Abstract
This study aimed to characterize the stereoselective pharmacokinetics of oral eflornithine in 25 patients with late-stage Trypanosoma brucei gambiense sleeping sickness. A secondary aim was to determine the concentrations of l- and d-eflornithine required in plasma or cerebrospinal fluid (CSF) for an efficient eradication of the T. brucei gambiense parasites. Patients were randomly allocated to receive either 100 (group I, n = 12) or 125 (group II, n = 13) mg/kg of body weight of drug every 6 h for 14 days. The concentrations of l- and d-eflornithine in the plasma and CSF samples were measured using a stereospecific liquid chromatographic method. Nonlinear mixed-effects modeling was used to characterize the plasma pharmacokinetics. The plasma concentrations of l-eflornithine were on average 52% (95% confidence interval [CI], 51, 54%; n = 321) of the d-enantiomer concentrations. The typical oral clearances of l- and d-eflornithine were 17.4 (95% CI, 15.5, 19.3) and 8.23 (95% CI, 7.36, 9.10) liters/h, respectively. These differences were likely due to stereoselective intestinal absorption. The distributions of eflornithine enantiomers to the CSF were not stereoselective. A correlation was found between the probability of cure and plasma drug exposure, although it was not more pronounced for the l-enantiomer than for that of total eflornithine. This study may explain why oral treatment for late-stage human African trypanosomiasis (HAT) patients with racemic eflornithine has previously failed; the more potent l-enantiomer is present at much lower concentrations in both plasma and CSF than those of the d-enantiomer. Eflornithine stereoselective pharmacokinetics needs to be considered if an oral dosage regimen is to be explored further.
INTRODUCTION
Human African trypanosomiasis (HAT), also known as sleeping sickness, is a parasitic disease transmitted by the tsetse fly and has a mortality rate of nearly 100% if left untreated (1, 2). The disease is prevalent in 36 sub-Saharan African countries, with 60 million people at risk of being infected (3). It has been ranked as the world's third most important parasitic disease when adjusted for life years lost (1). In 1997, an estimated 450,000 individuals were infected (4). Although this number has decreased in recent years, thanks to coordinated campaigns, it remains a neglected disease, with numerous patients not having access to treatment (5).
There are two parasitic subspecies that cause HAT, Trypanosoma brucei rhodsiense and T. brucei gambiense. The majority of the reported cases (>90%) are caused by the T. brucei gambiense subspecies (6). The disease is divided into two stages, an early hemolymphatic stage and a late encephalitic stage (1). For the late stage, there are few treatment options, i.e., melarsoprol and eflornithine. Eflornithine is administered either as monotherapy or in combination with orally administered nifurtimox (1, 2, 7). The arsenical drug melarsoprol, administered intravenously, causes severe side effects and shows signs of resistance (8, 9). As a result, eflornithine, which is effective mainly against the T. brucei gambiense subspecies, is more frequently recommended as a first-line treatment (2, 10–11). The main problem with eflornithine is the complicated mode of administration (intravenous infusion of 100 to 150 mg/kg of body weight every 6 h [QID] for 10 to 14 days), which leaves numerous patients untreated (11, 12). Although the introduction of nifurtimox-eflornithine combination treatment (NECT) has allowed the eflornithine dosage regimen to be reduced to intravenous infusions of 200 mg/kg of body weight every 12 h (BID) for 7 days while retaining efficacy, this dosage regimen still requires specialized health facilities and personnel. An oral eflornithine-based treatment would greatly reduce the burden on health care personnel and allow more patients to have access to treatment. However, previous attempts to develop an oral monotherapy treatment with eflornithine have failed (12).
Eflornithine elicits its trypanostatic effect on parasites manifested in both the hemolymphatic and central nervous system (CNS) (13). In the late stage of HAT infection, eflornithine has been proposed to elicit its main effect in the CNS (14). In addition, since eflornithine is a trypanostatic drug, it is rather slow acting and therefore needs to be present at sufficient levels in plasma or CNS for a certain time period. Eflornithine (α-difluoromethylornithine [DFMO]) is a 50:50 racemic mixture of its l- and d-enantiomers. It has been shown that the enantiomers display different potencies in vitro, with the l-enantiomer having up to a 20-fold-higher affinity for the target enzyme ornithine decarboxylase (15). The l-enantiomer also appears to be more potent in cultured T. brucei gambiense parasites (R. Brun, Swiss Tropical Institute, personal communication). Consequently, measuring exposure to the active enantiomer becomes important when investigating the possibilities of an oral-based treatment.
There are currently no peer-reviewed published studies with human data on the stereoselective pharmacokinetics of eflornithine; only a few published studies exist with data in rats after single oral and intravenous administration (16, 17). No difference in clearance was found between the enantiomers, but the l-enantiomer displayed a relatively lower bioavailability (30%) than that of the d-enantiomer (59%). Based on the results of the deconvolution of oral plasma concentration-time profiles, enantiomeric ratios in feces, and in vitro permeability measurements in Caco-2 cells, this difference was suggested to be due to a mechanism occurring in the gut or at the absorption site. Neither showed a stereoselective difference in bioavailability as a consequence of presystemic metabolism. Although the mechanistic explanation for the stereoselective difference in the bioavailability of eflornithine remains unidentified, these preclinical data might explain why previous attempts to develop an oral treatment have failed. In patients with HAT, it is possible that the systemic exposure of the more potent enantiomer is lower than expected.
The present investigation aimed to characterize the stereospecific pharmacokinetics of eflornithine in late-stage HAT patients receiving the oral racemic drug. In addition, the plasma and cerebrospinal fluid (CSF) concentrations of l-eflornithine or total eflornithine required for the eradication of T. brucei gambiense were investigated.
MATERIALS AND METHODS
Patients and study design.
The study was conducted during 2000 to 2002 at the Project de Recherches Cliniques sur la Trypanosomiase (PCRT), Daloa, Côte d'Ivoire. Prior to the study initiation, approval of the study protocol was obtained from the Ministry of Public Health, Côte d'Ivoire. Detailed descriptions of the study design, drug administration, clinical and parasitological assessment, and blood and CSF sampling protocol have been described (12). A total of 25 (16 males and 9 nonpregnant nonlactating females) late-stage T. brucei gambiense patients age 18 to 69 years and weighing 43 to 63 kg were included in the study. None were unconscious, critically ill, or had any condition that necessitated immediate and concomitant treatment, and none had a chronic medical illness. No patient had a history of trypanosomicidal pretreatment for the infection episode at the time of the study. A summary of the demographic, clinical, and laboratory data according to treatment group is presented in Table 1.
TABLE 1.
Summary of patient demographics according to dose group at study initiation
| Patient characteristica | Data for group: |
|
|---|---|---|
| I | II | |
| No. of patients | 12 | 13 |
| Age (median [95% CI]) (yr) | 27.5 (23, 39) | 25 (20, 40) |
| No. of males | 6 | 10 |
| No. of females | 6 | 3 |
| Wt (median [95% CI]) (kg) | 50 (45, 52) | 55 (49, 60) |
| Ht (median [95% CI]) (cm) | 167 (161, 174) | 168 (163, 175) |
| WBC in CSF (median [95% CI]) (cells/μl) | 142.0 (98, 620) | 366 (96, 704) |
| Hemoglobin (median [95% CI]) (g/dl) | 10.7 (9.8, 11.5) | 10.3 (8.6, 13.2) |
95% CI, 95% confidence interval; WBC, white blood cells; CSF, cerebrospinal fluid.
