoring the therapeutic efficacy of CQ is thus important. Here, we evaluated the therapeutic efficacy of CQ-PQ for P. vivax malaria in northeast Myanmar. We recruited 587 patients with P. vivax monoinfection attending local malaria clinics during 2012 to 2013. These patients received three daily doses of CQ at a total dose of 24 mg of base/kg of body weight and an 8-day PQ treatment (0.375 mg/kg/day) commencing at the same time as the first CQ dose. Of the 401 patients who finished the 28-day follow-up, the cumulative incidence of recurrent parasitemia was 5.20% (95% confidence interval [CI], 3.04% to 7.36%). Among 361 (61%) patients finishing a 42-day follow-up, the cumulative incidence of recurrent blood-stage infection reached 7.98% (95% CI, 5.20% to 10.76%). The cumulative risk of gametocyte carriage at days 28 and 42 was 2.21% (95% CI, 0.78% to 3.64%) and 3.93% (95% CI, 1.94% to 5.92%), respectively. Interestingly, for all 15 patients with recurrent gametocytemia, this was associated with concurrent asexual stages. Genotyping of recurrent parasites at the merozoite surface protein 3α gene locus from 12 patients with recurrent parasitemia within 28 days revealed that 10 of these were the same genotype as at day 0, suggesting recrudescence or relapse. Similar studies in 70 patients in the same area in 2007 showed no recurrent parasitemias within 28 days. The sensitivity to chloroquine of P. vivax in northeastern Myanmar may be deteriorating.
INTRODUCTION
Plasmodium vivax has the widest geographical distribution among the four human-infecting species, stretching from the Korean Peninsula to northern Argentina. An estimated 2.48 billion people lived at risk of P. vivax malaria in 2010, of which a large majority was in Central and Southeast Asia (1). Each year, P. vivax infects an estimated 130 to 391 million people (2, 3). Past eradication campaigns have witnessed the resilience of vivax malaria to control efforts. In areas where P. vivax and P. falciparum are coendemic, intensified control efforts have led to major changes in malaria epidemiology, and the problem of vivax malaria has become more prominent (4). With emerging global interests in malaria elimination (5), many nations in which vivax malaria is endemic will inevitably face greater challenges for the control and elimination of this parasite. For example, among the 34 malaria-eliminating countries, 26 have malaria burdens mainly or solely due to P. vivax (4).
The relative resilience of vivax malaria may be attributed to the formation of dormant hypnozoites in the livers of patients. These hypnozoites awaken in the weeks and months following a primary attack and cause new attacks, called relapses. Thus, treatment of P. vivax malaria requires drugs that target both the blood-stage parasites to relieve the acute illness and liver-stage hypnozoites to prevent relapses. This partnered chemotherapeutic strategy is called radical cure. For the past 60 years, the chloroquine (CQ) and primaquine (PQ) combination has been the frontline therapy for radical cure of vivax malaria. However, mounting evidence indicates that resistance to CQ by the asexual blood-stage P. vivax is spreading through Southeast Asia (6). This problem threatens the availability of a safe and effective radical cure against vivax malaria (7).
Clinical CQ-resistant (CQR) P. vivax was first reported from Papua New Guinea and Indonesia more than 20 years ago (8–12). While P. vivax CQ resistance has since been documented in many regions (6, 13), the problem appeared to be most serious in Indonesia, which led to the change to artemisinin combination therapy (ACT) with PQ for P. vivax therapy in 2009 (7). Despite the ample evidence of CQR parasites in the vast range of P. vivax malaria, the mechanisms of resistance remain unknown and appear to be different from that for CQR P. falciparum (6, 14). There are no validated molecular markers for the CQR phenotype in P. vivax, and ex vivo testing results are often difficult to interpret. Surveillance of CQR P. vivax has been reported relatively scarcely, likely owing to the relative difficulty of doing so, i.e., a requirement for clinical studies with prolonged follow-up of patients. The available data informing the current map of CQR P. vivax suggests that it occurs across most of its geographical range in lesser or greater frequencies (13). A better understanding of the threat imposed by CQR P. vivax would directly inform a strategy for improved management of vivax malaria therapy aimed at eliminating its transmission.
Myanmar has the heaviest malaria burden in the Greater Mekong Subregion (GMS) (15). Within Myanmar, malaria is distributed unevenly, and the regions with the highest malaria burdens border China, Thailand, and India and represent pools of infection which could frustrate efforts of neighboring countries in their progress toward malaria elimination (15, 16). CQ resistance in vivax malaria was documented in several regions of Myanmar as early as 1993 (17–19). Currently, CQ and PQ remain the frontline therapy for vivax malaria in Myanmar. As in other regions of the GMS acting aggressively against becoming an area where falciparum malaria is endemic, the area of northeast Myanmar bordering China's Yunnan province experienced a significant increase in the prevalence of vivax malaria (20). This occurred despite ramped up control measures. The possibility of CQR underpinning the surge in cases prompted us to evaluate the therapeutic response of vivax malaria to the CQ-PQ regimen applied in this region.
MATERIALS AND METHODS
Study site.
