Abstract
This study evaluated the safety, tolerability, and pharmacokinetics of a posaconazole i.v. (intravenous) solution. This was a single-center, 2-part, randomized, rising single- and multiple-dose study in healthy adults. In part 1, subjects received 0 (vehicle), 50, 100, 200, 250, or 300 mg posaconazole in a single dose i.v. by 30-min peripheral infusion (6 cohorts of 12 subjects each [9 active and 3 placebo], making a total of 72 subjects). Blood samples were collected until 168 h postdose. In part 2, subjects were to receive 2 peripheral infusions at a 12-h interval on day 1 followed by once-daily infusion for 9 days. However, part 2 was terminated early because of high rates of infusion site reactions with multiple dosing at the same infusion site. The pharmacokinetics results for part 1 (n = 45 subjects) showed that the mean posaconazole exposure (area under the concentration-time curve from time zero to infinity [AUC0–∞]) ranged from 4,890 to 46,400 ng · h/ml (range of coefficient of variation values, 26 to 50). The dose-proportionality slope estimate (90% confidence interval) for AUC0–∞ was 1.30 (1.19 to 1.41), indicating a greater-than-dose-proportional increase. The data for safety in part 1 show that 29/72 subjects had ≥1 adverse event. Infusion site reactions were reported in 2/9 vehicle subjects, 0/18 placebo subjects, and 7/45 i.v. posaconazole subjects. The data for safety in part 2 show that infusion site reactions were reported in 1/4 (25%) placebo subjects, 3/9 (33%) vehicle control subjects, and 4/5 (80%) i.v. posaconazole (100 mg) subjects (3 posaconazole recipients subsequently developed thrombophlebitis and were discontinued from treatment). In conclusion, the posaconazole i.v. solution showed a greater-than-dose-proportional increase in exposure, primarily at doses below 200 mg. When administered peripherally at the same infusion site, multiple dosing of i.v. posaconazole led to unacceptably high rates of infusion site reactions. Intravenous posaconazole was otherwise well tolerated. Single doses of i.v. posaconazole were tolerated when given through a peripheral vein over 30 min.
INTRODUCTION
Posaconazole (Noxafil) formulated as an oral suspension is a systemic triazole antifungal approved in more than 80 countries for use as therapy for refractory invasive fungal infection (IFI), prophylaxis of IFI in patients at high risk, and therapy for oropharyngeal candidiasis (1–5). The precise approved indications differ across the various countries in which posaconazole is available (6, 7). To enhance its gastric absorption, posaconazole oral suspension must be taken multiple times per day with a meal, a nutritional supplement, or an acidic carbonated beverage (8, 9). Although a new tablet formulation of posaconazole with improved absorption characteristics has been developed (10) and was recently approved in the United States and Europe, a limitation of any oral formulation is that patients at risk for IFI may be unable to take any formulation through the oral route because of vomiting, prohibition of enteral medications, or inability to swallow as a result of severe chemotherapy-induced mucositis (11, 12). In these patients, an intravenous (i.v.) formulation may be particularly useful.
The posaconazole i.v. formulation was developed as an aqueous solution containing the solubilizer sulfobutyl ether beta-cyclodextrin. The first study to evaluate the pharmacokinetics (PK), safety, and tolerability of i.v. posaconazole was terminated early because of a high incidence of postinfusion reactions when the drug was infused through a peripheral line over 90 min in healthy volunteers (6). Because local intolerability was likely caused by irritation associated with the low pH of the 90-min peripheral infusate, decreasing the infusion time may attenuate some of these effects.
This study evaluated the PK and safety of posaconazole i.v. solution administered through the peripheral route in healthy subjects. The primary objective of the study was to evaluate the safety and tolerability of posaconazole i.v. solution as a single dose and as multiple doses when administered by peripheral infusion to healthy volunteers over 30 min.
(Portions of these data were previously presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, 10 to 13 September 2013.)
MATERIALS AND METHODS
Study design.