The patients were randomly allocated to receive one of the two oral treatment regimens of racemic eflornithine as follows: group I (n = 12) received 100 mg/kg of body weight every 6 h for 14 days, and group II (n = 13) received 125 mg/kg of body weight every 6 h for 14 days. The doses from day 8 and onward were reduced from the initial calculated doses in five patients (three in group I and two in group II) due to weight loss in the patients, about 10 and 36% in four patients and one patient, respectively. Except for additional trypanosomicidal drugs (e.g., pentamidine, melarsoprol, and nifurtimox), all drugs necessary for the welfare of the patients were allowed. The signs and symptoms of trypanosomiasis were assessed during treatment, at the end of treatment (24 h after last dose), and at 1, 3, 6, and 12 months after the end of treatment. At these follow-ups, a lumbar puncture for CSF examination of T. brucei gambiense and white blood cell counts, including an examination of lymph node aspirate (if typical nodes were present) and blood for trypanosomes (using microhematocrit centrifugation and miniature anion-exchange centrifugation technique) were performed. Cure was defined as the disappearance of parasite in blood, lymph node, and CSF samples at the end of treatment, with no subsequent reappearance during the 12-month follow-up.
A total of 14 heparinized blood samples (3 ml each) were collected from each patient in each group before the first dose on day 1 and the second dose on day 10. Frequent blood samples were obtained following the last dose on day 15 at 0 (pretreatment), 10, 20, 30, and 45 min, and 1, 2, 3, 6, 9, 14, and 24 h after dosing. The CSF samples (3 ml each) were collected through a lumbar puncture before treatment and at 5 min before the second dose on day 10 and the last dose on day 15. The plasma and CSF samples were stored at −196°C in liquid nitrogen until they were transported (in dry ice) to Thammasat University, Thailand. At Thammasat University, the samples underwent nonstereoselective analysis and thereafter were stored at −196°C liquid nitrogen until they were transported (in 2008), under dry ice, to Gothenburg University, Sweden. At Gothenburg University, the samples were stored at −80°C until they were reanalyzed in 2009.
Drug analysis.
The concentrations of l- and d-eflornithine in plasma and CSF samples were determined according to the previously described stereospecific liquid chromatographic method (18). d-/l-Eflornithine (d,l-α-difluoromethylornithine) hydrochloride was provided by the WHO/Special Programme for Research and Training in Tropical Diseases (TDR) (Geneva, Switzerland). To evaluate potential racemization, the l-eflornithine and d-eflornithine enantiomers were synthesized by Lipitek International, Inc. (San Antonio, TX, USA). The mean accuracies (% error) for the l- and d-eflornithine assay in plasma at concentrations of 3, 400, and 1,000 μM were −7.3, 7.1, and 4.9% and −5.0, −6.8, and 4.0%, respectively. The corresponding values for intraday precision (% coefficient of variation [%CV]) were 7.1, 3.8, and 6.7%, and 5.4, 4.7, and 6.0%, respectively. The corresponding values for interday precision (%CV) were 8.9, 5.7, and 1.9%, and 5.3, 6.3, and 4.0%, respectively. The lower limit of quantification (LLOQ) was set to 1.5 μM. The quantification of l- and d-eflornithine in the CSF samples was identical to that described for plasma, except that artificial CSF was used for the calibration curve and quality control (QC) samples (19). Accuracy and precision were within 15% error for all concentrations (4.5, 35, and 150 μM each enantiomer) during the analytical runs. The LLOQ was set to 1.5 μM. Racemic eflornithine was previously shown to be stable for a minimum of 6 months at −80°C with no observed tendency toward degradation. Neither freeze nor thaw cycles (n = 3) degrade racemic eflornithine (20).
To investigate possible racemization, blank plasma was spiked with either l- or d-eflornithine corresponding to final concentrations of 390 and 450 μM, respectively. The prepared samples were divided into aliquots (75 μl) and stored, together with blank plasma, at either 8°C or −27°C for 3 months. The samples were then reanalyzed to investigate the stabilities of the separate enantiomers and to investigate whether the spiked enantiomer could form the other enantiomer. For the samples spiked with a separate enantiomer, the peak area corresponding to the retention time of the opposite enantiomer was integrated and compared to that of the blank plasma. The mean (standard error) peak areas (n = 3) for l-eflornithine when stored at 8°C and −27°C were 2,229 ± 50 mVs and 2,212 ± 99 mVs, respectively. The corresponding peak areas for d-eflornithine were 2,334 ± 113 mVs and 2,379 ± 77 mVs, respectively. In both the l- and d-eflornithine-spiked samples, no peak areas of the opposite enantiomer were detected in the samples stored at 8°C and −27°C, respectively. No further statistical analysis was done, as no difference in peak area was detected. Thus, eflornithine enantiomers were stable and did not show signs of racemization when stored at 8°C for 3 months.
Plasma and CSF concentrations of l- and d-eflornithine.
The sum of the determined plasma and CSF concentrations of l- and d-eflornithine were compared to the previously determined total concentrations (12) using three approaches: (i) a comparison of the average plasma concentration for each sampling time point of each enantiomer, (ii) an investigation of the number of samples that showed >30% and 15% difference, and (iii) a direct comparison of the newly determined concentrations (the sum of l- and d-enantiomer) and the previously determined total concentrations (12).
Pharmacokinetic and pharmacodynamic analysis. (i) Population pharmacokinetic modeling.