The study was conducted between March 2012 and December 2013 in two camps for internally displaced people, one nearby hospital, and four surrounding villages near Laiza City, Kachin State, Myanmar, along the China-Myanmar border (97.56°E, 24.75°N) (20). The camps were established in 2010 as a consequence of internal military conflict. Populations of the two camps were approximately 12,000 in December 2013 and consisted mostly of ethnic Kachin people. The four villages had approximately 1,100 residents. The regional hospital had a larger catchment area of nearly 100,000 people from surrounding smaller villages. In this subtropical region, malaria is hypoendemic, with an annual parasite incidence of ∼57 per 1,000 people/year. Malaria transmission is seasonal, peaking in June to September, coincident with the rainiest months (20).
Patient enrollment.
Patients 1 to 77 years of age who presented with fever or history of fever within the last 48 h and symptoms suggestive of malaria at four clinics were recruited into the study. Screening of patients for malaria infection was done by light microscopy of Giemsa-stained thin and thick blood smears. The smears were first read by field microscopists at the clinics and were further examined at a nearby field laboratory by two experienced microscopists. Any discrepancies were reevaluated to obtain a final consensus of the diagnoses. For parasite enumeration, the smears were examined by the two microscopists, who counted the number of parasites per 200 white blood cells (WBCs). The average number of parasites per 200 WBCs was used to estimate the parasite density (number of parasites/μl of blood), assuming 8,000 WBCs/μl of blood. Patients who were severely underweight (children), pregnant (verbally affirmed), or with underlying diseases were excluded. Qualified patients with P. vivax monoinfection and who provided informed consent/assent were enrolled in the study. The study protocol was approved by the Institutional Review Boards of Kunming Medical University, Kunming, Yunnan Province, China, and the Department of Health of Kachin, Myanmar.
Treatment and follow-up.
P. vivax treatment followed the locally prescribed regimen for acute vivax malaria, which included 3 daily doses of CQ at a total dose of 20 mg of base/kg of body weight, assuming an average 60-kg body weight for an adult (12 mg base/kg on day 0 and 6 mg/kg on day 1 and day 2). Members of the research team supervised each administration of CQ. Patient subjects also received an 8-day PQ regimen (0.375 mg/kg/day) that commenced at the same time as the first CQ dose. For proper drug dosage in children, the CQ and PQ tablets were split into four quarters. A dosing chart was used to indicate the dosing regimen, corresponding to body weights from 5 to 50 kg with increments of 2.5 kg. Children were weighed, and drugs were given based on the most closely matched body weight in the chart. Screening for glucose-6-phosphate dehydrogenase deficiency was not conducted, which was standard practice in the area. Subjects were cautioned on the risk of PQ toxicity and advised to stop PQ upon having dark urine. CQ and PQ were manufactured by Shanghai Zhongxi Pharmaceutical Co. Ltd. (Shanghai, China). Participants were asked to return for follow-up evaluations on days 1, 2, 3, 7, 14, 28, and 42. They were also encouraged to come back to the clinics for a check should they feel sick at any time during the follow-up period. During each visit, thin and thick blood smears were made for microscopic examinations, while filter paper blood spots were prepared for molecular genotyping of the parasites.
Parasite genotyping.
For recurrent malaria cases within the 42-day follow-up period, we genotyped the day 0 and recurrent parasites by using the polymorphic P. vivax merozoite protein 3α (pvmsp3α) gene, following a previously published PCR-RFLP (restriction fragment length polymorphism) method (21, 22). Briefly, parasite DNA was isolated from the filter paper blood by using a QIAamp DNA microkit (Qiagen, Germany) following the manufacturer's instructions. The pvmsp3α gene was amplified by nested PCR, and the PCR product was separated by electrophoresis on a 0.8% agarose gel. For RFLP analysis, the PCR product was digested with either HhaI or AluI, and the digested product was analyzed by electrophoresis on a 1.8% agarose gel. Alleles were distinguished based on the size of the PCR product and restriction banding patterns.
Data analysis.
Statistical analysis was performed using SPSS version 16.0 (SPSS, Chicago, IL). Kaplan-Meier survival analysis was done to evaluate the outcome of the treatment of patients during the follow-up period. To accurately summarize the estimated risk of treatment failure as well as the risk of gametocyte carriage, we used standard life table calculations to estimate the cumulative incidence of recurrent/persistent parasitemia, corrected for dropouts (23). This methodology adjusts risk estimate denominators by interval-specific losses to follow-up that occur in a study.
RESULTS
Patient enrollment.
During the 22-month enrollment period, 594 febrile patients with P. vivax single infection diagnosed by microscopic examination of blood smears and fulfilling the inclusion criteria were recruited into the study. Of these patients, seven were excluded from analysis: one developed P. falciparum malaria on day 2 and six had P. vivax gametocytemia without asexual parasites (Fig. 1). Table 1 shows the demographic information and clinical features of the patients. Men and women were enrolled in the study approximately equally. The median age was 9 years (ranging from 1 to 77 years), and 76.49% (449/587) were children of 15 years and below. On examination and screening, 82.96% (487/587) of the patients had fever with an axillary temperature above 37.5°C. The geometric mean of parasite density was 1,793, 2,026, and 1,158 asexual forms/μl for the three age groups of <5 years, 5 to 15 years, and >15 years, respectively (Table 1). At the time of admission, 67.63% (397/587) of patients had detectable gametocytemia, with a geometric mean gametocyte density of 44 gametocytes/μl of blood.