This was a single-center, 2-part, randomized, placebo-controlled, third-party blind, rising single- and multiple-dose study. The study was conducted in accordance with the principles of Good Clinical Practice, and written informed consent was obtained from each subject before any study-related procedures. The study was conducted at a single site in the Netherlands from September 2010 to May 2011.
Cannulation for administering the drug was performed by authorized staff in a sufficiently large vein in the forearm (vena cephalica or vena basilica) or elbow (vena mediana cubiti) using a 20- or 22-gauge cannula (Biovalve PUR polyurethane cannula; Vygon, Ecouen, France). Cannulation on each vein was performed once for each subject, and a transparent dressing was used to fixate the cannula to allow monitoring for thrombophlebitis. To stimulate peripheral blood flow, the room temperature was kept at approximately 22°C during the infusion, which was completed within exactly 30 min. Within 1 or 2 min after infusion, the infusion site was flushed with 20 ml of 5% dextrose in water, followed by a few milliliters of heparin at 1 U/ml. The catheter used for study drug administration remained in the vein for 24 h after infusion. The timing of assessments was adjusted relative to the end of each infusion.
In part 1, healthy adult volunteers were randomly assigned to 1 of 6 cohorts, with 12 subjects in each cohort (9 active and 3 placebo [5% dextrose]). Each subject received a single dose of i.v. posaconazole or placebo, administered by 30-min peripheral infusion. The cohorts were as follows: cohort 1, 0 mg posaconazole i.v. solution (cyclodextrin vehicle control only); cohort 2, 50 mg posaconazole i.v. solution, cohort 3, 100 mg posaconazole i.v. solution; cohort 4, 200 mg posaconazole i.v. solution; cohort 5, 250 mg posaconazole i.v. solution; and cohort 6, 300 mg posaconazole i.v. solution.
In part 2, subjects were randomly assigned to 1 of 4 cohorts with 12 subjects in each cohort (9 active and 3 placebo [5% dextrose]). Each subject was to receive 2 peripheral infusions 12 h apart on day 1, followed by once-daily infusions for 9 days. The intended doses and cohorts were as follows: cohort 7, 0 mg posaconazole i.v. solution (vehicle control); cohort 8, 100 mg posaconazole i.v. solution; cohort 9, 200 mg posaconazole i.v. solution; and cohort 10, 300 mg posaconazole i.v. solution. However, because of unacceptably high rates of thrombophlebitis after multiple i.v. infusions of 100 mg posaconazole (cohort 8), the study was terminated. Subjects were dosed in only 2 cohorts in part 2 before the study was terminated. These were cohort 7 (0 mg posaconazole i.v. solution [cyclodextrin vehicle control only]) and cohort 8 (100 mg posaconazole i.v. solution). The study was terminated before the completion of cohort 8.
Subjects.
Subjects were healthy men and women aged 18 to 65 years with a body mass index range from 19 to 35 kg/m2 inclusive. Subjects were free of any clinically significant disease and had laboratory test results that were within normal limits or were acceptable to the investigator. Vital sign measurements had to be within normal limits. QTc intervals were ≤430 ms (male subjects) and ≤450 ms (female subjects). Subjects had to have veins suitable for cannulation and no previous episodes of thrombophlebitis. Female subjects had to be postmenopausal or surgically sterile, or they had to use contraception for 2 months before and throughout the study. Male subjects had to agree to use contraception during and for 1 month after the study. The subjects could not have had any fungal infection (including onychomycosis) within 3 months of the study. Subjects were excluded if they had any surgical or medical condition that might significantly alter the absorption, distribution, metabolism, or excretion of any drug. Subjects were excluded if they had ever taken posaconazole, if they had taken over-the-counter medicine within 7 days or prescription medicine within 14 days of the study, or if they smoked more than 10 cigarettes (or equivalent tobacco use) per day, drank more than 21 units of alcohol per week, or consumed 5 units of caffeine per day. Subjects could not have a history of current or past drug or alcohol abuse or mental instability and could not have donated blood within the past 90 days.