In the pharmacokinetic modeling, per-protocol doses were used (for each enantiomer, 50% of the total racemic dose in molar units). The units for dose and concentrations were molar. The plasma concentrations of l- and d-eflornithine were modeled separately by nonlinear mixed-effect modeling using NONMEM version 7.2 (Icon Development Solutions, Ellicott City, MD, USA). The pharmacokinetic data were analyzed using first-order conditional estimation with interactions (FOCE INTER) (21). The R-based program Xpose4 and PsN were used throughout the modeling procedure for handling the model files and graphics (22–24). Several structural pharmacokinetic models were evaluated during the modeling process. For drug distribution, one-, two-, and three-compartment models with linear elimination clearance were evaluated. In addition, nonlinear intercompartmental clearance and nonlinear binding to the peripheral compartment were also assessed (17, 25, 26). For the absorption process, the models with a first-order absorption constant with and without lag time were evaluated. Alternatively, the lag time was replaced by manually adding a number of transit compartments (n = 1 to 15) prior to reaching the absorption site (27, 28). In addition, models with either sequential zero- to first-order absorption or simultaneous zero- and first-order absorption, both with or without lag time, were tested. Since patients received an oral dose only, the absolute bioavailabilities of the separate enantiomers in these patients were not determined. Thus, clearance, intercompartmental clearance, and volume of distribution were apparent parameters (oral) parameters. To explore the interindividual variability of the relative bioavailability (F), this value was fixed to one. Between-subject variability of all pharmacokinetic parameters was modeled as exponential models, assuming a log-normal distribution. Random residual variability was modeled as a proportional and an additive error of the observations. Throughout the modeling process, shrinkage was monitored for between-subject and random residual variability (29). The potential covariates for plasma pharmacokinetics (dose level, body weight, age, gender, concomitant medication, and hemoglobin level) were visually explored and added later on as linear or categorical covariates. The inclusion of covariates was assessed by using the log likelihood ratio, in which the difference in the objective function value (OFV) between the full and reduced models was asymptotically chi-square distributed. A decrease in the OFV of 3.84 between two nested models (1 degree of freedom) was considered to indicate a statistically superior model (P < 0.05). Model selection was based on structural diagnostic plots, mechanistic plausibility, OFV, and the precision of the parameter estimates. The diagnostic plot visual predictive checks (VPC) were also applied (30). Throughout the modeling process, the robustness of the parameter estimate was evaluated using bootstrap analyses. The final model was used to generate a VPC plot by simulating 1,000 new data sets based on the design of the original data set. These simulations were used to compute the 95% confidence intervals (95% CI) for the 10th, 50th (median), and 90th percentiles of the concentrations at each time point. These simulations were then compared graphically with the 10th, 50th, and 90th percentiles derived from the observed data. The robustness of the plasma pharmacokinetic parameters was evaluated using a nonparametric bootstrap of 1,000 data sets.
(ii) Cerebrospinal fluid analysis.
The ratios of the CSF concentrations of l- and d-eflornithine from day 10 onwards were determined in order to assess the steady-state status of each enantiomer. The correlation between the CSF and plasma concentrations of each enantiomer was evaluated using linear regression analysis. The correlation was considered statistically significant if the 95% CI of the slope was greater than zero.
(iii) Pharmacodynamic analysis.
Binary logistic regression was applied for an assessment of the relationship between the probability of relapse and plasma or the CSF levels of l-eflornithine and total eflornithine (the sum of l- and d-eflornithine) concentrations. For plasma, the average trough concentration at steady state (Css,min), the concentration at 3 h after the last dose, and the area under the concentration-time curve in a dosing interval (AUCτ) were used. The Css,min was calculated for each patient by averaging the concentrations attained on day 10, prior to the last dose on day 15, and that at 6 h after the last dose. AUCτ was calculated from the individual estimate of model-based oral clearance (CL/F) as AUCτ = dose/CL/F, where the dose corresponds to the micromolar dose of each enantiomer at a dosing interval. For CSF, the average of the concentrations from days 10 and 14 were used. The probability of being cured [P(cure)] was modeled as a binary function of the observed l- and total eflornithine concentrations, as follows: P(cure) = e(a + b × eflornithine)/[1 + e(a + b × eflornithine)], where a is the intercept, and b is the slope of the probability changes with an increase in eflornithine concentration or exposure (AUCτ). The pharmacodynamic data were analyzed using FOCE and the LAPLACE option in NONMEM 7.2. The parameter estimates and precision for the relationship between cure and drug exposure were obtained from a nonparametric bootstrap of 1,000 data sets. The correlation was considered statistically significant between the probability of being cured and drug exposure when the 5th and 95th percentiles of the slope were greater than zero.
RESULTS
Plasma and CSF concentrations of l- and d-eflornithine.
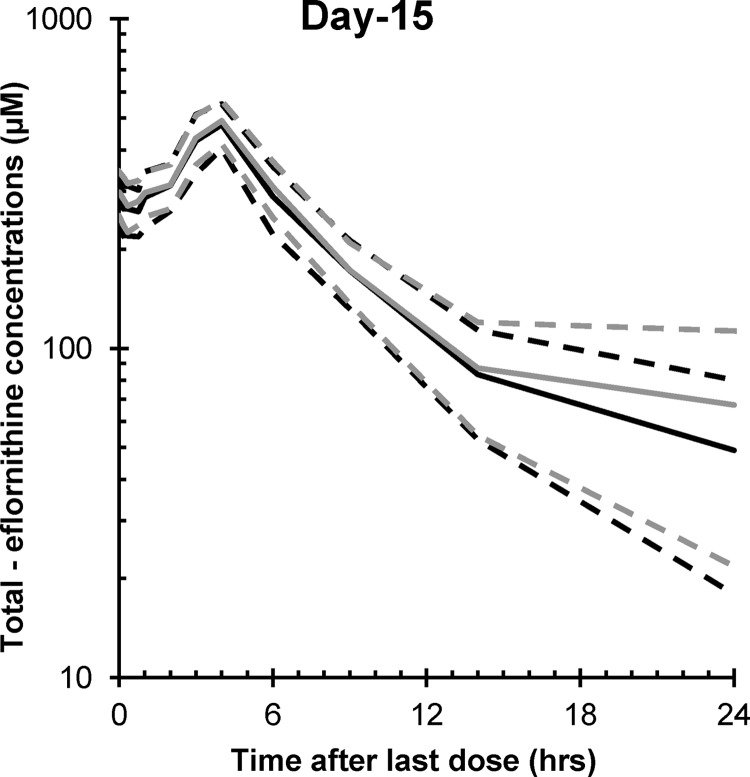
The sum of the l- and d-eflornithine [l+d] concentrations agreed well with the total [E] concentrations previously reported for the nonstereospecific analysis of eflornithine concentrations in the same group of patients (Fig. 1) (12). The mean (95% CI) [l+d]/[E] ratio in a total of 316 samples was 1.08 (0.99, 1.17). When regressing the sum of l- and d-eflornithine plasma concentrations against the total concentrations, the mean (95% CI) slope and intercept were 0.97 (0.93, 1.01) and 6.8 (−6.1, 19.6), respectively. Of the measured samples, 33 and 16% deviated from the previously reported total concentrations by >15 and 30%, respectively. This was primarily at total concentrations of <100 μM. Two individual measurements were 40% higher than the previously reported concentrations. Five samples collected at the last sampling points that were previously reported to be below the LLOQ were measurable with the enantiospecific method. For the CSF samples, the corresponding mean ratio of [l+d]/[E] was 1.12 (95% CI, 1.05, 1.19; n = 50). The mean slope and intercept for the regression equation were 0.98 (95% CI, 0.84, 1.11) and 5.03 (95% CI, −1.49, 11.5), respectively.
FIG 1.
The average plasma concentration profiles of total (d+l) eflornithine after the last dose on day 15, with oral administration of racemic eflornithine at 100 or 125 mg/kg in late-stage HAT patients. The gray lines correspond to the previously published data, where the solid line is the average and the dashed lines correspond to the 95% CI of the average concentration profile. The black lines correspond to the sum of l- and d-eflornithine, where the solid and dashed lines correspond to the average and the 95% confidence intervals, respectively.
l- and d-eflornithine plasma pharmacokinetics.