FIG 1.
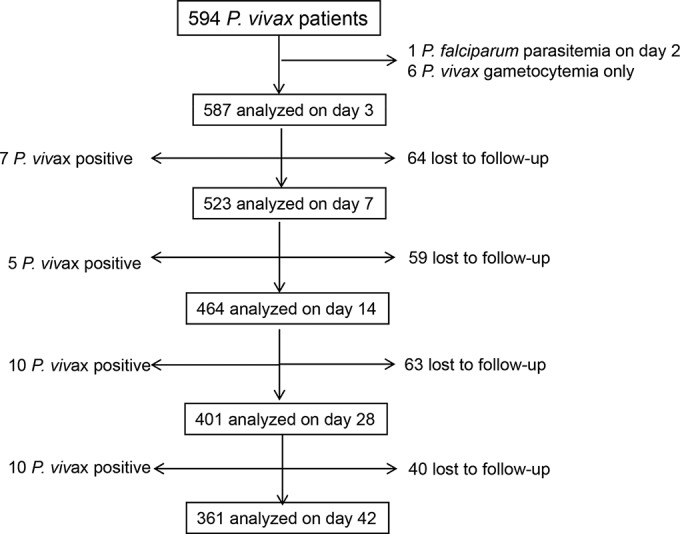
Flow chart of the chloroquine-primaquine efficacy study of P. vivax patients during 2012 to 2013, northeast Myanmar.
TABLE 1.
Demographic and clinical features of the P. vivax patients at the time of enrollment (day zero)
| Patient feature | Value |
|---|---|
| Total no. of patients (% male) | 587 (53.15) |
| Median age (yrs) (range) | 9 (1–77) |
| % patients feverish on day zero (axillary temp >37.5°C) | 82.96 (487/587) |
| Mean temp (°C) (range) | 38.6 (35.5–41.7) |
| Asexual parasite density on day zero (geometric mean [range]) in age group | |
| <5 yrs (n = 64) | 1,793/μl (60–40,000) |
| 5–15 yrs (n = 385) | 2,026/μl (20–54,600) |
| >15 yrs (n = 138) | 1,158/μl (40–32,280) |
| % patients presenting with gametocytes on day zero | 67.63 (397/587) |
| Gametocyte density on day zero (geometric mean [range]) | 44/μl (0–2,940) |
Parasitological responses.
The regimen of the CQ-PQ combination proved effective for treating the majority of P. vivax malaria cases. After initiation of the treatment, P. vivax parasitemia decreased rapidly during the first 3 days, with 99.5% (584/587) of patients who received this treatment showing cleared asexual blood parasitemia (Fig. 2). With regard to the early therapeutic response, 31.34% (184/587), 2.73% (16/587), and 0.51% (3/587) patients remained positive for asexual parasitemia on days 1, 2 and 3, respectively. All patients were free of malaria symptoms on day 3 after the treatment. Of the 587 patients enrolled, 401 and 361 patients finished the 28-day and 42-day follow-up periods, respectively (Fig. 1). Among the three patients who had parasitemia on day 3, a 7-year-old boy remained parasitemic on day 7, but he responded well to a repeated standard regimen of CQ and thereafter remained aparasitemic for the rest of the 42-day follow-up. Six other patients who had cleared parasitemia on both day 2 and 3 developed recurrent parasitemia on day 7 (Fig. 1). In total, between day 4 and 28, 22 subjects had P. vivax asexual parasitemia (1 persistent and 21 recurrent): 7 positives by day 7, 5 more positives between days 7 and 14, and 10 positives between days 14 and 28. Based on the WHO definition (limited to 28-day follow-up), the 22 recurrences between days 7 and 28 were considered late parasitological failure (LPF). The cumulative incidence of treatment failure at days 7, 14, and 28 was 1.43%, 2.56% and 5.20%, respectively (Table 2). Between days 28 and 42, there were an additional 10 cases that showed recurrent P. vivax parasitemia, and the accumulative incidence of recurrent blood-stage infection reached 7.98%. All recurrent cases received a repeated standard regimen of CQ, and all were responsive. In addition, since an overwhelming majority of patients were children, children under 15 years old were more likely to have recurrent vivax parasitemia between days 14 and 42 (odds ratio of 2.04; 95% confidence interval, 0.85 to− 4.31), although this increase was not statistically significant (P = 0.08873, Fisher's exact test).
FIG 2.
