Abstract
Candida parapsilosis is the second or third most common cause of candidemia in many countries. The Infectious Diseases Society of America recommends fluconazole as the primary therapy for C. parapsilosis candidemia. Although the rate of fluconazole resistance among C. parapsilosis isolates is low in most U.S. institutions, the resistance rate can be as high as 7.5%. This study was designed to assess the mechanisms of fluconazole resistance in 706 incident bloodstream isolates from U.S. hospitals. We sequenced the ERG11 and MRR1 genes of 122 C. parapsilosis isolates with resistant (30 isolates; 4.2%), susceptible dose-dependent (37 isolates; 5.2%), and susceptible (55 isolates) fluconazole MIC values and used real-time PCR of RNA from 17 isolates to investigate the regulation of MDR1. By comparing these isolates to fully fluconazole-susceptible isolates, we detected at least two mechanisms of fluconazole resistance: an amino acid substitution in the 14-α-demethylase gene ERG11 and overexpression of the efflux pump MDR1, possibly due to point mutations in the MRR1 transcription factor that regulates MDR1. The ERG11 single nucleotide polymorphism (SNP) was found in 57% of the fluconazole-resistant isolates and in no susceptible isolates. The MRR1 SNPs were more difficult to characterize, as not all resulted in overexpression of MDR1 and not all MDR1 overexpression was associated with an SNP in MRR1. Further work to characterize the MRR1 SNPs and search for overexpression of other efflux pumps is needed.
INTRODUCTION
Candida species in general and Candida parapsilosis in particular are opportunistic pathogens frequently responsible for hospital-acquired infections (1–3). While the burden of C. parapsilosis varies geographically and by patient population, C. parapsilosis is responsible for about 12 to 17% of cases of candidemia in the United States (4–6) and is identified in many studies to be the second or third most common cause of candidemia both in the United States and internationally, with the average mortality rate being 29% (range, 4% to 45%) (7). C. parapsilosis is particularly notable for the risk that it poses to neonates, among whom it is estimated to be responsible for 34% of all cases of candidemia in the United States and 33% internationally, with the average crude mortality rate being 10% (8).
The Infectious Diseases Society of America recommends fluconazole as the primary therapy for C. parapsilosis candidemia (9). The majority of clinical isolates of C. parapsilosis are susceptible to fluconazole; the rates of resistance in the United States from two surveillance studies range regionally from 0 to 7.5% (4, 5), though at least one hospital has reported higher rates of resistance (10). One small study of invasive fungal infections in liver transplant patients conducted antifungal susceptibility testing on 6 of 16 C. parapsilosis isolates and found that all were resistant to fluconazole according to current CLSI breakpoints. The authors noted that this coincided with a hospital-wide peak in the incidence of fluconazole-resistant C. parapsilosis, which later subsided (10).
Despite the potentially rising incidence of C. parapsilosis (11) and the threat that fluconazole resistance could pose in a clonally expanding population, little is known about the molecular mechanisms of C. parapsilosis fluconazole resistance. Fluconazole prevents fungal cell growth by inhibiting 14-α-demethylase, which is responsible for the production of an ergosterol precursor and is encoded by the gene ERG11. C. albicans, whose resistance mechanisms are well characterized, evades the effects of fluconazole in four known ways: (i) the upregulation of drug efflux pumps, primarily CDR1, CDR2, and MDR1, which transport fluconazole out of the cell; (ii) mutational changes to 14-α-demethylase that reduce its affinity to fluconazole; (iii) upregulation of ERG11 to dilute fluconazole binding; and (iv) other alterations to the cell's sterol pathway (12).
To date, there has been only a single study on the fluconazole resistance mechanisms of C. parapsilosis (13). In that study, which used isolates with in vitro-induced resistance, the authors found that MDR1, a drug efflux pump, was upregulated 19-fold in an isolate with induced fluconazole resistance compared to the level of regulation of its susceptible parent. This corresponded to a point mutation in the MRR1 gene, a transcription factor for MDR1. The authors therefore hypothesized that fluconazole resistance in C. parapsilosis was achieved through a gain-of-function mutation in MRR1 that upregulated MDR1 and removed fluconazole to an extent sufficient to prevent effective buildup within the cell. However, it was not clear whether the results were generalizable to resistant isolates from patients.
Using isolates collected during population-based U.S. surveillance of candidemia, we focused on two potential mechanisms of resistance: an MRR1 gain-of-function mutation and alterations to ERG11. To determine if either of these mechanisms was present in clinical isolates, we sequenced the ERG11 and MRR1 genes of 122 C. parapsilosis patient isolates with resistant, susceptible dose-dependent (SDD), and susceptible fluconazole MIC values. Upon finding alterations in the MRR1 sequences of 23 isolates, we conducted real-time PCR (RT-PCR) to determine whether any of these corresponded to an upregulation of MDR1. Additionally, we performed microsatellite analysis to determine whether isolates with shared mutations came from a shared lineage. Our results suggest that ERG11 mutations are a frequent cause of fluconazole resistance in C. parapsilosis.
MATERIALS AND METHODS
Isolates and susceptibility testing.
Isolates were selected from a pool of C. parapsilosis isolates collected as part of population-based candidemia surveillance in the metropolitan Atlanta, GA, area (from March 2008 to May 2013, n = 397), Baltimore City and County, MD (from June 2008 to May 2013, n = 262), Knox County, TN (from January 2011 to May 2013, n = 19), and the metropolitan Portland, OR, area (from January 2011 to May 2013, n = 28) (4, 11). All isolates were C. parapsilosis sensu stricto; no C. orthopsilosis or C. metapsilosis isolates were included in the study. Isolates were stored frozen at −70°C until needed. Susceptibility testing was performed as previously described for this collection (4). The final isolates were chosen either by having a nonsusceptible fluconazole MIC (MIC ≥ 4 μg/ml) or randomly from those with a susceptible fluconazole MIC distribution.