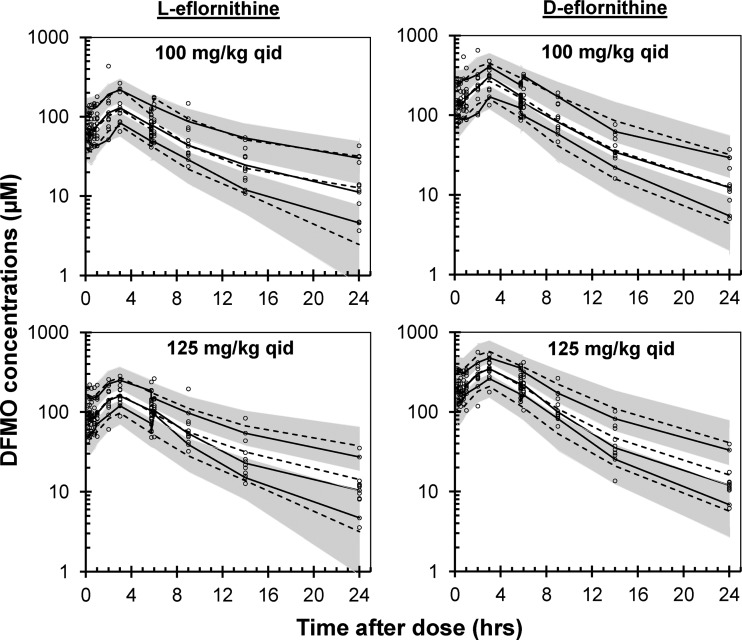
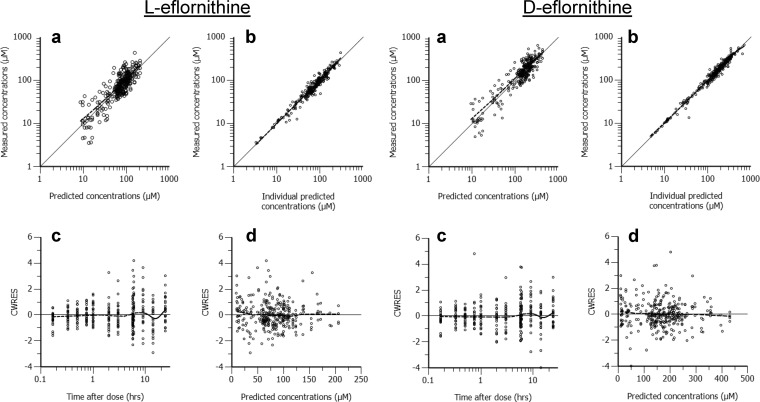
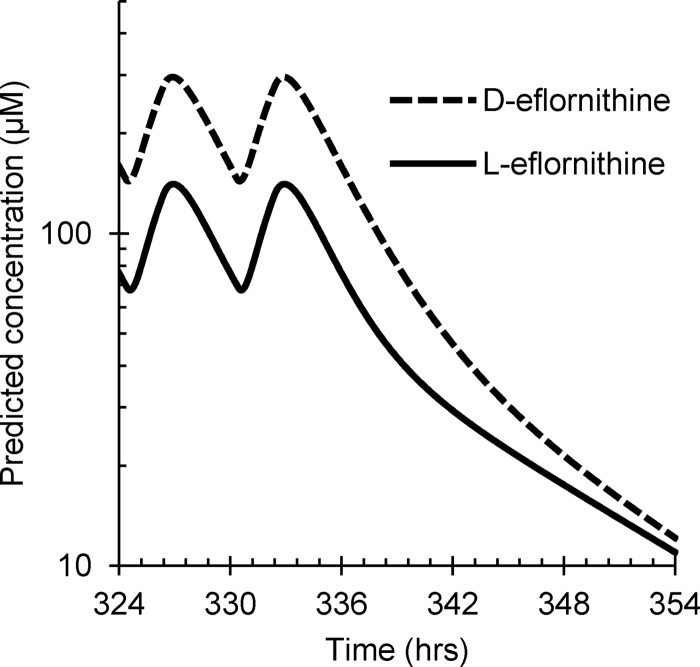
No chromatographic peak corresponding to l- or d-eflornithine was detected in the plasma samples collected from all patients prior to drug administration. The plasma concentrations of l-eflornithine were, on average, 52% (95% CI, 51, 54%; n = 321) of the d-enantiomer concentrations. The plasma pharmacokinetics of both enantiomers were best described by a two-compartment model with linear clearance and zero- to first-order absorption rate with lag time (Fig. 2, 3, and 4 and Table 2). The median simulated and observed data of each enantiomer agreed well in the two dosing groups (Fig. 2).
FIG 2.
Visual predictive check of the final l- (left) and d-eflornithine (right) model according to treatment group. The circles are the measured concentrations, and the black solid lines are the 10th, 50th, and 90th percentiles of the observed data. The dashed lines are the model-simulated 10th, 50th, and 90th percentiles. The gray shaded areas are the 95% confidence intervals around the 10th and 90th simulated percentiles.
FIG 3.
Goodness-of-fit plots for the l-eflornithine (left four graphs) and d-eflornithine (right four graphs) pharmacokinetic model. (Panels a) Measured eflornithine concentrations versus population predictions; (panels b) measured eflornithine concentrations versus individual predictions; (panels c) conditional weighted residuals (CWRES) versus time after dose; (panels d) CWRES versus population predicted concentrations. The solid line corresponds to the line of unity, and the dashed line is a nonparametric smoother (LOESS) (panels a and b, each set of graphs). Ideally, the LOESS line should fall on the line of unity. The dashed line is the LOESS and should not give a pronounced slope along the x axis (panels c and d, each set of graphs).
FIG 4.
Simulated plasma concentration-time profile of l- (solid line) and d eflornithine (dashed line) based on the population pharmacokinetic parameter estimates at a dose of 100 mg/kg of racemic eflornithine given every 6 h. Depicted are the last two doses during the study period.
TABLE 2.