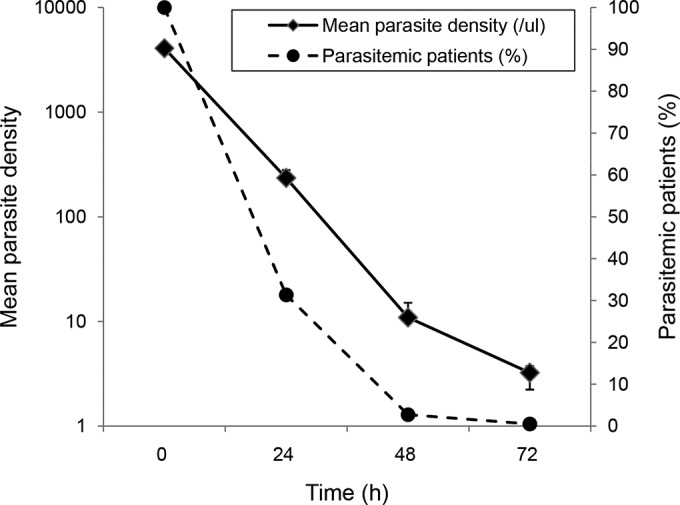
Mean P. vivax parasite density for 587 infected patients and the percentage of patients who remained parasitemic, tested before and after chloroquine-primaquine treatment. The negative blood smears were entered as 2.5 parasites/μl of blood. The y axis for mean parasite density is on a log scale. The error bars indicate standard errors of the geometric means.
TABLE 2.
Life table for cumulative incidence of recurrent parasitemia in P. vivax patients treated with chloroquine-primaquine
| Day | Na | No. P. vivax positive (failure) | LFUb | IRc | CIRPd (95% CI) |
|---|---|---|---|---|---|
| 0 | 587 | 0 | 0 | 0 | 0 |
| 3 | 587 | 0 | 0 | 0 | 0 |
| 7 | 523 | 7 | 64 | 0.0143 | 1.43 (0.41–2.44) |
| 14 | 464 | 5 | 59 | 0.0115 | 2.56 (1.13–4.00) |
| 28 | 401 | 10 | 63 | 0.0271 | 5.20 (3.04–7.36) |
| 42 | 361 | 10 | 40 | 0.0293 | 7.98 (5.20–10.76) |
The number of patients remaining at risk on the indicated day post-study enrollment.
LFU, number of patients lost to follow-up
IR, interval risk, calculated as i[N − (LFU/2)]−1.
CIRPn, the cumulative incidence of recurrent parasitemia, calculated as follows: 1 − {[1 − IRn] × [1 − CIRP(n − 1)]}, where n is the day of the test and n − 1 is the prior interval.
Following treatment with CQ and PQ, gametocyte clearance was also rapid. Of the initial 397 patients with gametocytemia at enrollment, 85.14% (338/397) showed gametocytemia cleared by day 1, whereas 9.47% (18/190) patients without gametocytemia on admission developed gametocytemia on day 1. On day 2, 7 patients remained gametocytemic, whereas 3 without gametocytemia on admission developed gametocytemia. Only 1 individual did not clear gametocytemia by day 3. Between day 7 and day 42, a total of 15 (53.57%) participants had patent gametocytemia, and interestingly, all had concurrent asexual-stage recurrence. The day 28 and day 42 cumulative risk of gametocyte carriage was 2.21% (95% confidence interval [CI], 0.78% to 3.64%) and 3.93% (95% CI, 1.94% to 5.92%).
Genotyping of P. vivax cases with recurrent parasitemia.
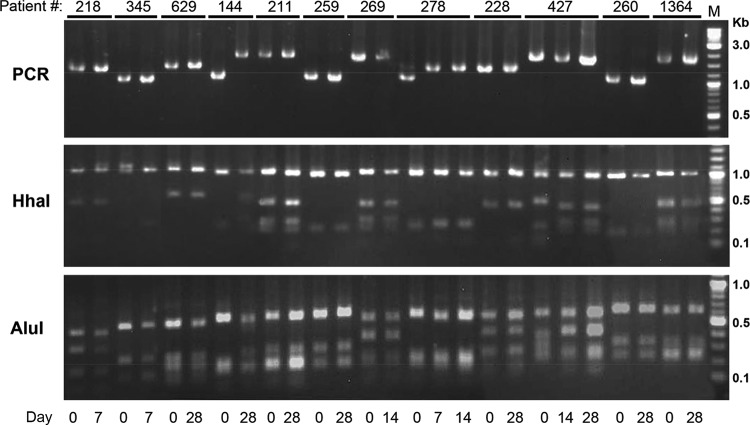
There were 22 patients considered to have LPF, with recurrent parasitemias between days 7 and 28, among samples from 16 patients that were available on day 0 and the day of recurrent parasitemia. Genotyping of the pvmsp3α gene in paired samples was successful for samples from 12 patients. PCR and restriction digestions revealed at least 8 banding patterns. For 10 patients, the genotypes on day 0 and the day of recurrence were the same, suggesting that these recurrent parasitemias could have been due to recrudescence (Fig. 3). However, it is noteworthy that genotyping of pvmsp3α alone would show an ∼12% risk of reinfection occurring with the same genotype. The genotypes for patient 144 at day 0 and day 28 were different. For patient 278, the parasites on days 7 and 14 had the same genotype, but this was different from that on day 0. Since relapses may be of heterologous or homologous genotypes relative to the primary parasitemia, these analyses do not prove a failure to clear asexual blood stages, but in most cases they provide evidence that is at least consistent with that explanation.