Sequencing of ERG11 and MRR1.
DNA was prepared using an UltraClean microbial DNA isolation kit (MO BIO Laboratories, Carlsbad, CA). Amplification of ERG11 and MRR1 was performed using the Roche master mix, as described by the manufacturer (Roche Diagnostics, Indianapolis, IN). Annealing temperatures and the extension time varied by primer (Table 1). PCR products were treated with the ExoSAP-IT reagent (Affymetrix, Santa Clara, CA) per the manufacturer's instruction and sequenced with sequencing primers (Table 1) using a BigDye Terminator kit (Applied Biosystems, Foster City, CA). The sequences were analyzed using Sequencher (version 5.1) software (Gene Codes Corporation, Ann Arbor, MI) and compared to the C. parapsilosis isolate ATCC 22019 wild-type ERG11 sequence and C. parapsilosis isolate CDC317 wild-type MRR1 sequence, respectively, using the Clustal W program. Mann-Whitney and Pearson chi-square tests were performed using SPSS software (IBM, Armonk, NY).
TABLE 1.
Primers for amplification and sequencinga
| Gene (purpose) | Primer name | Sequence | Annealing temp (°C) | Extension time (min) |
|---|---|---|---|---|
| ERG11 (PCR and sequencing) | ERG11 F1F | TAG TGG GAT CGG TGG ATC TT | 60 | 1 |
| ERG11 F2R | CTT TAT CTA AAT CAG CAT ACA ATT GAG | |||
| ERG11 F3F | TCT AGA TCC TTA TTA GGA GAA GCA ATG | 60 | 1 | |
| ERG11 F4R | ACT GAC TCC TGC CCT CAG ATT | |||
| ERG11 (sequencing only) | ERG11 F1R | ATG ATG TTG TAA ATG AAA GGA GCA | ||
| ERG11 F2F | TTA GCC CTT CAT GGG TAC AAC T | |||
| ERG11 F3R | TAC TTT GTG TTT GGC ACA ACC | |||
| ERG11 F4F | AAA AGT TGT TTC TCC CTT GGT TG | |||
| MRR1 (PCR and sequencing) | MRR1 F1F | CTG TAT GGA GAG TGA GAT TTT AGG TT | 60 | 1.25 |
| MRR1 F3R | TCC TTG GTT ACC TCA TTG CTC | |||
| MRR1 F4F | ATG GAG ACC ATT AAT TTT TTT GAC A | 60 | 1.25 | |
| MRR1 F6R | GAA TGA CTT CAT TGA AAT GTA ATG CT | |||
| MRR1 F7F | AAG AAA ATT CTT AGC TTA ACT GGA | 53 | 1.25 | |
| MRR1 F9R | AGA AAA TCT AAT TGG TAA AGA AGA AAG GA | |||
| MRR1 (sequencing only) | MRR1 F1R | TAA AAC CTT CTT CGT CAT AAC AAC A | ||
| MRR1 F2F | ACC TCA AAC GAA TGA AAT AAA GGA | |||
| MRR1 F2R | ATA ACA GAG GTT GAA TCG TTG GC | |||
| MRR1 F3F | CTA ATT CGT TGC TTGA GAT CAA AA | |||
| MRR1 F4R | CCA ATG CCA AGT CTA GTC TTT TCT | |||
| MRR1 F5F | TAG AAT AAG AAG GAC TCT TCC AAG C | |||
| MRR1 F5R | CCA AGA TGA TTC TTT CTC TTA TCT GTT | |||
| MRR1 F6F | GCA AGT TTG CCT TTG ATT CAA | |||
| MRR1 F7R | GAA TGA CTC TTT GTC AAT TTC CA | |||
| MRR1 F8F | CTA CAG ATT AAA ATC TCA GCC TGA CC | |||
| MRR1 F8R | CTG CGA GAT GCC GTA GTT C | |||
| MRR1 F9F | TCC ACT CCG ACT AGT GAT ACA TC |
The source of all primer sequences was this study.
Microsatellite amplification and analysis.
The microsatellite loci amplified were those described by Reiss and coworkers (14). The amplification mix consisted of 13.3 μl water, 2 μl 10× PCR buffer (Roche), 0.2 mM deoxynucleoside triphosphate mix (Roche), 1 μl dimethyl sulfoxide, 0.6 U Taq DNA polymerase (Roche), 0.2 pM forward and reverse primers (14), and 2 μl DNA per reaction mixture. PCR was performed using a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA) using the following conditions: 4 min denaturation at 96°C; 30 cycles of 30 s at 95°C, 30 s at 58°C, and 30 s at 72°C; and a final 30-min extension at 72°C. Amplified sequences were sized using an ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA) and compared to the GeneScan 500 6-carboxytetramethylrhodamine size standard (Applied Biosystems) in the 35- to 500-nucleotide range. Results were read using PeakScanner (version 2.0) software (Applied Biosystems, Foster City, CA) and analyzed using Microsatellite Analyser software (15) to determine Nei's chord distances (16). An unweighted-pair group method using average linkages (UPGMA) tree was constructed from the resulting distance matrix using the PHYLIP Neighbor executable (version 3.6) program (University of Washington, Seattle, WA) and edited using Geneious (version 6.1.6) software (Biomatters, Auckland, New Zealand).
Quantitative real-time PCR.