Eflornithine pharmacokinetic parameter estimates of l- and d-eflornithine after oral racemic doses of 100 or 125 mg/kg QID for 14 days in patients with African trypanosomiasis
| Parametera | Original data setb |
Bootstrap data setc |
||
|---|---|---|---|---|
| Estimate (95% CI) | IIV % (RSE) | Median estimate (2.5th, 97.5th percentile) | Median IIV % (median RSE) | |
| l-Eflornithine | ||||
| CL/F (liters/h) | 17.4 (15.5, 19.3)d | 17.9 (32.1) | 17.24 (15.6, 19.3)d | 16.6 (61.4) |
| Vc/F (liters) | 55.3 (44.3, 66.3)d | 55.0 (39.2, 71.4)d | ||
| Vp/F (liters) | 64.8 (42.3, 87.3)d | 81.1 (20.0) | 66.3 (42.4, 108)d | 77.1 (40.5) |
| CLD/F (liters/h) | 8.05 (4.61, 11.5)d | 8.01 (3.31, 15.0)d | ||
| F | 1 (FIX) | 26.1 (18.8) | 1 (FIX) | 24.7 (36.0) |
| Lag time (h) | 0.433 (0.312, 0.554) | 0.447 (0.359, 0.581) | ||
| Ka (1/h) | 1.098 (0.68, 1.51) | 109 (11.0) | 1.16 (0.654, 2.27) | 108 (32.3) |
| Duration (h) | 1.96 (1.54, 2.38) | 2.00 (1.72, 2.96) | ||
| Proportional residual error (%) | 18.9 (15.7, 22.1) | 18.8 (15.5, 21.9) | ||
| d-Eflornithine | ||||
| CL/F (liters/h) | 8.23 (7.36, 9.10)d | 17.9 (33.5) | 8.21 (7.50, 8.95)d | 18.4 (53.9) |
| Vc/F (liters) | 30.6(22.9, 38.3)d | 31.6 (26.3, 37.9)d | ||
| Vp/F (liters) | 13.5 (9.95, 17.05)d | 51.4 (26.4) | 14.3 (9.99, 19.2)d | 55.1 (46.2) |
| CLD/F (liters/h) | 1.39 (0.686, 2.09)d | 1.27 (0.477, 2.28)d | ||
| F | 1 (FIX) | 28.8 (25.6) | 1 (FIX) | 28.0 (48.9) |
| Lag time (h) | 0.340 (0.202, 0.478) | 0.344 (0.184, 0.520) | ||
| Ka (1/h) | 1.39 (0.834, 1.95) | 115 (13.6) | 1.44 (0.925, 2.83) | 114 (30.0) |
| Duration (h) | 2.06 (1.86, 2.26) | 2.08 (1.88, 2.38) | ||
| Proportional residual error (%) | 15.6 (12.8, 18.4) | 15.0 (12.3, 18.3) | ||
The parameters correspond to a typical body weight of 52.4 kg. CL/F, apparent oral clearance; Vc/F, apparent central volume of distribution; Vp/F, apparent peripheral volume of distribution; CLD/F, apparent intercompartmental clearance; F, relative bioavailability, set to 1; lag time, time after when absorption starts; Ka, absorption rate constant; duration, zero-order absorption time.
CI, confidence interval; RSE, relative standard error; IIV, interindividual variability.
Bootstrap estimates correspond to the parameters by bootstrapping 1,000 data sets, of which 946 and 943 runs for l- and d-eflornithine were successful, respectively. The successful runs were used to calculate the percentiles.
Pharmacokinetic parameters that were significantly greater (95% CI not overlapping and 2.5th and 97.5th percentiles not overlapping) for l-eflornithine than for d-eflornithine.
The population-estimated absorption model parameters lag time (Tlag), zero-order drug transfer to the absorption compartment (duration), and the first-order absorption constant (Ka) were nearly identical for l- and d-eflornithine. Other pharmacokinetic parameters of the l-enantiomer were significantly (95% CIs not overlapping) greater than those of the d-enantiomer (Table 2). The shapes of the plasma concentration-time profiles were very similar for the two enantiomers, although the actual concentrations of l-eflornithine were about half of those of d-eflornithine (Fig. 4). A marked interindividual variability (IIV) was observed for Ka and the peripheral volume of distribution (Vp/F). Random residual variability was modeled as proportional to the observed values. The additive part of the residual error was set to zero, as it was not identified. The shrinkages for interindividual and random residual variability were between 7.5 and 18% for all parameters, except for IIV on CL/F for the l-enantiomer, for which shrinkage was 26%. The exclusion of the two individuals that deviated by >40% from the previously reported concentrations did not affect the population estimates, and the data were therefore retained in the population analysis. The parameter estimates after bootstrapping agreed well with the original model (Table 2). None of the covariates evaluated (dose level, body weight, age, gender, concomitant medication, and hemoglobin level) was found to be associated with the kinetic parameters (OFV < 3.84).
Cerebrospinal fluid analysis.
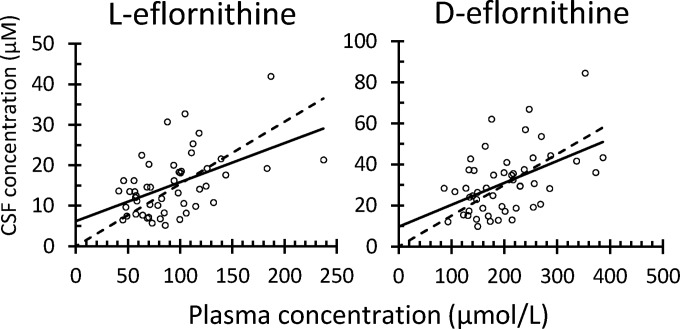
No peak corresponding to l-eflornithine was observed in the chromatograms of CSF samples obtained prior to administration of the first dose. For d-eflornithine, however, a peak was found, and this was on average 9% (n = 50; standard deviation [SD] = 8%; range = 1 to 36%; 95% CI, 7, 9%) of the measured levels on days 10 and 15. This area was therefore subtracted from the measured peaks on days 10 and 15. The CSF concentrations of the two enantiomers on days 10 and 15 were similar, suggesting the attainment of steady-state level at least from day 10 onwards. The mean (95% CI) concentration ratios between days 15 and 10 were 0.99 (0.83, 1.15) and 0.99 (0.84, 1.16) for l- and d-eflornithine, respectively. The concentrations of the l-enantiomer on days 10 and 15 were on average 49% (95% CI, 47, 50%) of the concentrations of the d-enantiomer. A significant correlation was observed between the CSF and plasma concentrations of the two enantiomers (95% CI of slope greater than zero, Fig. 5). There was no difference in the CSF-to-plasma concentration ratios between the two enantiomers.
FIG 5.
Correlation between cerebrospinal fluid (CSF) and plasma concentrations for l- (left) and d-eflornithine (right). The solid line constitutes the best line of fit allowing a fitted intercept, and the dashed line corresponds to the best line of fit using origin as an intercept. The mean slope and intercept for l-eflornithine were 0.096 (95% CI, 0.047, 0.145) and 6.22 (95% CI, 1.34, 11.1). For d-eflornithine, the mean slope and intercept were 0.107 (95% CI, 0.050, 0.163) and 9.70 (95% CI, −2.29, 21.7), respectively. The slopes for l- and d-eflornithine when using origin as an intercept were 0.15 (95% CI, 0.134, 0.174) and 0.15 (95% CI, 0.131, 0.169), respectively.
Pharmacodynamic analysis.