FIG 3.
Genotyping of the parasite isolates from patients with recurrent parasitemias within 28 days after chloroquine-primaquine treatment. P. vivax parasites at day zero and the day of parasite recurrence (day 7, 14, and 28) were genotyped by PCR amplification of the pvmsp3α locus and by restriction digestion with either HhaI or AluI of the PCR products.
DISCUSSION
This study estimated the frequency of therapeutic failure with CQ-PQ in northeastern Myanmar to be about 6% and 8% at days 28 and 42, respectively. The estimated efficacy of CQ-PQ at day 28 against the asexual parasites responsible for acute vivax malaria was marginal at 94.80% (95% CI, 92.64% to 96.97%). This is worrying with regard to CQR per se, because use of the 8-day PQ regimen in the current study very likely resulted in augmented killing of asexual blood forms (24). Since PQ is also active against P. vivax blood-stage infections (25) and it synergizes the activity of CQ against CQR P. falciparum (26), PQ may be synergistic with CQ for the treatment of vivax malaria. In fact, in Indonesia, where CQR P. vivax is prevalent, the combination of PQ with CQ dramatically improved the therapeutic efficacy for CQR P. vivax parasites (13, 27, 28). In other words, if our subjects had consumed CQ only, the rates of failure due to CQR would very likely have been higher, perhaps by a wide margin (28). One caveat of this study is that we did not measure the blood CQ level in patients at the time of recurrent parasitemia, leaving us unable to conclude whether these cases were true CQR. Therefore, future studies are needed to confirm the existence of CQR P. vivax in this region and determine its prevalence.
Most recent studies conducted in the GMS have suggested a declining trend of P. vivax sensitivity to CQ. Clinical observations in Thailand in the past showed either complete sensitivity (29–32) or only small proportions of patients developing recurrent parasitemia within 28 days (33, 34). Similarly, in the neighboring region of China's Yunnan Province, longitudinal monitoring suggested that CQ efficacy remained relatively unchanged, with 1.5% (9/603) patients experiencing late clinical failure (35). However, a study conducted in 2007 to 2008 in western Thailand reported a clinical failure rate of more than 8% within 28 days of CQ treatment (36). Recently, one case report from the Thai-Myanmar border thoroughly documented CQR P. vivax (37). In Myanmar, sporadic CQR P. vivax cases were reported more than 20 years ago (17, 18) and also, more recently, in Dawei District, south of our study site (19). Compared with the 34% treatment failures observed at Dawei, our studies estimated a much lower frequency (∼5%), but of course we also applied PQ whereas the other investigators did not. Six years ago, Liang et al. performed a treatment efficacy study in the same place as our study site; in that study, 70 P. vivax-infected patients received a total dosage of either 1,200 or 1,500 mg of CQ base without PQ (38). Follow-up to day 28 showed that both treatment regimens achieved a 100% cure rate. Therefore, compared to that study, our data suggest degrading sensitivity of P. vivax strains to CQ in northeastern Myanmar. This may be related to the influx of refugees that occurred in the interim.
At our study site, we noticed a significant portion (76.63%) of the patients were children 15 years old and younger (20). Consequently, children were more likely to have recurrent vivax parasitemia during the follow-up. The occurrence of CQR P. vivax mostly in children has been observed in other areas where vivax malaria is endemic, such as Indonesia, Peru, and Thailand (36, 39–41). The reasons for such a bias are not completely clear but may be related to underdeveloped immunity in young children. Nevertheless, the increased malaria burden among children requires that they be acknowledged as a vulnerable group and that they be specifically targeted in control efforts.
One reason that P. vivax is more difficult to eliminate is gametocyte carriage by infected individuals prior to the manifestation of clinical symptoms, which enables transmission before drug treatment. Consistently, 67.63% patients in our study had detectable gametocytemia at the time of enrollment. Since P. vivax sexual forms are susceptible to all blood schizontocides (42), gametocyte clearance was rapid, and only one patient remained gametocytemic by day 3. However, for patients with recurrent asexual parasitemia between day 7 and day 42, 53.57% had patent gametocytemia. The propensity of gametocyte carriage during recurrence is consistent with findings from earlier clinical trials (43). Thus, it is important that therapies for vivax malaria effectively clear both the blood and liver stages, so as to prevent recurrences (recrudescence and relapse), as well as the associated gametocytes.
Of the 361 patients who completed the 42-day follow-up, 10 patients developed recurrent P. vivax parasitemia between days 28 and 42. Since CQ levels in the blood of patients beyond a month of the start of treatment normally fall below the minimum effective concentration (100 ng/ml) against sensitive P. vivax parasites, the recurrent P. vivax parasitemia occurring in this period could have resulted from relapse or reinfection of a CQ-sensitive or -resistant strain. Although genotyping based on pvmsp3α provided some evidence suggesting recrudescence or relapse of parasites, genotying of pvmsp3α alone could not rule out reinfection with the same genotype. In fact, it is difficult or impossible to judge from genotyping whether these parasitemias were due to new infections or recrudescence and relapse, since P. vivax relapses can often result from activation of heterologous hypnozoites in the liver (44). Further studies are urgently needed to investigate whether the regimen of PQ applied in Myanmar is also failing for radical cure of vivax malaria.