Four milliliters of Sabouraud dextrose broth was inoculated with C. parapsilosis to a concentration of 7 × 104 to 25 × 104 cells/ml and incubated in a rotary shaker at 37°C for approximately 18 h. Concentrations were checked by use of a hemocytometer within 2 h of harvesting to ensure a maximum final concentration of 1.0 × 108 cells/ml, indicative of semilogarithmic growth. RNA extraction was performed using a RiboPure yeast kit (Ambion, Austen, TX) according to the manufacturer's instructions. RNA integrity was checked visually by nondenaturing gel electrophoresis and quantitated using a NanoDrop 1000 spectrophotometer (Thermo Scientific, Waltham, MA). As quantitative real-time PCR (qRT-PCR) controls without reverse transcriptase showed evidence of genomic DNA contamination, some RNAs were subjected to DNase digestion followed by RNA cleanup with an RNeasy minikit (Qiagen, Venlo, Netherlands), according to the manufacturer's instructions, or by repeating the RiboPure yeast kit DNase protocol using twice the recommended volume of DNase I and incubating for twice the recommended time, after which all were treated with a Turbo DNA-free kit (Invitrogen, Carlsbad, CA), according to the manufacturer's rigorous DNase treatment procedure, and cleaned up with the RNeasy minikit as described above. A lack of DNA contamination was confirmed by reverse transcriptase-free quantitative PCR with primers TUB4-F-A and TUB4-R-A and TUB4 probe A (Table 2). The absence of interfering mutations in the primer-probe region of each gene was confirmed by sequencing using the primers and conditions described in Table 3.
TABLE 2.
Primers and probes for qRT-PCRa
| Gene | Primer name | Sequence | 5′ label | 3′ label | Efficiency (%) | Dynamic range (in quantification cycles) |
|---|---|---|---|---|---|---|
| MDR1 | MDR1-F-2 | CCC TTG TCG TTG GCA TTA | 94.5 | 21.57–32.89 | ||
| MDR1-R-2 | GCC TTC CTA GCA AGC AAT GTA | |||||
| MDR1 probe 2 | AGC TGG CTG GAG ATG GTG | FAM | BHQ1 | |||
| ACT1 | ACT1-F-2 | CGA ACG TGG TTA CGG TTT CTC CAC TA | 81.3 | 18.56–33.58 | ||
| ACT1-R-2 | ACT TGA CCA TCT GGC AAT TCG TAT | |||||
| ACT1 probe | TGC TTT GGA CTT TGA ACA AGA AAT GCA AAC CTC AT | HEX | BHQ1 | |||
| TUB4 | TUB4-F-A | CGG TGG CAC CAT TCA ACA | 83.2 | 21.21–36.37 | ||
| TUB4-R-A | CAT CTG ACA ATT CCA AAA ACA TGT C | |||||
| TUB4 probe A | CCA GTC GCA CCA CAA CTA CAT CAA CGA G | HEX | BHQ1 |
FAM, 6-carbocyfluorescein; BHQ1, black hole quencher 1; HEX, 6-carboxy-2′,4,4′,5′,7,7′-hexachlorofluorescein.
TABLE 3.
Primers for sequencing of qRT-PCR genesa
| Gene | Primer name | Sequence |
|---|---|---|
| MDR1 (partial) | MDR1seq F | CTG GGT TTT GTA TCC TTA GAT TCC T |
| MDR1seq R | AAG CGC CTC GAC CAA AAT | |
| ACT1 (partial) | ACT1seq F | TTC AGG TGA TGG TGT CAC TCA |
| ACT1seq R | AGT CAC ACT TCA TGA TAG AGT TGA AAG | |
| TUB4 (partial) | TUB4seq F | CTA CTT CGT TTC AAG GCA CAA AC |
| TUB4seq R | TTG TAC GTG CTT GAA CTT TCA AA |
The source of all primer sequences was this study, and for all primers the annealing temperature was 55°C and the extension time was 30 s.
qRT-PCR was run on a CFx-96 real-time PCR detection system (Bio-Rad, Hercules, CA) using a QuantiTect multiplex RT-PCR kit (Qiagen) in a 20-μl reaction volume, according to the manufacturer's instructions, with the following exceptions: the MDR1 primers and ACT1 probe were used at a final concentration of 0.3 μM. Triplicate reactions were run in singleplex using the primers and probes listed in Table 2. Primers and probes were designed using LightCycler probe design (version 2.0) software (Roche) and Primer Express (version 2.0) software (ABI, Foster City, CA). Sample replicates with standard deviations above 0.35 were repeated. The constitutively expressed ACT1 and TUB4 genes were used as a reference for normalizing the relative gene expression levels. Normalized gene expression analysis was performed using CFx manager software (Bio-Rad), which also performed interrun normalization using a common calibrator sample.
RESULTS
Identification of resistant and SDD isolates.
A total of 706 isolates of C. parapsilosis from 80 hospitals were tested for fluconazole susceptibility. There were 30 isolates that were resistant (MIC ≥ 8 μg/ml) and 37 isolates that were susceptible dose-dependent (SDD) (MIC = 4 μg/ml) to fluconazole. The majority of fluconazole-resistant or -nonsusceptible isolates were collected from patients in Atlanta hospitals (70.0% of resistant isolates, 64.9% of SDD isolates), followed by Baltimore (26.7% of resistant isolates, 27.0% of SDD isolates), Portland (0.0% of resistant isolates, 8.1% of SDD isolates), and Knox County (3.3% of resistant isolates, 0.0% of SDD isolates). The proportions of resistance and dose-dependent susceptibility were 5.3% and 6.1%, respectively, for the Atlanta study area and 3.0% and 3.8%, respectively, for the Baltimore study area.
Sequencing of ERG11.
The ERG11 genes of 122 isolates (30 resistant isolates, 37 susceptible dose-dependent isolates, and 55 randomly chosen susceptible isolates) were amplified and sequenced. By comparison to the sequence of the wild-type C. parapsilosis isolate ATCC 22019, five different single nucleotide polymorphisms (SNPs) in 54 isolates were identified. One of the SNPs, A395T (here called SNP 1; amino acid substitution, Y132F), was present in 17 of 30 resistant isolates (56.7%; heterozygous in 1 isolate, homozygous in 16 isolates) but none of the SDD or susceptible isolates. These isolates, listed in Table 4, were found in five different hospitals but primarily concentrated in three, with 71% of the isolates occurring in two hospitals in Atlanta and 18% occurring in one hospital in Baltimore. Two other SNPs, C−111T in the 5′ untranslated region and G1193T (R398I), were found together in 36 isolates (6 resistant, 13 SDD, and 17 susceptible isolates). Finally, two SDD isolates had one SNP each, T533C (M178T) and A847T (N283Y), respectively, the latter of which was heterozygous. The geometric mean MIC values of isolates homozygous for and without SNP 1 were 14.7 μg/ml and 1.92 μg/ml, respectively. The difference between the two groups was statistically significant (P < 0.0005; two-tailed, Mann Whitney U test value = 1,604.500). Isolates containing ERG11 SNP 1 accounted for 67% of resistant isolates in Atlanta and 38% in Baltimore.