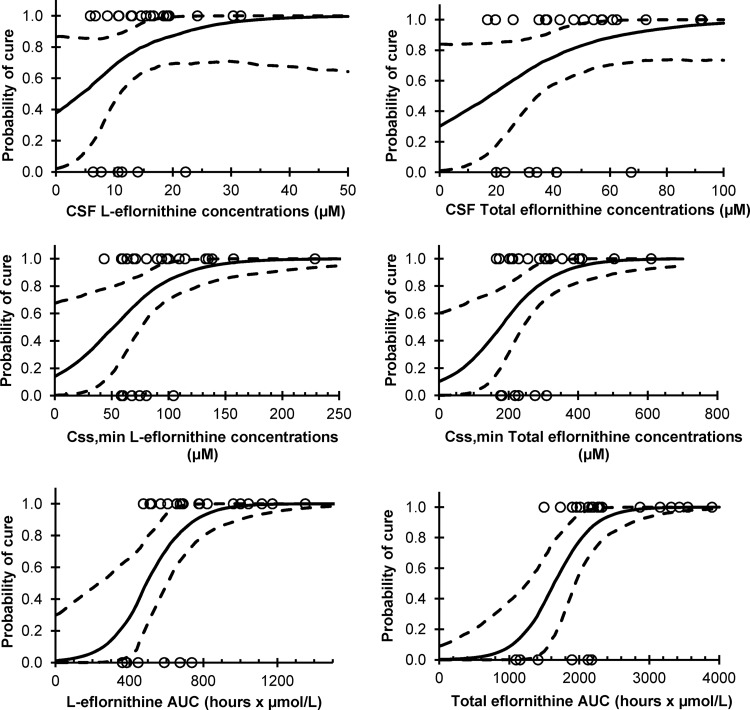
Of the 25 patients included in the study, six were reinfected within 6 months after the end of treatment. There appeared to be an association (although not statistically significant) between the probability of being cured and CSF concentrations of >23 μM l-eflornithine and 68 μM total eflornithine, respectively (Table 3 and Fig. 6, top). For plasma (Table 3 and Fig. 6, middle), Css,min was significantly associated with cure (5th and 95th percentiles greater than zero; Table 3 and Fig. 6, middle), and concentrations of >105 μM l-eflornithine and 310 μM total eflornithine cured all patients.
TABLE 3.
Parameter estimates for logistic regression model for probability of being cured versus CSF, Css,min, and model estimated plasma AUC in a 6-h dosing intervala
| Parameter by enantiomerb | Median (5th and 95th percentiles): |
|
|---|---|---|
| Slope | Intercept | |
| CSF | ||
| l-Eflornithine | 0.116 (−0.0278, 0.417) | −0.499 (−3.86, 31.5) |
| Total eflornithine | 0.0460 (−0.00448, 0.155) | −0.841 (−4.70, 1.66) |
| Css,min | ||
| l-Eflornithine | 0.0343 (0.0103, 0.0891) | −1.82 (−5.83, 0.741) |
| Total eflornithine | 0.0125 (0.00393, 0.0327) | −2.17 (−6.80, 0.423) |
| AUCτ | ||
| l-Eflornithine | 0.00865 (0.00345, 0.0272) | −4.47 (−15.2, −0.843) |
| Total eflornithine | 0.00368 (0.00178, 0.0914) | −6.07 (−16.8, −2.32) |
CSF, cerebrospinal fluid; Css,min, average trough concentration at steady state. The estimates are presented as the median and 5th and 95th percentiles based on 1,000 data sets generated by a nonparametric bootstrap of the original data set.
CSF concentrations are the average concentrations on days 10 and 15 (n = 2). Css,min is the average plasma concentration prior to the next dose or 6 h after the last dose (n = 3). AUCτ is the area under the plasma concentration-time curve in the dosing interval (τ = 6 h). The total eflornithine is the sum of the l- and d-eflornithine concentrations. The bootstrap estimates correspond to parameters by bootstrapping 1,000 data sets for each enantiomer and exposure measurement. Runs without successful conclusion (“MINIMIZATION SUCCESSFUL”) were omitted for analysis (n = 1 to 29); 979 to 999 runs were included when calculating the percentiles.
FIG 6.
Probability of cure versus l-eflornithine (left) and the sum of l- and d-eflornithine (total eflornithine, right) drug exposures. The top row depicts the probability of cure regressed against the average observed cerebrospinal fluid concentration (CSF) from days 10 and 15. The second row shows the probability of cure regressed against the average plasma concentration prior to dose administration (Css,min). The bottom row displays the probability of cure regressed against the model-estimated AUC in a dosing interval (AUCτ). The solid lines correspond to the median values, and the dashed lines correspond to the 95th and 5th percentile model fits obtained from 1,000 nonparametric bootstrap data sets.
Plasma samples taken 3 h after the last dose did not provide a clearer relationship than did Css,min (data not shown). AUCτ (Table 3 and Fig. 6, bottom) appeared to provide the best relationship to the probability of being cured; AUCτ values of 750 and 2,200 h · μmol/liter for l- and total eflornithine, respectively, were associated with a cure response in all patients.
DISCUSSION
Eflornithine and eflornithine-based treatments are commonly recommended as first-line treatments for late-stage HAT (2, 10, 11). For an efficient eradication of the Trypanosoma parasites, sufficient levels of eflornithine must be achieved in both plasma and the CNS (14, 32). In vitro data suggest that it is the l-form that is the principal active enantiomer when eflornithine enantiomers are present in equimolar amounts (15; R. Brun, Swiss Tropical Institute, personal communication). Taken together, these data suggest that the stereoselective pharmacokinetics of both enantiomers need to be addressed when evaluating the possibility of developing a simplified mode of eflornithine dosing. The present study is the first report of stereoselective pharmacokinetics of eflornithine enantiomers in patients with late-stage HAT.
It has been an issue of discussion on how different analytical methods for drug concentration data should be most appropriately compared to identify possible biases between the methods (33). In the present study, three approaches were applied, and the results showed a higher deviation between the measurements at concentrations of <100 μM. Whether this was a consequence of poor precision of the enantiospecific or the nonenantiospecific assay was not definitely concluded. However, since the results of the two methods generally agreed well for plasma and CSF, no further investigations of this were deemed necessary. For the d-eflornithine enantiomer, a peak was found in the CSF samples prior to dose administration. Although the peak was minor in relation to the measured d-eflornithine concentrations (on average, 9%), it suggests that should this bioanalytical method be used for future studies, the endogenous peak should be identified and CSF samples should be taken prior to the administration of eflornithine.
The pharmacokinetics of l- and d-eflornithine were modeled using a zero- to first-order absorption rate with lag time and a two-compartment model with linear clearance. Based on this absorption model, the results suggest that the absorption rate expressed in absolute amounts of eflornithine enantiomers increased up to approximately 2 h after dosing and thereafter displayed a monoexponential decline. This absorption/input profile is very similar to that previously observed in rats following single doses and the deconvolution of oral plasma concentration profiles of both enantiomers (17). Physiologically, this might be a consequence of drug transport along the gastrointestinal tract, although these types of mechanisms are usually described with a first-order process (27, 34). The zero- to first-order absorption model can also be parameterized, so that the zero-order rate is in proportion to amount of the administered drug (amount per unit time) that would result in a dose dependency of time to reach maximum plasma concentrations. In a rat after single oral dose, there was a dose dependency in the time to reach maximum plasma concentrations when covering a wide dose range (40 to 3,000 mg/kg of body weight of racemic eflornithine) (17). In the present study, with a relatively narrow dose range (100 to 125 mg/kg of body weight QID), there was no improvement in the model fits using this reparameterization, which was therefore omitted. The multicompartmental (three or four) profile of eflornithine in humans has been reported after short-term (5-min) intravenous infusions (35). In rats, the pharmacokinetic profile after intravenous infusions (60 to 420 min, at the doses of 100 to 2,700 mg/kg of body weight) exhibited a three-compartmental model with saturable binding to one of the peripheral compartments (17). These data suggest a more complex multicompartmental profile of eflornithine; however, further extension of the pharmacokinetic model was not supported by the present data. It is also of note that if the present model is applied for the subsequent planning of future oral eflornithine-based clinical studies, the terminal phase after the last dose during the 2-week QID dosing period has minimal impact on drug efficacy, due to its low contribution to the total AUC, for the treatment of late-stage HAT patients.