Blood collection for assessment of posaconazole PK parameters.
Blood samples for PK evaluation of posaconazole in plasma were collected for each cohort at the following times: 0 h (predose) and 0.5, 1, 1.5, 2, 3, 4, 6, 12, 24, 48, 72, 120, and 168 h after the initiation of infusion on day 1 (PK samples were not collected from cohort 1 or cohort 7 [vehicle control subjects]).
PK parameters were calculated using WinNonlin Enterprise (Mountain View, CA) version 5.2.1. Individual posaconazole plasma concentration data per dose group and actual sampling times were used to determine values for the following parameters after single-dose administration: maximum observed plasma drug concentration (Cmax), time to Cmax (tmax), area under the concentration-time curve from time zero to the time of the last measurable sample (AUC0–last), AUC from time zero to infinity (AUC0–∞), terminal-phase (elimination) rate constant (λz), terminal-phase half-life (t1/2), total body clearance (CL), and volume of distribution during the terminal phase (Vz).
Approximately 4 ml of blood was collected per time point into tubes containing EDTA; samples were kept on ice or were refrigerated until centrifugation (within 2 h of collection) for 10 min at 1,500 × g at approximately 4°C. Plasma samples (duplicate sets) were immediately frozen to at least −20°C and were maintained in the frozen state until analyzed. Plasma samples were assayed for posaconazole using a validated detection method in which liquid chromatography is coupled to tandem mass spectrometry (range of detection, 5 to 5,000 ng/ml) (13).
Safety.
Safety monitoring included adverse event (AE) reporting, clinical laboratory tests, vital sign measurements, 12-lead electrocardiograms, ejection fraction measurements from echocardiography, and physical examinations. Infusion site reactions were closely monitored, and a semiquantitative scoring scale was used to grade the severity of the infusion site reactions. A definition of phlebitis was established on a point system using the following local complications: tenderness >4 cm from insertion site, 1 point; warmth, 1 point; erythema 3 to 6 cm from site, 1 point; erythema more than 6 cm from site, 2 points; and induration or swelling, 2 points. Phlebitis was defined as 3 or more points on a modified version of the scoring scale (14).
RESULTS
Disposition and demographics.
Seventy-two subjects were enrolled in 1 of 6 single-dose cohorts in part 1 (n = 12 per cohort); all 72 subjects completed the study.
Eighteen subjects were enrolled in 1 of 2 multiple-dose cohorts (cohorts 7 and 8) in part 2. Twelve subjects were enrolled in cohort 7 (vehicle group [cyclodextrin only]), including 9 subjects receiving vehicle control and 3 subjects receiving placebo; 10 subjects completed the study. Six subjects were enrolled in cohort 8, including 5 subjects receiving 100 mg posaconazole i.v. and 1 subject receiving placebo; none completed the study. All 8 subjects who did not complete part 2 (2 placebo subjects in cohort 7 and 6 subjects in cohort 8) were discontinued prematurely either because of an AE (4 subjects) or because of early study termination (4 subjects).
In part 1, 54% of the subjects were female, 94% were white, and none were Hispanic or Latino; the age range was 18 to 56 years. In part 2, 28% of the subjects were female, 94% were white, and <1% were Hispanic or Latino; the age range was 19 to 64 years.
PK evaluations.
PK analyses were conducted only in part 1 because of the early termination of part 2.
All 45 subjects in part 1 who received posaconazole were included in the PK analyses. After single-dose administration of 50 to 300 mg i.v. posaconazole, the mean exposure (AUC0–∞) ranged from 4,890 to 46,400 ng · h/ml (percent coefficient of variation [%CV] range, 26 to 50), and the Cmax plasma concentration ranged from 313 to 2,840 ng/ml (%CV range, 26 to 30) (Table 1).
TABLE 1.