TABLE 4.
MICs of isolates with ERG11 SNP 1a
| Isolate | Hospitalb | MIC (μg/ml)c |
|
|---|---|---|---|
| Fluconazole | Voriconazole | ||
| CAS08-0490 | ATL05 | 16 (R) | 1 (R) |
| CAS08-0796 | ATL05 | 8 (R) | 0.5 (I) |
| CAS09-0912 | ATL05 | 8 (R) | 0.25 (I) |
| CAS09-0959 | BAL09 | 32 (R) | 1 (R) |
| CAS09-1107 | ATL05 | 8 (R) | 0.5 (I) |
| CAS09-1291 | ATL05 | 16 (R) | 1 (R) |
| CAS09-1321 | BAL09 | 64 (R) | 1 (R) |
| CAS09-1504 | BAL09 | 32 (R) | 2 (R) |
| CAS09-1783 | ATL05 | 16 (R) | 0.25 (I) |
| CAS10-1966 | ATL05 | 8 (R) | 0.25 (I) |
| CAS10-2364 | ATL10 | 16 (R) | 0.25 (I) |
| CAS10-2602 | ATL10 | 8 (R) | 0.5 (I) |
| CAS11-3037 | ATL17 | 16 (R) | 0.25 (I) |
| CAS11-3324 | ATL10 | 16 (R) | 1 (R) |
| CAS11-3362 | ATL05 | 8 (R) | 0.125 (S) |
| CAS12-3954 | ATL10 | 16 (R) | 0.25 (I) |
| CAS12-3992 | ATL14 | 8 (R) | 0.125 (S) |
SNP 1 is the A395T substitution (amino acid substitution, Y132F).
ATL, an Atlanta-area hospital; BAL, a Baltimore-area hospital; KNX, a Knoxville-area hospital; POR, a Portland-area hospital.
R, resistant; I, intermediate; S, susceptible.
Sequencing of MRR1.
The MRR1 genes of the same 122 isolates described above were sequenced. Comparison against the MRR1 sequence of wild-type C. parapsilosis identified 23 (18.9%) isolates with SNPs (Table 5). These included nine different nonsynonymous SNPs (including one nonsense mutation), a synonymous SNP, a 5′ untranslated region SNP, and a 5′ untranslated region insertion. Of the six SNPs that occurred in multiple isolates, none occurred exclusively in resistant isolates, although two MRR1 polymorphisms, G−53A and C1856T (A619V), occurred only in resistant and SDD isolates. Three MRR1 polymorphisms, G2575A (A859T), −102_−101insT (where −101insT indicates insertion of a T nucleotide at position −101), and G2337T (L779F), occurred in one resistant isolate each, and another nonsynonymous SNP, G1436A (R478K), occurred in one SDD isolate. At least one MRR1 polymorphism was present in 12.7% of susceptible isolates (n = 7), 16.2% of SDD isolates (n = 6), and 33.3% of resistant isolates (n = 10). The proportions of susceptible and resistant isolates with and without MRR1 SNPs were significantly different (P = 0.023). These polymorphisms were not concentrated in any particular hospitals.
TABLE 5.
Isolates with MRR1 polymorphisms
| Nucleotide (amino acid) polymorphism | Isolate | Hospital | Fluconazole MICa (μg/ml) | Note |
|---|---|---|---|---|
| G−53A | CAS08-0060 | ATL12 | 16 (R) | |
| CAS08-0419 | ATL05 | 16 (R) | ||
| CAS10-2578 | ATL14 | 4 (SDD) | ||
| C1856T (A619V) | CAS09-1299 | ATL01 | 8 (R) | Heterozygous |
| CAS09-1761 | ATL01 | 4 (SDD) | Heterozygous | |
| G1214A (R405K) | CAS10-1852 | BAL05 | 8 (R) | |
| CAS09-1025 | ATL05 | 4 (SDD) | ||
| CAS13-4604 | ATL14 | 0.5 (S) | ||
| G531T (K177N) | CAS10-1830 | BAL06 | 8 (R) | |
| CAS12-4406 | KNX01 | 8 (R) | ||
| CAS08-0029 | ATL01 | 0.5 (S) | ||
| CAS10-2116 | BAL06 | 0.5 (S) | ||
| CAS12-4166 | BAL09 | 2 (S) | ||
| C3157T (Q1053X) | CAS10-1830 | BAL06 | 8 (R) | Heterozygous |
| CAS08-0029 | ATL01 | 0.5 (S) | Heterozygous | |
| CAS10-2116 | BAL06 | 0.5 (S) | Heterozygous | |
| A1844G (D615G) | CAS11-3108 | ATL03 | 8 (R) | Heterozygous |
| CAS09-0941 | BAL01 | 4 (SDD) | Heterozygous | |
| CAS10-1841 | BAL09 | 4 (SDD) | Heterozygous | |
| CAS09-1196 | ATL03 | 0.25 (S) | ||
| CAS10-2702 | BAL02 | 0.5 (S) | ||
| G2575A (A859T) | CAS08-0339 | BAL02 | 8 (R) | Heterozygous |
| C744T (no change) | CAS09-1025 | ATL05 | 4 (SDD) | Heterozygous |
| C1139A (P380H) | CAS12-4003 | POR01 | 1 (S) | Heterozygous |
| −102_−101insT | CAS12-4480 | BAL04 | 8 (R) | |
| G2337T (L779F) | CAS12-4342 | ATL03 | 32 (R) | |
| G1436A (R478K) | CAS13-4861 | ATL05 | 4 (SDD) | Heterozygous |
R, resistant; SDD, susceptible dose dependent; S, susceptible.