No covariates under investigation were found to significantly affect the pharmacokinetic parameters of the two enantiomers of eflornithine. A slightly lower clearance was observed in females (approximately 5% for both l- and d-eflornithine), and clearance was also slightly positively correlated with body weight. Eflornithine is primarily eliminated by renal excretion (fraction excreted, >80%); no metabolites have been found, and the drug does not bind to plasma proteins (36). Its intravenous clearance is low (1.2 to 2 ml/min/kg) and is similar to the glomerular filtration rate (14, 36, 37). It is believed that these observed relationships, although not significant, might be a consequence of the influence of gender and body weight on the glomerular filtration rate (38).
The difference in the systemic exposure of each enantiomer of eflornithine was clearly observed. A close-to-identical observation was also found in rats, which was shown to be due to the difference in the extent of the stereoselective absorption of each enantiomer (16, 17). The underlying mechanism was suggested to be either stereoselective paracellular absorption, chiral chemical complex formation, or active uptake in the gastrointestinal tract (17). The CSF-to-plasma concentration ratios were similar for the two enantiomers, suggesting that entry into and out of CSF is a nonstereoselective process. The CSF-to-plasma concentration ratio agreed with the previously published nonstereospecific analysis (12). A sufficient concentration of eflornithine in the CNS has been suggested to be a determinant of treatment efficacy in late-stage HAT patients (32, 39). It is noted that the CSF samples used in the present study were collected from each patient by lumbar puncture, and the concentrations at this site may not always correlate well with the concentrations closer to the brain. It has been recommended that CSF concentrations of >50 μmol/liter are required to be maintained during therapy to avoid treatment failure (11). The recommendation is based on the information on CSF levels after intravenous administration of racemic eflornithine. Based on the available data from studies in rats (17), it is likely that steady-state plasma and CSF concentrations for the enantiomers are equivalent after intravenous dosing in humans. Considering that the l-enantiomer is the principal active enantiomer, the minimum CSF concentration of l-eflornithine required to eradicate the parasite is considered to be 25 μM following oral administration of racemic eflornithine. The results from the present study showed that CSF concentration was not a reliable predictor of treatment response (nonsignificant relationship), although steady-state CSF concentrations of >23 μM l-enantiomer and 68 μM total eflornithine cured all patients. The lack of correlation might be explained by sampling or analytical error, or by an inadequate number of study subjects. The present data also indicate that Css,min might be used instead of CSF concentration as a predictor of treatment response.
Plasma AUCτ appeared to be the parameter that was most correlated to the probability of being cured. The concentrations of the l-enantiomer did not reveal a clearer relationship than that with total eflornithine. Based on the logistic regression, an AUCτ of l-eflornithine of ≥800 h · μmol/liter is required for a cure. This would necessitate an oral racemic dose of 125 mg/kg of body weight QID for 14 days. Considering the estimated variability in oral clearance and the relative bioavailability in this study (estimated through Monte Carlo simulations), a racemic dose of up to 205 mg/kg of body weight QID for 14 days (820 mg/kg of body weight/day) would be required to ensure that this exposure level is maintained in >95% of the patients. A tolerability assessment of this dose level is essential. Approaches to increase the bioavailability of eflornithine should be considered, in particular for the more potent l-enantiomer.
The recent introduction of nifurtimox-eflornithine combination treatment (NECT) has allowed the intravenous infusion of eflornithine to be reduced from 100 to 150 mg/kg of body weight QID for 14 days to 200 mg/kg of body weight BID for 7 days (40). With this dose regimen, it is expected that the total drug exposure of eflornithine, defined as the daily AUC times the treatment period, would be reduced by half. Whether this might enable the combination of oral eflornithine and nifurtimox to be used as an effective treatment requires further investigation. A pharmacokinetic investigation of each enantiomer, particularly the l-enantiomer, is required.
The results of the present study may explain why the oral treatment of late-stage human HAT patients with racemic eflornithine has hitherto failed, and it emphasizes that stereoselective pharmacokinetics needs to be considered when any oral eflornithine-based dosage regimen is explored.
REFERENCES
- 1.Kennedy PG. 2008. The continuing problem of human African trypanosomiasis (sleeping sickness). Ann Neurol 64:116–126. doi: 10.1002/ana.21429. [DOI] [PubMed] [Google Scholar]
- 2.Lutje V, Seixas J, Kennedy A. 2010. Chemotherapy for second-stage human African trypanosomiasis. Cochrane Database Syst Rev (8):CD006201. doi: 10.1002/14651858.CD006201.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Rodgers J. 2009. Human African trypanosomiasis, chemotherapy and CNS disease. J Neuroimmunol 211:16–22. doi: 10.1016/j.jneuroim.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy PG. 2006. Human African trypanosomiasis: in and out of Africa. Neurology 66:962–963. doi: 10.1212/01.wnl.0000208221.55385.55. [DOI] [PubMed] [Google Scholar]
- 5.WHO. 2014. Trypanosomiasis, human African (sleeping sickness). World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs259/en/. [Google Scholar]
- 6.Kennedy PG. 2006. Human African trypanosomiasis—neurological aspects. J Neurol 253:411–416. doi: 10.1007/s00415-006-0093-3. [DOI] [PubMed] [Google Scholar]
- 7.Priotto G, Kasparian S, Mutombo W, Ngouama D, Ghorashian S, Arnold U, Ghabri S, Baudin E, Buard V, Kazadi-Kyanza S, Ilunga M, Mutangala W, Pohlig G, Schmid C, Karunakara U, Torreele E, Kande V. 2009. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet 374:56–64. doi: 10.1016/S0140-6736(09)61117-X. [DOI] [PubMed] [Google Scholar]
- 8.Docampo R, Moreno SN. 2003. Current chemotherapy of human African trypanosomiasis. Parasitol Res 90(Suppl 1):S10–S13. [DOI] [PubMed] [Google Scholar]
- 9.Chappuis F. 2007. Melarsoprol-free drug combinations for second-stage Gambian sleeping sickness: the way to go. Clin Infect Dis 45:1443–1445. doi: 10.1086/522983. [DOI] [PubMed] [Google Scholar]