Posaconazole pharmacokinetic parameters after single-dose i.v. administration in healthy volunteers
| Cohort | Posaconazole dose (mg) | No. of subjects | Arithmetic mean (%CV) or value as indicated fora: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| λz (h) | t1/2 (h) | tmax (h) [median (range)] | Cmax (ng/ml) | AUC0–last (ng · h/ml) | AUC0–∞ (ng · h/ml) | AUC% | Vz (liters) | CL (liters/h) | |||
| 2 | 50 | 9 | 0.0410 (34) | 18.7 (34) | 0.6 (0.5–0.7) | 313 (30) | 4,620 (31) | 4,890 (30) | 5.95 (62) | 294 (39) | 10.9 (25) |
| 3 | 100 | 9 | 0.0360 (14) | 19.6 (16) | 0.5 (0.5–0.5) | 1,330 (27) | 10,800 (27) | 11,200 (26) | 4.47 (47) | 262 (22) | 9.40 (23) |
| 4 | 200 | 9 | 0.0307 (23) | 23.6 (23) | 0.5 (0.5–24)b | 2,250 (29) | 34,600 (52) | 35,400 (50) | 2.75 (97) | 226 (38) | 6.54 (32) |
| 5 | 250 | 9 | 0.0279 (21) | 26.0 (23) | 0.5 (0.5–0.5) | 2,260 (26) | 40,600 (39) | 41,500 (41) | 1.84 (85) | 245 (33) | 6.68 (29) |
| 6 | 300 | 9 | 0.0292 (20) | 24.6 (20) | 0.5 (0.5–1.0) | 2,840 (30) | 45,500 (26) | 46,400 (26) | 1.74 (46) | 236 (17) | 6.90 (27) |
AUC0–∞, area under the concentration-time curve from time zero to infinity; AUC0–last, AUC from time zero to the time of the last measurable sample; AUC%, percentage of AUC0–∞ that is extrapolated; CL, total body clearance; Cmax, maximum observed plasma drug concentration; %CV, percent coefficient of variation; i.v., intravenous; tmax, time to Cmax; t1/2, terminal-phase half-life; λz, terminal-phase (elimination) rate constant; Vz, volume of distribution during the terminal phase.
One subject had a high concentration at the 24-h sample; therefore, tmax is 24 h for that subject.
Figure 1 shows the log-linear arithmetic mean posaconazole plasma concentration-time profiles after single-dose administration of 50- to 300-mg posaconazole i.v. solutions. The dose proportionality slope estimates (90% confidence interval) for Cmax, AUC0–last, and AUC0–∞ were 1.16 (1.02 to 1.30), 1.33 (1.22 to 1.44), and 1.30 (1.19 to 1.41), respectively, indicating a more-than-dose-proportional increase (Table 2). Visual inspection shows that this more-than-dose-proportional increase occurs primarily at doses below 200 mg rather than at doses from 200 to 300 mg (Fig. 2).
FIG 1.
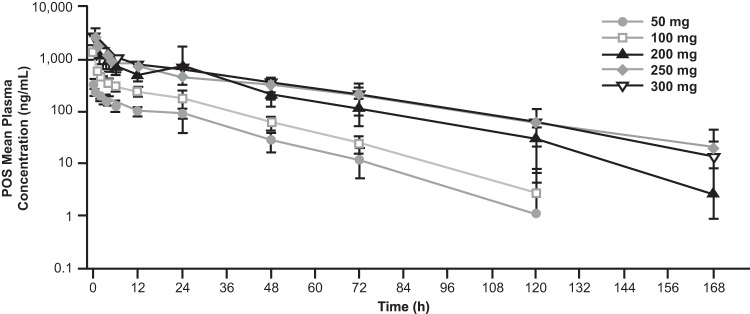
Arithmetic mean (±standard deviation) posaconazole (POS) plasma concentration-time profiles per dose after single-dose administration of 50 to 300 mg of posaconazole i.v. (intravenous) solution (log-linear scale).
TABLE 2.
