Abstract
LFF571 is a novel semisynthetic thiopeptide antibacterial that is undergoing investigation for safety and efficacy in patients with moderate Clostridium difficile infections. LFF571 inhibits bacterial protein synthesis by interacting with elongation factor Tu (EF-Tu) and interrupting complex formation between EF-Tu and aminoacyl-tRNA. Given this mechanism of action, we hypothesized that concentrations of LFF571 below those necessary to inhibit bacterial growth would reduce steady-state toxin levels in C. difficile cultures. We investigated C. difficile growth and toxin A and B levels in the presence of LFF571, fidaxomicin, vancomycin, and metronidazole. LFF571 led to strain-dependent effects on toxin production, including decreased toxin levels after treatment with subinhibitory concentrations, and more rapid declines in toxin production than in inhibition of colony formation. Fidaxomicin, which is an RNA synthesis inhibitor, conferred a similar pattern to LFF571 with respect to toxin levels versus viable cell counts. The incubation of two toxigenic C. difficile strains with subinhibitory concentrations of vancomycin, a cell wall synthesis inhibitor, increased toxin levels in the supernatant over those of untreated cultures. A similar phenomenon was observed with one metronidazole-treated strain of C. difficile. These studies indicate that LFF571 and fidaxomicin generally result in decreased C. difficile toxin levels in culture supernatants, whereas treatment of some strains with vancomycin or metronidazole had the potential to increase toxin levels. Although the relevance of these findings remains to be studied in patients, reducing toxin levels with sub-growth-inhibitory concentrations of an antibiotic is hypothesized to be beneficial in alleviating symptoms.
INTRODUCTION
Clostridium difficile infection (CDI) is a serious gastrointestinal disease. Approximately 500,000 cases occur in the United States each year, making CDI the most common hospital-acquired infection (1, 2). The ability of C. difficile to form spores makes it difficult to remove from surfaces and allows it to spread easily within a health care setting. Recently, the incidence of the disease has been increasing, and hyper-virulent strains, such as B1/NAP1/027, have been recognized (reviewed in reference 3). The epidemiology of CDI has also been changing, and the disease is now more commonly seen outside the hospital environment. The standard of care for CDI is treatment with the antibiotic metronidazole or vancomycin. Fidaxomicin (FDX) was approved in 2011 for the treatment of C. difficile-associated diarrhea.
C. difficile is an anaerobe that opportunistically colonizes the gut, often after treatment with broadly acting antibacterials. CDI is an enterotoxin-mediated disease that can be subclinical or produce symptoms ranging from mild diarrhea to severe pseudomembranous colitis, megacolon, bowel perforation, sepsis, and death (1). Toxins encoded by C. difficile are termed A, B, and CDT. There has been substantial debate about the roles of each toxin in pathogenesis, and some consider toxins A and B to be essential virulence factors (4, 5). Toxins A and B are large, multidomain proteins that catalyze the glucosylation of Rho GTPases. Inactivation of the cellular enzymes leads to deregulation of cytoskeleton arrangement and cell death, followed by mucosal inflammation and diarrhea (6). Toxin CDT is an ADP-ribosylating binary toxin with an unclear contribution to disease (6).
LFF571 (7) is a semisynthetic thiopeptide antibiotic with potent in vitro activity against C. difficile (8, 9), and it is efficacious in the treatment of C. difficile infection in animal models (10). LFF571 was recently shown to be noninferior to vancomycin in patients with moderate C. difficile infections (24). LFF571 inhibits bacterial protein synthesis by binding to elongation factor Tu (EF-Tu) and preventing this translation factor from delivering an aminoacyl-tRNA to the ribosome (11, 12). Because of this mechanism of action, we hypothesized that LFF571 would prevent toxin production at doses below the growth-inhibitory concentration of the compound. In theory, this may reduce toxin-mediated diarrheal symptoms at lower concentrations and may provide an advantage over other antibiotics with unrelated modes of action. Here, we show that subinhibitory or inhibitory concentrations of LFF571 and fidaxomicin decrease toxin levels in supernatants of C. difficile cultures, while subinhibitory to inhibitory levels of vancomycin and metronidazole increase toxin levels in select strains.
MATERIALS AND METHODS
Antibiotics.
LFF571 and fidaxomicin (lipiarmycin A3, prepared by fermentation of Catellatospora sp. strain Bp3323-81) were obtained from Novartis. Vancomycin and metronidazole were purchased from US Pharmacopeia (Rockville, MD).