- 10.Balasegaram M, Harris S, Checchi F, Ghorashian S, Hamel C, Karunakara U. 2006. Melarsoprol versus eflornithine for treating late-stage Gambian trypanosomiasis in the Republic of the Congo. Bull World Health Organ 84:783–791. doi: 10.2471/BLT.06.031955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balasegaram M, Young H, Chappuis F, Priotto G, Raguenaud ME, Checchi F. 2008. Effectiveness of melarsoprol and eflornithine as first-line regimens for gambiense sleeping sickness in nine Médecins Sans Frontières programmes. Trans R Soc Trop Med Hyg 103:280–290. doi: 10.1016/j.trstmh.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Na-Bangchang K, Doua F, Konsil J, Hanpitakpong W, Kamanikom B, Kuzoe F. 2004. The pharmacokinetics of eflornithine (alpha-difluoromethylornithine) in patients with late-stage T.b. gambiense sleeping sickness. Eur J Clin Pharmacol 60:269–278. doi: 10.1007/s00228-004-0759-7. [DOI] [PubMed] [Google Scholar]
- 13.Barrett MP, Boykin DW, Brun R, Tidwell RR. 2007. Human African trypanosomiasis: pharmacological re-engagement with a neglected disease. Br J Pharmacol 152:1155–1171. doi: 10.1038/sj.bjp.0707354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burri C, Brun R. 2003. Eflornithine for the treatment of human African trypanosomiasis. Parasitol Res 90(Suppl 1):S49–S52. doi: 10.1007/s00436-002-0766-5. [DOI] [PubMed] [Google Scholar]
- 15.Qu N, Ignatenko NA, Yamauchi P, Stringer DE, Levenson C, Shannon P, Perrin S, Gerner EW. 2003. Inhibition of human ornithine decarboxylase activity by enantiomers of difluoromethylornithine. Biochem J 375:465–470. doi: 10.1042/BJ20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansson R, Malm M, Roth C, Ashton M. 2008. Enantioselective and nonlinear intestinal absorption of eflornithine in the rat. Antimicrob Agents Chemother 52:2842–2848. doi: 10.1128/AAC.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson CC, Gennemark P, Artursson P, Äbelö A, Ashton M, Jansson-Löfmark R. 2013. Population pharmacokinetic modeling and deconvolution of enantioselective absorption of eflornithine in the rat. J Pharmacokinet Pharmacodyn 40:117–128. doi: 10.1007/s10928-012-9293-x. [DOI] [PubMed] [Google Scholar]
- 18.Jansson-Löfmark R, Römsing S, Albers E, Ashton M. 2010. Determination of eflornithine enantiomers in plasma by precolumn derivatization with o-phthalaldehyde-N-acetyl-l-cysteine and liquid chromatography with UV detection. Biomed Chromatogr 24:768–773. doi: 10.1002/bmc.1361. [DOI] [PubMed] [Google Scholar]
- 19.Oka K, Yamamoto M, Nonaka T, Tomonaga M. 1996. The significance of artificial cerebrospinal fluid as perfusate and endoneurosurgery. Neurosurgery 38:733–736. doi: 10.1227/00006123-199604000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Hanpitakpong W, Kamanikom B, Banmairuroi V, Na-Bangchang K. 2003. High-performance liquid chromatographic method for determination of 2-difluoromethyl-dlornithine in plasma and cerebrospinal fluid. J Chromatogr B Analyt Technol Biomed Life Sci 788:221–231. doi: 10.1016/S1570-0232(02)00438-5. [DOI] [PubMed] [Google Scholar]
- 21.Icon Development Solutions. 1986-2006. NONMEM users guide. Icon Development Solutions, Ellicott City, MD. [Google Scholar]
- 22.Lindbom L, Pihlgren P, Jonsson EN. 2005. PsN-Toolkit–a collection of computer intensive statistical methods for nonlinear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79:241–257. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Jonsson EN, Karlsson MO. 1999. Xpose–an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51–64. [DOI] [PubMed] [Google Scholar]
- 24.R Core Development Team. 2009. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org. [Google Scholar]
- 25.Karlsson MO, Molnar V, Freijs A, Nygren P, Bergh J, Larsson R. 1999. Pharmacokinetic models for the saturable distribution of paclitaxel. Drug Metab Dispos 27:1220–1223. [PubMed] [Google Scholar]
- 26.Mager DE, Jusko WJ. 2001. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 28:507–532. doi: 10.1023/A:1014414520282. [DOI] [PubMed] [Google Scholar]
- 27.Savic RM, Jonker DM, Kerbusch T, Karlsson MO. 2007. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34:711–726. doi: 10.1007/s10928-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 28.Shen J, Boeckmann A, Vick A. 2012. Implementation of dose superimposition to introduce multiple doses for a mathematical absorption model (transit compartment model). J Pharmacokinet Pharmacodyn 39:251–262. doi: 10.1007/s10928-012-9247-3. [DOI] [PubMed] [Google Scholar]
- 29.Savic RM, Karlsson MO. 2009. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J 11:558–569. doi: 10.1208/s12248-009-9133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsson MO, Holford N. 2008. A tutorial on visual predictive checks, abstr 1434 Abstracts of the Annual Meeting of the Population Approach Group in Europe, 18 to 20 June 2008, Marseille, France. [Google Scholar]
- 31.Reference deleted.
- 32.Milord F, Loko L, Ethier L, Mpia B, Pépin J. 1993. Eflornithine concentrations in serum and cerebrospinal fluid of 63 patients treated for Trypanosoma brucei gambiense sleeping sickness. Trans R Soc Trop Med Hyg 87:473–477. doi: 10.1016/0035-9203(93)90044-Q. [DOI] [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. 2007. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 17:571–582. doi: 10.1080/10543400701329422. [DOI] [PubMed] [Google Scholar]
- 34.Zhou H. 2003. Pharmacokinetic strategies in deciphering atypical drug absorption profiles. J Clin Pharmacol 43:211–227. doi: 10.1177/0091270002250613. [DOI] [PubMed] [Google Scholar]
- 35.Bonate PL. 2011. Pharmacokinetic-pharmacodynamic modeling and simulation, 2nd ed. Springer, Deerfield, IL. [Google Scholar]
- 36.Sanderson L, Dogruel M, Rodgers J, Bradley B, Thomas SA. 2008. The blood-brain barrier significantly limits eflornithine entry into Trypanosoma brucei brucei infected mouse brain. J Neurochem 107:1136–1146. doi: 10.1111/j.1471-4159.2008.05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haegele KD, Alken RG, Grove J, Schechter PJ, Koch-Weser J. 1981. Kinetics of alpha-difluoromethylornithine: an irreversible inhibitor of ornithine decarboxylase. Clin Pharmacol Ther 30:210–217. doi: 10.1038/clpt.1981.150. [DOI] [PubMed] [Google Scholar]
- 38.Stevens LA, Coresh J, Greene T, Levey AS. 2006. Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med 354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 39.Milord F, Pepin J, Loko L, Ethier L, Mpia B. 1992. Efficacy and toxicity of eflornithine for treatment of Trypanosoma brucei gambiense sleeping sickness. Lancet 340:652–655. doi: 10.1016/0140-6736(92)92180-N. [DOI] [PubMed] [Google Scholar]
- 40.Yun O, Priotto G, Tong J, Flevaud L, Chappuis F. 2010. NECT is next: implementing the new drug combination therapy for Trypanosoma brucei gambiense sleeping sickness. PLoS Negl Trop Dis 4:e720. doi: 10.1371/journal.pntd.0000720. [DOI] [PMC free article] [PubMed] [Google Scholar]