Dose-proportionality assessment of posaconazole after i.v. administration of a single doseb
| Parameter | Slope | 90% CI |
Estimated fold increase (vs 6-fold dose increase)a | 90% CI |
||
|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||
| Cmax | 1.159 | 1.018 | 1.300 | 7.979 | 6.197 | 10.27 |
| AUC0–last | 1.328 | 1.215 | 1.441 | 10.8 | 8.824 | 13.21 |
| AUC0–∞ | 1.302 | 1.191 | 1.414 | 10.32 | 8.452 | 12.59 |
The dose range was 50 to 300 mg, i.e., a 6-fold increase from lowest to highest dose.
AUC0–∞, area under the concentration-time curve from time zero to infinity; AUC0–last, AUC from time zero to the time of the last measurable sample; CI, confidence interval; Cmax, maximum observed plasma drug concentration.
FIG 2.
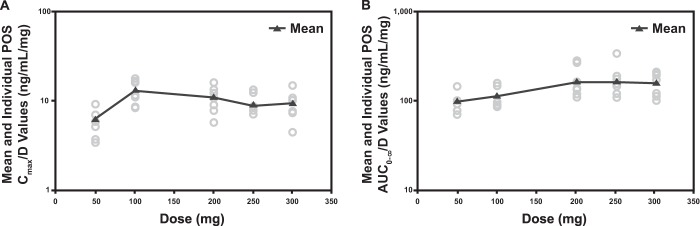
Individual (circles) and mean (triangles) Cmax (A) and AUC0–∞ (B) dose-adjusted values by dose of posaconazole after single-dose i.v. administration in healthy volunteers. AUC0–∞, area under the concentration-time curve from time zero to infinity; Cmax, maximum observed plasma drug concentration; POS, posaconazole; D, drug.
Safety. (i) Part 1: single-dose administration.
In part 1 (n = 72 subjects), peripheral posaconazole i.v. administration in single doses up to 300 mg infused over 30 min was generally safe and well tolerated by healthy adults. All AEs were mild to moderate, and there was no apparent increase in treatment-related AEs with the 250-mg or 300-mg doses compared with the rates of AEs with the lower doses. Overall, 29 of 72 subjects (40%) had ≥1 AE (placebo, 6/18 [33%]; vehicle, 5/9 [56%]; 50 mg posaconazole, 4/9 [44%]; 100 mg posaconazole, 3/9 [33%]; 200 mg posaconazole, 4/9 [44%]; 250 mg posaconazole, 3/9 [33%]; and 300 mg posaconazole, 4/9 [44%]). Seven (7/18 [39%]) subjects receiving a single dose of 250 mg or 300 mg posaconazole i.v. reported a treatment-emergent AE. Infusion site reactions after the 50-mg (n = 1), 200-mg (n = 1), 250-mg (n = 2), and 300-mg (n = 3) doses were reported in 2 of 9 subjects receiving vehicle (cyclodextrin) only (22%), 0 of 18 subjects receiving placebo, and 7 of 45 subjects receiving posaconazole (16%). Two of these subjects had thrombophlebitis (1 each in the groups receiving 200 mg and 300 mg posoconazole) that was considered probably related to the study drug. All infusion site reactions were mild or moderate in severity and resolved within 1 week of treatment. Frequently reported symptoms in patients with infusion site reactions included tenderness, erythema, pain, induration/swelling, and warmth. A summary of thrombophlebitis and infusion site reactions during part 1 (single dose) is shown in Table 3. Apart from infusion site reactions, including thrombophlebitis, the most frequent AEs were nasopharyngitis (n = 5 [7%]), headache (n = 4 [6%]), nausea (n = 3 [4%]), and catheter site pain (n = 3 [4%]). Most AEs were of mild severity. There were no serious AEs or AEs that resulted in treatment discontinuation in part 1.
TABLE 3.