Resistance to voriconazole.
Of all 706 C. parapsilosis isolates with MIC data, 6 were resistant (MIC ≥ 1 μg/ml) to voriconazole. All six of the voriconazole-resistant isolates were also resistant to fluconazole and contained ERG11 SNP 1. The distributions of voriconazole MIC values of isolates with homozygous SNP 1 and without SNP 1 (listed in Table 4) differed significantly (P < 0.0005). The geometric mean MICs for the two groups were 0.48 μg/ml and 0.04 μg/ml, respectively.
qRT-PCR of MDR1.
To determine whether any of the SNPs identified in MRR1 were correlated with the upregulation of MDR1, qRT-PCR quantification of MDR1 RNA was conducted on all isolates with one of the six MRR1 nonsynonymous SNPs that were present only in resistant or SDD isolates, as well as in eight isolates without an MRR1 SNP (four resistant, two SDD, and two susceptible isolates). The efficiencies and ranges of detection of each primer set are reported in Table 2. The coefficients of variation of reference genes ACT1 and TUB4 were 0.146 and 0.174, respectively, and their M value was 0.455, indicating that the genes were sufficiently stable (17).
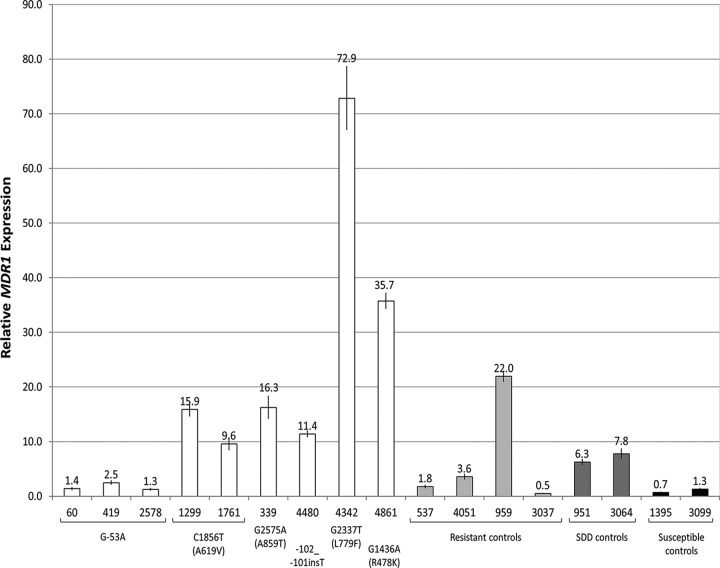
Relative gene expression analysis results are presented in Fig. 1. Compared to the averaged expression of susceptible control isolates, RNA from nine isolates showed at least a 5-fold upregulation in MDR1 expression. Six of these isolates had an MRR1 SNP, and three did not. These included both isolates with C1856T (A619V) and each of the four nonsusceptible isolates with a unique nonsynonymous SNP, G2575A (A859T), −102_−101insT, G2337T (L779F), and G1436A (R478K). The isolates containing L779F or R478K exhibited particularly high levels of MDR1 expression, with 72.9-fold and 35.7-fold increases, respectively. Of the four resistant isolates without MRR1 SNPs included as controls, one had increased expression, as did both of the control SDD isolates without MRR1 SNPs.
FIG 1.
Relative MDR1 expression analysis from qRT-PCR of isolates with MRR1 SNPs exclusive to fluconazole-nonsusceptible isolates. MDR1 values were normalized to each isolate's level of ACT1 and TUB4 expression, and the average for two susceptible controls was used as the control value and defined as 1-fold expression. Error bars represent 1 standard error of the mean. Bars are grouped by the isolate's shared MRR1 SNP, which is indicated beneath each group or individual by base change and, when applicable, amino acid change (in parentheses). Control isolates without MRR1 SNPs are grouped and shaded by resistance level.
Microsatellite analysis.