In conclusion, this work has documented a degraded efficacy of CQ-PQ for treating vivax malaria at the China-Myanmar border area, and this is likely a consequence of worsening parasite resistance to CQ. These findings bear upon strategies for the elimination of malaria by most nations of the GMS and China. CQ is in many respects the ideal antimalarial: inexpensive, widely available, and safely tolerated by almost everyone. Stepping away from CQ in favor of other therapies, like ACTs, would come with greater costs, complexity, clinical risks, and contraindications (45). Doing so will require firm evidence of therapeutic inadequacy. Evidence from studies like ours reported here may inform crucial and difficult decisions in the coming decades and potentially lead to the elimination of malaria from the GMS.
ACKNOWLEDGMENTS
This study was supported by NIAID, NIH (U19AI089672). Z.Y. was supported by the National Science Foundation of China (U1202226, 81161120421, and 31260508) and the Ministry of Education of China (20125317110001). L.C. was also supported by Yunnan Provincial Funds (2013HA026). J.K.B. is supported by the Wellcome Trust (grant B9RJIXO).
We acknowledge the data collection by staff from the local hospital and malaria clinics, slide reading by microscopists at our field station, and Jianhua Duan for overall supervision of the case follow-up activities.
REFERENCES
- 1.Gething PW, Elyazar IR, Moyes CL, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HF, Price RN, Mueller I, Baird JK, Hay SI. 2012. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis 6:e1814. doi: 10.1371/journal.pntd.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. 2004. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis 4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. 2007. Vivax malaria: neglected and not benign. Am J Trop Med Hyg 77(6 Suppl):79–87. [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, Gueye CS, Fullman N, Gosling RD, Feachem RG. 2013. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet 382:900–911. doi: 10.1016/S0140-6736(13)60310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feachem RG, Phillips AA, Hwang J, Cotter C, Wielgosz B, Greenwood BM, Sabot O, Rodriguez MH, Abeyasinghe RR, Ghebreyesus TA, Snow RW. 2010. Shrinking the malaria map: progress and prospects. Lancet 376:1566–1578. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baird JK. 2009. Resistance to therapies for infection by Plasmodium vivax. Clin Microbiol Rev 22:508–534. doi: 10.1128/CMR.00008-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baird JK. 2011. Resistance to chloroquine unhinges vivax malaria therapeutics. Antimicrob Agents Chemother 55:1827–1830. doi: 10.1128/AAC.01296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rieckmann KH, Davis DR, Hutton DC. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183–1184. [DOI] [PubMed] [Google Scholar]
- 9.Schuurkamp GJ, Spicer PE, Kereu RK, Bulungol PK, Rieckmann KH. 1992. Chloroquine-resistant Plasmodium vivax in Papua New Guinea. Trans R Soc Trop Med Hyg 86:121–122. doi: 10.1016/0035-9203(92)90531-G. [DOI] [PubMed] [Google Scholar]
- 10.Baird JK, Basri H, Purnomo Bangs MJ, Subianto B, Patchen LC, Hoffman SL. 1991. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am J Trop Med Hyg 44:547–552. [DOI] [PubMed] [Google Scholar]
- 11.Collignon P. 1991. Chloroquine resistance in Plasmodium vivax. J Infect Dis 164:222–223. doi: 10.1093/infdis/164.1.222. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz IK, Lackritz EM, Patchen LC. 1991. Chloroquine-resistant Plasmodium vivax from Indonesia. N Engl J Med 324:927. doi: 10.1056/NEJM199103283241317. [DOI] [PubMed] [Google Scholar]
- 13.Price RN, von Seidlein L, Valecha N, Nosten F, Baird JK, White NJ. 2014. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis 14:982–991. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price RN, Auburn S, Marfurt J, Cheng Q. 2012. Phenotypic and genotypic characterisation of drug-resistant Plasmodium vivax. Trends Parasitol 28:522–529. doi: 10.1016/j.pt.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, Fan Q, Fang Q, Jongwutiwes S, Parker D, Sirichaisinthop J, Kyaw MP, Su XZ, Yang H, Yang Z, Wang B, Xu J, Zheng B, Zhong D, Zhou G. 2012. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Trop 121:227–239. doi: 10.1016/j.actatropica.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delacollette C, D'Souza C, Christophel E, Thimasarn K, Abdur R, Bell D, Dai TC, Gopinath D, Lu S, Mendoza R, Ortega L, Rastogi R, Tantinimitkul C, Ehrenberg J. 2009. Malaria trends and challenges in the Greater Mekong Subregion. Southeast Asian J Trop Med Public Health 40:674–691. [PubMed] [Google Scholar]