Number of subjects with thrombophlebitis and infusion site reactions after single-dose i.v. administration of placebo, vehicle, or posaconazole in part 1
| Type of adverse event | No. (%) of subjects with thrombophlebitis/infusion site reactions who received: |
Total (n = 72) | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 18) | Posaconazole (mg) |
|||||||
| 0a (n = 9) | 50 (n = 9) | 100 (n = 9) | 200 (n = 9) | 250 (n = 9) | 300 (n = 9) | |||
| Thrombophlebitisb | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (11) | 0 (0) | 1 (11) | 2 (3) |
| Infusion site reactions | 0 (0) | 2 (22) | 1 (11) | 0 (0) | 1 (11) | 2 (22) | 3 (33) | 9 (13) |
Subjects received vehicle (cyclodextrin) only.
In both subjects, thrombophlebitis was preceded by infusion site reactions that started on day 1.
(ii) Part 2: multiple-dose administration.
In part 2 (n = 18 subjects), peripheral posaconazole i.v. infusion in multiple doses was not well tolerated; thrombophlebitis was reported in 5 of 18 subjects (28%), as follows: 1 of 4 subjects (25%) who received placebo, 1 of 9 subjects (33%) who received vehicle only, and 3 of 5 subjects (60%) who received 100 mg of the posaconazole i.v. solution. In 4 of the 5 subjects, thrombophlebitis was preceded by infusion site reactions that started on day 1 or 2. Four of the 5 subjects had thrombophlebitis of moderate intensity (vehicle, n = 1, and 100 mg i.v. posaconazole, n = 3), and 1 had thrombophlebitis of mild intensity (placebo, n = 1). All instances were considered drug related, and all subjects were discontinued from treatment (thrombophlebitis, n = 4, and administrative reasons, n = 1). One subject was treated with acetaminophen (vehicle-only group) and one with flucloxacillin (due to fever associated with thrombophlebitis; 100 mg i.v. posaconazole group); all subjects recovered between 5 and 17 days after the start of treatment.
Overall, 7 subjects (39%) reported infusion site reactions; 3/9 subjects (33%) received vehicle only, and 4/5 subjects (80%) received 100 mg posaconazole i.v. These infusion site reactions started between days 1 and 4 and were commonly associated with tenderness, pain, and erythema. As described above, 4 of the 7 subjects subsequently developed thrombophlebitis (total thrombophlebitis score, >2), on days 2, 3, 4, and 9 (Table 4). The high incidence of thrombophlebitis and infusion site reactions led to the decision to discontinue part 2 (the multiple-dose portion) of the study before the full enrollment of cohort 8.
TABLE 4.
Number of subjects with thrombophlebitis and infusion site reactions after multiple-dose i.v. administration of placebo, vehicle, or posaconazole in part 2
| Type of adverse event | No. (%) of subjects with thrombophlebitis/infusion site reactions who received: |
Total (n = 18) | ||
|---|---|---|---|---|
| Placebo (n = 4) | Posaconazole (mg) |
|||
| 0a (n = 9) | 50 (n = 5) | |||
| Thrombophlebitisb | 1 (25) | 1 (11) | 3 (60) | 5 (28) |
| Infusion site reactions | 0 (0) | 3 (33) | 4 (80) | 7 (39) |
Subjects received vehicle (cyclodextrin) only.
In 4 of 5 subjects, thrombophlebitis was preceded by infusion site reactions that started on day 1 or 2.
In addition to infusion site reactions, the most frequently reported AE was headache (5 subjects [28%]). Apart from infusion site reactions, no significant AEs in either part of the study and no decreases in ejection fraction or liver function test abnormalities that were of clinical concern were reported. No clinically significant changes in laboratory values, vital signs, or 12-lead electrocardiography findings occurred.
DISCUSSION
A new i.v. formulation of posaconazole has been developed to ensure adequate exposure in patients at high risk for IFI who cannot tolerate or absorb oral medication. The aim of this study was to evaluate the PK and safety of the posaconazole i.v. solution by peripheral administration and the safety and tolerability of posaconazole i.v. solution after single and multiple doses by peripheral administration in healthy volunteers. Intravenous posaconazole is now approved for the prophylaxis of invasive Aspergillus and Candida infections, administered over approximately 90 min via a central venous catheter at a dose of 300 mg twice a day on day 1 and once daily thereafter until recovery from neutropenia or immunosuppression (administration of a single dose via a peripheral vein is also acceptable in advance of central venous line placement).