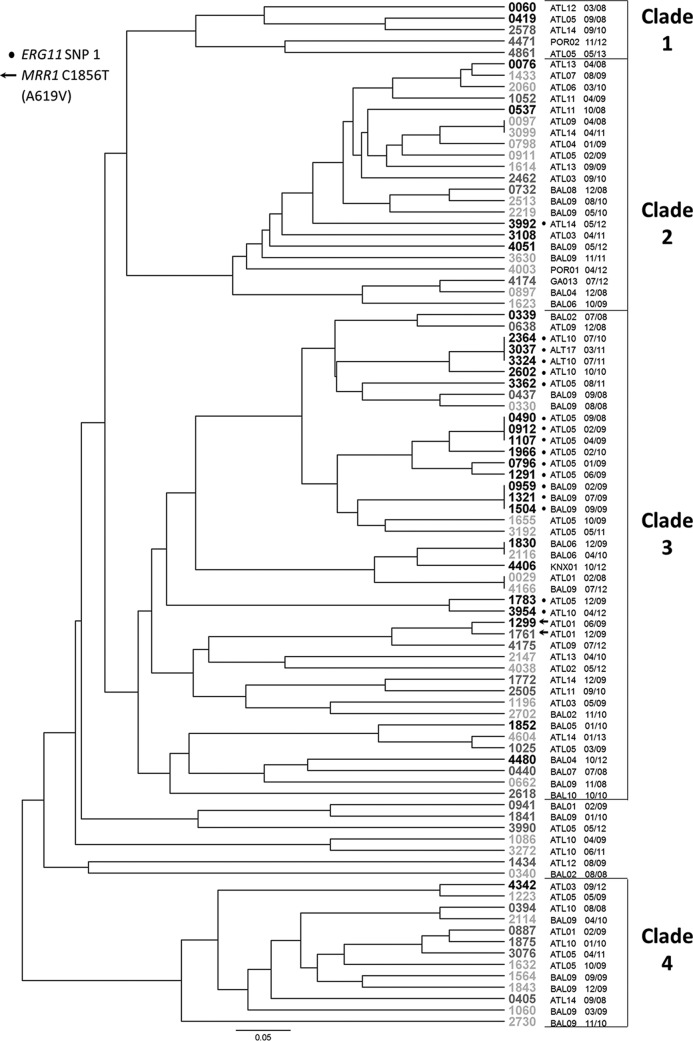
To understand whether resistance or shared SNPs were a function of shared ancestry, microsatellite analysis was conducted on all isolates with a polymorphism in either ERG11 or MRR1, all additional resistant isolates, and all isolates from the three hospitals with multiple resistant isolates (hospitals ATL05, ATL10, and BAL09), for a total of 92 isolates: 30 resistant, 26 SDD, and 36 susceptible isolates. Two of these, one SDD isolate and one susceptible isolate, returned triploid results for one locus, which could not be analyzed using our methodology, and were therefore excluded. The 90 remaining isolates produced 81 unique genotypes, including 3 instances of clonal pairs and 3 instances of clonal sets of three. All but seven of the isolates were distributed in four clades (Fig. 2). Isolates with ERG11 SNP 1 showed a tight cluster, with all but 1 isolate (of 17) occurring in clade 3. For the most part, clustering was not a function of geography or institution, with isolates from the same hospitals being disbursed across the tree. There were, however, two notable clusters of isolates with ERG11 SNP 1 within clade 3, one consisting of six isolates from hospital ATL05 collected over an 18-month period and the other consisting of three identical isolates from hospital BAL09 collected over an 8-month period.
FIG 2.
Results of microsatellite analysis presented as a UPGMA tree. Isolate names are given at the end of each branch, with the font color indicating the fluconazole susceptibility level: black letters, resistant isolates; dark gray letters, susceptible dose-dependent isolates; light gray letters, susceptible isolates. Hospital codes are given to the right of each isolate name, with ATL indicating an Atlanta-area hospital, BAL indicating a Baltimore-area hospital, KNX indicating a Knoxville-area hospital, and POR indicating a Portland-area hospital. The month and year of the isolate's collection are given to the right of the hospital code. Isolates with ERG11 SNP 1 are indicated with a black circle next to the isolate name. Those with C1856T (A619V), the only MRR1 SNP to occur exclusively in multiple nonsusceptible isolates with MDR1 expression elevated more than 5-fold, are indicated with an arrow.
DISCUSSION
Although fluconazole is the drug of choice for the treatment of C. parapsilosis, prior to this study we knew almost nothing about the mechanisms of C. parapsilosis patient isolate resistance to fluconazole. We addressed this problem in three ways. The first was the detection of mutations in ERG11, the target of fluconazole. The second was detection of mutations in MRR1, a gene that regulates a major fluconazole efflux pump. The third method was the detection of overexpression of MDR1, a major fluconazole efflux pump in C. parapsilosis.
The sole ERG11 SNP that was found exclusively in fluconazole-resistant isolates, SNP 1, may be responsible for a sizeable portion of C. parapsilosis fluconazole resistance. The strong association between this SNP and fluconazole resistance in this study is bolstered by the fact that this SNP was reported in C. albicans by Perea et al. (18), who found it to confer fluconazole resistance when the C. albicans ERG11 gene containing this SNP was transformed into otherwise susceptible Saccharomyces cerevisiae isolates. In another study, it was also found to decrease the susceptibility of C. albicans to voriconazole, mirroring the significantly increased voriconazole MICs that we found in C. parapsilosis isolates with ERG11 SNP 1 (19). The same SNP has subsequently been identified in other studies of fluconazole resistance in C. albicans and C. tropicalis (20, 21). A different substitution at the same amino acid in C. albicans Erg11p has been demonstrated to diminish the protein's ability to bind to fluconazole without affecting its enzymatic activity (22), and modeling of the same substitution as SNP 1 in C. tropicalis Erg11p has suggested that it would produce the same result (21). The MIC values of the isolates with ERG11 SNP 1, shown in Table 1, also validate the new species-specific breakpoints for C. parapsilosis and fluconazole (23, 24). These data confirm that isolates with known mutations in genes involved in fluconazole resistance have MIC values well below the previous resistance values of 64 μg/ml but above the current limit for susceptibility of 4 μg/ml.
The sequencing of transcription factor MRR1 and qRT-PCR quantification of MDR1 expression revealed five polymorphisms that were present exclusively in a resistant or SDD isolate or isolates with upregulated MDR1 expression: amino acid substitutions A619V, A859T, L779F, and R478K and promoter insertion −101insT. None of these can be definitively said to cause either the MDR1 upregulation or the reduced fluconazole susceptibility, as MDR1 upregulation was also found in three isolates without an MRR1 SNP, indicating the existence of MDR1 upregulation mechanisms beyond alterations in MRR1. Nonetheless, they represent the first set of potentially MDR1-upregulating mutations in clinical isolates of C. parapsilosis, and their precise activities should be further explored.
Of the five polymorphisms, only A619V occurred in multiple isolates, one resistant isolate with 16-fold-increased MDR1 levels and one SDD isolate with 10-fold-increased MDR1 levels, suggesting that the SNP may have moderate gain-of-function activity. Aligning the protein sequences of wild-type C. albicans and C. parapsilosis MRR1p revealed that one of the unique SNPs (A859T) is located at the amino acid equivalent to that of a C. albicans SNP (A880E) that has been demonstrated to increase MDR1 expression (25). This SNP lies within a hot spot ranging from C. albicans amino acids 873 to 896 (C. parapsilosis amino acids 852 to 875), within which seven demonstrated or putative C. albicans gain-of-function mutations and one C. dubliniensis mutation have been found (25–28). The alignment also showed that L779F, the SNP present in an isolate with 73-fold MDR1 upregulation, is located only 3 amino acids away from an amino acid equivalent to the position of C. albicans N803D, another SNP shown to cause MDR1 upregulation (25). The notably high expression, combined with the isolate's particularly elevated MIC, 32 μg/ml, suggests that if it can be linked to an MRR1 gain of function, L779F may be a particularly potent resistance mechanism.