- 17.Myat-Phone-Kyawm, Myint-Oo, Myint-Lwin, Thaw-Zin, Kyin-Hla-Aye, Nwe-Nwe-Yin. 1993. Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma). Trans R Soc Trop Med Hyg 87:687. doi: 10.1016/0035-9203(93)90294-Z. [DOI] [PubMed] [Google Scholar]
- 18.Marlar-Than, Myat-Phone-Kyaw, Aye-Yu-Soe, Khaing-Khaing-Gyi, Ma-Sabai, Myint-Oo. 1995. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans R Soc Trop Med Hyg 89:307–308. doi: 10.1016/0035-9203(95)90556-1. [DOI] [PubMed] [Google Scholar]
- 19.Guthmann JP, Pittet A, Lesage A, Imwong M, Lindegardh N, Min Lwin M, Zaw T, Annerberg A, de Radigues X, Nosten F. 2008. Plasmodium vivax resistance to chloroquine in Dawei, southern Myanmar. Trop Med Int Health 13:91–98. doi: 10.1111/j.1365-3156.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- 20.Li N, Parker DM, Yang Z, Fan Q, Zhou G, Ai G, Duan J, Lee MC, Yan G, Matthews SA, Cui L, Wang Y. 2013. Risk factors associated with slide positivity among febrile patients in a conflict zone of north-eastern Myanmar along the China-Myanmar border. Malar J 12:361. doi: 10.1186/1475-2875-12-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruce MC, Galinski MR, Barnwell JW, Snounou G, Day KP. 1999. Polymorphism at the merozoite surface protein-3α locus of Plasmodium vivax: global and local diversity. Am J Trop Med Hyg 61:518–525. [DOI] [PubMed] [Google Scholar]
- 22.Cui L, Mascorro CN, Fan Q, Rzomp KA, Khuntirat B, Zhou G, Chen H, Yan G, Sattabongkot J. 2003. Genetic diversity and multiple infections of Plasmodium vivax malaria in western Thailand. Am J Trop Med Hyg 68:613–619. [DOI] [PubMed] [Google Scholar]
- 23.Baird JK, Wiady I, Fryauff DJ, Sutanihardja MA, Leksana B, Widjaya H, Kysdarmanto Subianto B. 1997. In vivo resistance to chloroquine by Plasmodium vivax and Plasmodium falciparum at Nabire, Irian Jaya, Indonesia. Am J Trop Med Hyg 56:627–631. [DOI] [PubMed] [Google Scholar]
- 24.Pukrittayakamee S, Imwong M, Chotivanich K, Singhasivanon P, Day NP, White NJ. 2010. A comparison of two short-course primaquine regimens for the treatment and radical cure of Plasmodium vivax malaria in Thailand. Am J Trop Med Hyg 82:542–547. doi: 10.4269/ajtmh.2010.09-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pukrittayakamee S, Vanijanonta S, Chantra A, Clemens R, White NJ. 1994. Blood stage antimalarial efficacy of primaquine in Plasmodium vivax malaria. J Infect Dis 169:932–935. doi: 10.1093/infdis/169.4.932. [DOI] [PubMed] [Google Scholar]
- 26.Bray PG, Deed S, Fox E, Kalkanidis M, Mungthin M, Deady LW, Tilley L. 2005. Primaquine synergises the activity of chloroquine against chloroquine-resistant P. falciparum. Biochem Pharmacol 70:1158–1166. doi: 10.1016/j.bcp.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Baird JK, Wiady I, Sutanihardja A, Suradi Purnomo Basri H, Sekartuti Ayomi E, Fryauff DJ, Hoffman SL. 2002. Short report: therapeutic efficacy of chloroquine combined with primaquine against Plasmodium falciparum in northeastern Papua, Indonesia. Am J Trop Med Hyg 66:659–660. [DOI] [PubMed] [Google Scholar]
- 28.Baird JK, Basri H, Subianto B, Fryauff DJ, McElroy PD, Leksana B, Richie TL, Masbar S, Wignall FS, Hoffman SL. 1995. Treatment of chloroquine-resistant Plasmodium vivax with chloroquine and primaquine or halofantrine. J Infect Dis 171:1678–1682. doi: 10.1093/infdis/171.6.1678. [DOI] [PubMed] [Google Scholar]
- 29.Luxemburger C, van Vugt M, Jonathan S, McGready R, Looareesuwan S, White NJ, Nosten F. 1999. Treatment of vivax malaria on the western border of Thailand. Trans R Soc Trop Med Hyg 93:433–438. doi: 10.1016/S0035-9203(99)90149-9. [DOI] [PubMed] [Google Scholar]