Single-dose administration of 50 to 300 mg posaconazole i.v. resulted in a mean Cmax range of 313 to 2,840 ng/ml and a mean exposure (AUC0–∞) range of 4,890 to 46,400 ng · h/ml, which covers the range of exposures known to be effective for prophylaxis and treatment of IFIs. The dose-proportionality slope estimates (90% confidence interval) were 1.16 (1.02 to 1.30) for Cmax and 1.30 (1.19 to 1.41) for AUC0–∞, indicating a more-than-dose-proportional increase over the 6-fold increase in dose. This more-than-dose-proportional increase appears to occur primarily at doses below 200 mg rather than at the more clinically relevant doses of 200 to 300 mg.
Peripheral posaconazole i.v. administration in single doses up to 300 mg infused over 30 min was generally safe and well tolerated in healthy adults in this study. Infusion site reactions were reported in 7 (16%) subjects receiving a single dose of posaconazole i.v. Otherwise, the incidence of AEs was similar at all single doses evaluated, and there appeared to be no increase in infusion site reactions compared with the rate in subjects receiving vehicle alone. Given that the rates of infusion site reactions at the 250-mg (2/9) and 300-mg (3/9) doses were comparable to those in patients receiving placebo (2/9), it is not believed that rates of infusion site reactions increase with dose but, rather, that any suggested increase (3 versus 2) reflects random variability due to the low numbers of subjects rather than an actual effect. In contrast, in an earlier study that used peripheral infusion of posaconazole i.v. solution over 90 min, there was an unacceptably high rate of infusion site reactions (data on file at Merck & Co., Inc., as CSR P04985). The results of the present study show that single-dose i.v. posaconazole can be administered peripherally over 30 min with careful infusion site monitoring.
Multiple doses of peripheral posaconazole i.v. infusion were not well tolerated; infusion site reactions were reported in 25% of placebo subjects, 33% of vehicle-only subjects, and 80% of subjects treated with 100 mg posaconazole i.v. solution. Thrombophlebitis was reported in 25% of placebo subjects, 11% of vehicle-only subjects, and 60% of subjects administered 100 mg posaconazole i.v. solution. In these subjects, the maximum grades according to the grading scale of Soifer et al. (14) ranged from 3 to 6. These infusion site reactions led to early study termination. Apart from infusion site reactions, no significant AEs were reported in either the single- or the multiple-dose parts of this study, and no new safety concerns arose.
Decreasing the infusion time from 90 to 30 min led to reduced rates of infusion site reactions and thrombophlebitis when posaconazole was administered as a single-dose i.v. infusion but not when it was administered as a multiple-dose i.v. infusion. Although thrombophlebitis still occurred after single-dose peripheral administration of posaconazole i.v. solution over 30 min, the thrombophlebitis rate was sufficiently low and was considered acceptable with careful clinical monitoring of local infusion site reactions/thrombophlebitis. This result is relevant when treatment is needed in advance of central venous line placement or to bridge the period during which a central venous line is replaced or in use for other i.v. treatments. In the multiple-dose part of this study, however, the results show that decreasing the time of infusion still led to an unacceptably high rate of thrombophlebitis. Therefore, multiple-dose administration of posaconazole i.v. solution through peripheral administration from the same i.v. site is considered not feasible with the current i.v. formulation. When multiple dosing is required, infusion should be performed by way of a central line.
Summary and conclusions.
Posaconazole i.v. solution showed a more-than-dose-proportional increase in exposure, though this occurred primarily at doses below 200 mg rather than at the clinically relevant doses of 200 to 300 mg. When administered peripherally at the same infusion site, multiple dosing of posaconazole i.v. led to unacceptably high rates of infusion site reactions. The posaconazole i.v. solution was otherwise well tolerated in healthy volunteers. Given through a peripheral vein and with careful clinical monitoring of local infusion site reactions/thrombophlebitis, single dosing of posaconazole i.v. is tolerated when administered over 30 min.