Interestingly, we found resistant isolates to be significantly more likely to contain an MRR1 mutation than susceptible isolates. This disparity became especially apparent when resistant isolates that contained ERG11 SNP 1 (and that therefore already had a putative mechanism of resistance), none of which contained an MRR1 polymorphism, were excluded. Of the resistant isolates without ERG11 SNP1, 76.9% contained at least one MRR1 polymorphism, whereas only 12.7% of susceptible isolates contained at least one MRR1 polymorphism. Even after excluding isolates with the five potentially MDR1 overexpression-linked SNPs, 66.7% of resistant isolates without ERG11 SNP 1 contained an MRR1 SNP. The persistent disproportionate presence of MRR1 mutations in resistant isolates suggests that they may play a role in C. parapsilosis fluconazole resistance wider than that which can be demonstrated in this research. By discounting SNPs that were present exclusively in isolates with reduced susceptibility and MDR1 upregulation, it is possible that SNPs that may be selectively upregulating MDR1 in conjunction with some other, unidentified mechanism were overlooked. Research has also indicated that in C. albicans, MRR1 can increase the expression of many genes beyond MDR1, and hyperactive MRR1 reduces the susceptibility of isolates even in MDR1 knockouts (29). Therefore, some SNPs that were shown not to cause MDR1 overexpression could still potentially reduce fluconazole susceptibility through the regulation of other genes.
Microsatellite analysis revealed that most of the SNPs identified in ERG11 and MRR1 were found in isolates that tended to be closely related and concentrated in a small number of hospitals. This was particularly noticeable for ERG11 SNP 1, which was, with one exception, exclusively present in one large clade and in three groups of three isolates that appeared to be clonally related by the methods employed in this study. Three small clusters consisted of fluconazole-resistant isolates from the same hospital, suggesting the persistence of a strain within a hospital or within the general geographic area. This result may also imply that our results may apply only to our small catchment area and may not be generalizable to other areas of the United States or to other countries. Interestingly, in resistant isolates without ERG11 SNP 1, no hospital specificity was detected, suggesting perhaps that ERG11 SNP 1 or other associated factors in the clonal isolates may enable those strains to be particularly resilient.
There are several limitations to this study. The first is that we did not try to detect MDR1 overexpression in the presence of fluconazole induction. It is possible that the presence of fluconazole could be a trigger for MDR1 overexpression, and the lack of overexpression of MDR1 for some of the Mrr1p mutations may reflect this limitation. Another limitation is that we have data only for in vitro resistance. It is not clear whether this would have translated to treatment failure in each case. Finally, we did not perform any transformation experiments to see if the mutations that we describe could confer resistance to a susceptible isolate.
Here we described the first mutations in clinical isolates of C. parapsilosis that confer fluconazole resistance. More alarmingly, we showed that the most prevalent mutation, ERG11 SNP 1, is present in small clonal clusters and may show a propensity to persist in particular hospitals or communities. With its ability to remain on the hands of health care workers and its perceived current increase in abundance in U.S. hospitals, further surveillance for C. parapsilosis isolates harboring these mutations is warranted.
ACKNOWLEDGMENTS
This research was supported in part by an appointment to the Emerging Infectious Diseases (EID) Fellowship Program administered by the Association of Public Health Laboratories (APHL) and funded by the Centers for Disease Control and Prevention (CDC).
We acknowledge the Candidemia Surveillance Group, which consists of Joyce Peterson, Shirley McClinton, Ben Park, Mary Brandt, Tom Chiller; Eun Ji (Stacey) Ahn, Vinod Bhullar, Angie Trujillo, and Vladimir Loparev at the Centers for Disease Control and Prevention; Monica M. Farley, Wendy Baughman, Betsy Stein, and hospitals in Georgia Health District 3; Lee H. Harrison, Rosemary Hollick, Kim Holmes, and the Baltimore surveillance hospitals; William Schaffner, Brenda Barnes, Caroline Graber, and the Knoxville surveillance hospitals; and Zintars G. Beldavs, Magdalena Kendall, and the Portland surveillance hospitals, for submission of isolates.