- 30.Pukrittayakamee S, Chantra A, Simpson JA, Vanijanonta S, Clemens R, Looareesuwan S, White NJ. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob Agents Chemother 44:1680–1685. doi: 10.1128/AAC.44.6.1680-1685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tasanor O, Ruengweerayut R, Sirichaisinthop J, Congpuong K, Wernsdorfer WH, Na-Bangchang K. 2006. Clinical-parasitological response and in-vitro sensitivity of Plasmodium vivax to chloroquine and quinine on the western border of Thailand. Trans R Soc Trop Med Hyg 100:410–418. doi: 10.1016/j.trstmh.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Muhamad P, Ruengweerayut R, Chacharoenkul W, Rungsihirunrat K, Na-Bangchang K. 2011. Monitoring of clinical efficacy and in vitro sensitivity of Plasmodium vivax to chloroquine in area along Thai Myanmar border during 2009-2010. Malar J 10:44. doi: 10.1186/1475-2875-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vijaykadga S, Rojanawatsirivej C, Congpoung K, Wilairatana P, Satimai W, Uaekowitchai C, Pumborplub B, Sittimongkol S, Pinyorattanachote A, Prigchoo P. 2004. Assessment of therapeutic efficacy of chloroquine for vivax malaria in Thailand. Southeast Asian J Trop Med Public Health 35:566–569. [PubMed] [Google Scholar]
- 34.Congpoung K, Satimai W, Sujariyakul A, Intanakom S, Harnpitakpong W, Pranuth Y, Cholpol S, Bualombai P. 2011. In vivo sensitivity monitoring of chloroquine for the treatment of uncomplicated vivax malaria in four bordered provinces of Thailand during 2009-2010. J Vector Borne Dis 48:190–196. [PubMed] [Google Scholar]
- 35.Liu H, Yang HL, Tang LH, Li XL, Huang F, Wang JZ, Li CF, Wang HY, Nie RH, Guo XR, Lin YX, Li M, Xu JW. 2014. Monitoring Plasmodium vivax chloroquine sensitivity along China-Myanmar border of Yunnan Province, China during 2008-2013. Malar J 13:364. doi: 10.1186/1475-2875-13-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phyo AP, Lwin KM, Price RN, Ashley EA, Russell B, Sriprawat K, Lindegardh N, Singhasivanon P, White NJ, Nosten F. 2011. Dihydroartemisinin-piperaquine versus chloroquine in the treatment of Plasmodium vivax malaria in Thailand: a randomized controlled trial. Clin Infect Dis 53:977–984. doi: 10.1093/cid/cir631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rijken MJ, Boel ME, Russell B, Imwong M, Leimanis ML, Phyo AP, Muehlenbachs A, Lindegardh N, McGready R, Renia L, Snounou G, Singhasivanon P, Nosten F. 2011. Chloroquine resistant vivax malaria in a pregnant woman on the western border of Thailand. Malar J 10:113. doi: 10.1186/1475-2875-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang GL, Sun XD, Wang J, Zhang ZX. 2009. Sensitivity of Plasmodium vivax to chloroquine in Laza City, Myanmar. Chin J Parasitol Parasitic Dis 27:175–176. (In Chinese.) [PubMed] [Google Scholar]
- 39.Murphy GS, Basri H, Purnomo Andersen EM, Bangs MJ, Mount DL, Gorden J, Lal AA, Purwokusumo AR, Harjosuwarno S, et al. 1993. Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet 341:96–100. doi: 10.1016/0140-6736(93)92568-E. [DOI] [PubMed] [Google Scholar]
- 40.Ruebush TK II, Zegarra J, Cairo J, Andersen EM, Green M, Pillai DR, Marquino W, Huilca M, Arevalo E, Garcia C, Solary L, Kain KC. 2003. Chloroquine-resistant Plasmodium vivax malaria in Peru. Am J Trop Med Hyg 69:548–552. [PubMed] [Google Scholar]
- 41.Graf PC, Durand S, Alvarez Antonio C, Montalvan C, Galves Montoya M, Green MD, Santolalla ML, Salas C, Lucas C, Bacon DJ, Fryauff DJ. 2012. Failure of supervised chloroquine and primaquine regimen for the treatment of Plasmodium vivax in the Peruvian Amazon. Malar Res Treat 2012:936067. doi: 10.1155/2012/936067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pukrittayakamee S, Imwong M, Singhasivanon P, Stepniewska K, Day NJ, White NJ. 2008. Effects of different antimalarial drugs on gametocyte carriage in P. vivax malaria. Am J Trop Med Hyg 79:378–384. [PubMed] [Google Scholar]
- 43.Douglas NM, Simpson JA, Phyo AP, Siswantoro H, Hasugian AR, Kenangalem E, Poespoprodjo JR, Singhasivanon P, Anstey NM, White NJ, Tjitra E, Nosten F, Price RN. 2013. Gametocyte dynamics and the role of drugs in reducing the transmission potential of Plasmodium vivax. J Infect Dis 208:801–812. doi: 10.1093/infdis/jit261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imwong M, Snounou G, Pukrittayakamee S, Tanomsing N, Kim JR, Nandy A, Guthmann JP, Nosten F, Carlton J, Looareesuwan S, Nair S, Sudimack D, Day NP, Anderson TJ, White NJ. 2007. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis 195:927–933. doi: 10.1086/512241. [DOI] [PubMed] [Google Scholar]
- 45.Douglas NM, Anstey NM, Angus BJ, Nosten F, Price RN. 2010. Artemisinin combination therapy for vivax malaria. Lancet Infect Dis 10:405–416. doi: 10.1016/S1473-3099(10)70079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]