ACKNOWLEDGMENTS
This study was funded by Merck & Co., Inc., Whitehouse Station, NJ. Medical writing and editorial assistance were provided by Tim Ibbotson and David Gibson of ApotheCom, San Francisco, CA, and funded by Merck & Co., Inc., Whitehouse Station, NJ.
T.V.I., U.N., E.O., and H.W. conceived, designed, or planned the study. H.W., M.C., and M.L.P.S.V.I. analyzed the data. W.M.K., T.V.I., U.N., and H.W. collected or assembled the data. W.M.K., E.O., H.W., M.C., and M.L.P.S.V.I. interpreted the results. W.M.K. and H.W. drafted the manuscript. All authors provided substantive suggestions for revision or critically reviewed subsequent iterations of the manuscript, and all authors reviewed and approved the final version of the manuscript.
W.M.K., U.N., and M.C. are Merck employees, and M.L.P.S.V.I. was an employee of Merck at the time the study was conducted. E.O. and H.W. are Merck employees with stock options. T.V.I. has nothing to disclose.
REFERENCES
- 1.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 2.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 3.Keating GM. 2005. Posaconazole. Drugs 65:1553–1567. [DOI] [PubMed] [Google Scholar]
- 4.Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik JA, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis 44:2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 5.Raad II, Hachem RY, Herbrecht R, Graybill JR, Hare R, Corcoran G, Kontoyiannis DP. 2006. Posaconazole as salvage treatment of invasive fusariosis in patients with underlying hematologic malignancy, and other conditions. Clin Infect Dis 42:1398–1403. doi: 10.1086/503425. [DOI] [PubMed] [Google Scholar]
- 6.Merck & Co., Inc. 2014. Noxafil (posaconazole) injection, delayed release tablets, and oral suspension. Prescribing information. Merck & Co., Inc., Whitehouse Station, NJ: http://www.merck.com/product/usa/pi_circulars/n/noxafil/noxafil_pi.pdf. [Google Scholar]
- 7.Merck Sharp & Dohme Limited. 2012. Noxafil (posaconazole) oral suspension. Prescribing information; Merck Sharp & Dohme Limited, Hertfordshire, United Kingdom. [Google Scholar]
- 8.Courtney R, Wexler D, Radwanski E, Lim J, Laughlin M. 2004. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br J Clin Pharmacol 57:218–222. doi: 10.1046/j.1365-2125.2003.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishna G, Moton A, Ma L, Medlock MM, McLeod J. 2009. The pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother 53:958–966. doi: 10.1128/AAC.01034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishna G, Ma L, Martinho M, O'Mara E. 2012. Single-dose phase I study to evaluate the pharmacokinetics of posaconazole in new tablet and capsule formulations relative to oral suspension. Antimicrob Agents Chemother 56:4196–4201. doi: 10.1128/AAC.00222-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pille S, Bohmer D. 1998. Options for artificial nutrition of cancer patients. Strahlenther Onkol 174(Suppl 3):52–55. [PubMed] [Google Scholar]
- 12.Sansone-Parsons A, Krishna G, Calzetta A, Wexler D, Kantesaria B, Rosenberg MA, Saltzman MA. 2006. Effect of a nutritional supplement on posaconazole pharmacokinetics following oral administration to healthy volunteers. Antimicrob Agents Chemother 50:1881–1883. doi: 10.1128/AAC.50.5.1881-1883.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen JX, Krishna G, Hayes RN. 2007. A sensitive liquid chromatography and mass spectrometry method for the determination of posaconazole in human plasma. J Pharm Biomed Anal 43:228–236. doi: 10.1016/j.jpba.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Soifer NE, Borzak S, Edlin BR, Weinstein RA. 1998. Prevention of peripheral venous catheter complications with an intravenous therapy team, a randomized controlled trial. Arch Intern Med 158:473–477. doi: 10.1001/archinte.158.5.473. [DOI] [PubMed] [Google Scholar]