The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Romeo O, Delfino D, Cascio A, Lo Passo C, Amorini M, Romeo D, Pernice I. 2013. Microsatellite-based genotyping of Candida parapsilosis sensu stricto isolates reveals dominance and persistence of a particular epidemiological clone among neonatal intensive care unit patients. Infect Genet Evol 13:105–108. doi: 10.1016/j.meegid.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Diab-Elschahawi M, Forstner C, Hagen F, Meis JF, Lassnig AM, Presterl E, Klaassen CH. 2012. Microsatellite genotyping clarified conspicuous accumulation of Candida parapsilosis at a cardiothoracic surgery intensive care unit. J Clin Microbiol 50:3422–3426. doi: 10.1128/JCM.01179-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, Baughman W, Stein B, Hollick R, Park BJ, Chiller T. 2012. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol 50:3435–3442. doi: 10.1128/JCM.01283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfaller MA, Jones RN, Castanheira M. 2014. Regional data analysis of Candida non-albicans strains collected in United States medical sites over a 6-year period, 2006-2011. Mycoses 57:602–611. doi: 10.1111/myc.12206. [DOI] [PubMed] [Google Scholar]
- 6.Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, Rotstein C, Franks B, Azie NE. 2014. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004-2008. PLoS One 9:e101510. doi: 10.1371/journal.pone.0101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trofa D, Gacser A, Nosanchuk JD. 2008. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev 21:606–625. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pammi M, Holland L, Butler G, Gacser A, Bliss JM. 2013. Candida parapsilosis is a significant neonatal pathogen: a systematic review and meta-analysis. Pediatr Infect Dis J 32:e206–e216. doi: 10.1097/INF.0b013e3182863a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghuram A, Restrepo A, Safadjou S, Cooley J, Orloff M, Hardy D, Butler S, Koval CE. 2012. Invasive fungal infections following liver transplantation: incidence, risk factors, survival, and impact of fluconazole-resistant Candida parapsilosis (2003-2007). Liver Transplant 18:1100–1109. doi: 10.1002/lt.23467. [DOI] [PubMed] [Google Scholar]
- 11.Cleveland AA, Farley MM, Harrison LH, Stein B, Hollick R, Lockhart SR, Magill SS, Derado G, Park BJ, Chiller TM. 2012. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008-2011. Clin Infect Dis 55:1352–1361. doi: 10.1093/cid/cis697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanglard D, Odds FC. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect Dis 2:73–85. doi: 10.1016/S1473-3099(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 13.Silva AP, Miranda IM, Guida A, Synnott J, Rocha R, Silva R, Amorim A, Pina-Vaz C, Butler G, Rodrigues AG. 2011. Transcriptional profiling of azole-resistant Candida parapsilosis strains. Antimicrob Agents Chemother 55:3546–3556. doi: 10.1128/AAC.01127-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiss E, Lasker BA, Lott TJ, Bendel CM, Kaufman DA, Hazen KC, Wade KC, McGowan KL, Lockhart SR. 2012. Genotyping of Candida parapsilosis from three neonatal intensive care units (NICUs) using a panel of five multilocus microsatellite markers: broad genetic diversity and a cluster of related strains in one NICU. Infect Genet Evol 12:1654–1660. doi: 10.1016/j.meegid.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Dieringer D, Schlötterer C. 2003. Microsatellite Analyser (MSA): a platform independent analysis tool for large microsatellite data sets. Mol Ecol Notes 3:167–169. doi: 10.1046/j.1471-8286.2003.00351.x. [DOI] [Google Scholar]
- 16.Nei M, Tajima F, Tateno Y. 1983. Accuracy of estimated phylogenetic trees from molecular data. II. Gene frequency data. J Mol Evol 19:153–170. [DOI] [PubMed] [Google Scholar]
- 17.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perea S, Lopez-Ribot JL, Kirkpatrick WR, McAtee RK, Santillan RA, Martinez M, Calabrese D, Sanglard D, Patterson TF. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 45:2676–2684. doi: 10.1128/AAC.45.10.2676-2684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang MJ, Liu JY, Ni PH, Wang S, Shi C, Wei B, Ni YX, Ge HL. 2013. Erg11 mutations associated with azole resistance in clinical isolates of Candida albicans. FEMS Yeast Res 13:386–393. doi: 10.1111/1567-1364.12042. [DOI] [PubMed] [Google Scholar]
- 20.Morio F, Loge C, Besse B, Hennequin C, Le Pape P. 2010. Screening for amino acid substitutions in the Candida albicans Erg11 protein of azole-susceptible and azole-resistant clinical isolates: new substitutions and a review of the literature. Diagn Microbiol Infect Dis 66:373–384. doi: 10.1016/j.diagmicrobio.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Forastiero A, Mesa-Arango AC, Alastruey-Izquierdo A, Alcazar-Fuoli L, Bernal-Martinez L, Pelaez T, Lopez JF, Grimalt JO, Gomez-Lopez A, Cuesta I, Zaragoza O, Mellado E. 2013. Candida tropicalis antifungal cross-resistance is related to different azole target (Erg11p) modifications. Antimicrob Agents Chemother 57:4769–4781. doi: 10.1128/AAC.00477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly SL, Lamb DC, Kelly DE. 1999. Y132H substitution in Candida albicans sterol 14alpha-demethylase confers fluconazole resistance by preventing binding to haem. FEMS Microbiol Lett 180:171–175. doi: 10.1016/S0378-1097(99)00478-4. [DOI] [PubMed] [Google Scholar]
- 23.Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D. 2010. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat 13:180–195. doi: 10.1016/j.drup.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2012. M27-S4 reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 25.Dunkel N, Blass J, Rogers PD, Morschhauser J. 2008. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol Microbiol 69:827–840. doi: 10.1111/j.1365-2958.2008.06309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eddouzi J, Parker JE, Vale-Silva LA, Coste A, Ischer F, Kelly S, Manai M, Sanglard D. 2013. Molecular mechanisms of drug resistance in clinical Candida species isolated from Tunisian hospitals. Antimicrob Agents Chemother 57:3182–3193. doi: 10.1128/AAC.00555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morio F, Pagniez F, Besse M, Gay-andrieu F, Miegeville M, Le Pape P. 2013. Deciphering azole resistance mechanisms with a focus on transcription factor-encoding genes TAC1, MRR1 and UPC2 in a set of fluconazole-resistant clinical isolates of Candida albicans. Int J Antimicrob Agents 42:410–415. doi: 10.1016/j.ijantimicag.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Schubert S, Rogers PD, Morschhauser J. 2008. Gain-of-function mutations in the transcription factor MRR1 are responsible for overexpression of the MDR1 efflux pump in fluconazole-resistant Candida dubliniensis strains. Antimicrob Agents Chemother 52:4274–4280. doi: 10.1128/AAC.00740-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schubert S, Barker KS, Znaidi S, Schneider S, Dierolf F, Dunkel N, Aid M, Boucher G, Rogers PD, Raymond M, Morschhauser J. 2011. Regulation of efflux pump expression and drug resistance by the transcription factors Mrr1, Upc2, and Cap1 in Candida albicans. Antimicrob Agents Chemother 55:2212–2223. doi: 10.1128/AAC.01343-10. [DOI] [PMC free article] [PubMed] [Google Scholar]