Organisms.
Bacterial strains used in this study (Table 1) were from the American Type Culture Collection (ATCC) or kindly provided by D. Low (Mount Sinai Hospital, Toronto, Canada). Each strain was routinely cultured on brucella agar containing 5% sheep blood, 0.5 μg/ml vitamin K, and 5 μg/ml hemin. Cultures were incubated at 37°C in an anaerobic chamber (Coy Laboratory Products, Inc.) with a gas mix environment of 10% hydrogen, 10% carbon dioxide, and a balance of nitrogen.
TABLE 1.
C. difficile strains and relevant phenotypes
| Strain | Source | Description | Toxin phenotype | MIC (μg/ml) fora: |
|||
|---|---|---|---|---|---|---|---|
| LFF571 | FDX | VAN | MET | ||||
| NB95009 | ATCC 700057 | VPI 11186b | A− B− | 0.5 | 0.03 | 0.5 | 0.25 |
| NB95013 | ATCC 43255 | VPI 10463 | A+ B+ | 0.25 | 0.06 | 0.5 | 0.25 |
| NB95016 | Clinical isolatec | MOH003, ribotype AE; NAP 1 | A+ B+ CDT+ | 0.5 | 0.125 | 0.5 | 2 |
| NB95029 | Clinical isolatec | MOH118, ribotype AA | A− B+ | 0.5 | 0.125 | 0.5 | 2 |
| NB95031 | Clinical isolatec | MOH082, ribotype AA | A+ B+ | 0.5 | 0.125 | 0.5 | 2 |
FDX, fidaxomicin; VAN, vancomycin; MET, metronidazole.
CLSI MIC quality control strain.
Kindly provided by D. Low (Mt. Sinai Hospital, Toronto, Canada).
In vitro susceptibility testing, cell titer determinations, and toxin sampling.
MICs of the test agents were determined using the agar dilution method recommended by the Clinical and Laboratory Standards Institute (CLSI) (13). To select the appropriate test agent concentration ranges for toxin level and cell titer determinations, antibacterial activity was determined under the specific growth conditions used to generate the toxin assay samples. Briefly, 5 to 6 colonies of C. difficile strains from an overnight brucella agar plate incubation were suspended in prereduced tryptone yeast plus sodium thioglycolate (TY) broth supplemented with 0.5 μg/ml vitamin K and 5.0 μg/ml hemin. Duplicate suspensions of C. difficile (0.5 ml) were transferred to 96 deep-well plates, and serial 2-fold dilutions of the test agents were added to the appropriate wells. Cultures were incubated for 24 h at 37°C anaerobically, unless otherwise indicated.
Once the optimal test agent concentration range was determined, experimental samples were generated from 0.5-ml cultures inoculated and incubated as described above. Viable cells were quantified by removing 110 μl and plating 10-fold serial dilutions onto brucella agar plates, followed by incubating anaerobically at 37°C for 48 h unless stated otherwise. The remaining culture was centrifuged for 10 min at 3,500 × g, and the supernatants were removed and stored at −20°C for 48 to 96 h prior to assaying for toxin A and B levels.
Toxin A and B analysis.
Total toxin A and B levels in C. difficile culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA) (Wampole, TechLab, Blacksburg, VA), according to the manufacturer's recommendations. The assay uses microtiter plates precoated with polyclonal goat antibodies against toxins A and B. Briefly, 50 μl of horseradish peroxidase (HRP)-conjugated secondary antibodies (mouse monoclonal anti-toxin A and goat polyclonal anti-toxin B) was added to each well, immediately followed by 100 μl of undiluted culture supernatant. The plates were incubated for 60 min at 37°C before washing five times with phosphate-buffered saline (PBS). Subsequently, 100 μl of substrate (tetramethyl benzidine) was added, and the samples were incubated for 10 min at room temperature. The reactions were stopped by the addition of 50 μl H2SO4, and signals were detected at A450 using a spectrophotometer (SpectraMax; Molecular Devices, Sunnyvale, CA). Purified toxin A and toxin B (tgcBiomics) were used as controls. The lower limits of detection in this assay were 1.25 ng/ml of purified toxin A and 6 ng/ml of purified toxin B.
To monitor toxin A and B levels separately, culture supernatants were analyzed by ELISA (tgcBiomics, Mainz, Germany) according to the manufacturer's instructions. Briefly, 100 μl of undiluted C. difficile culture supernatants was transferred to microtiter plates coated with antibodies to toxins A and B. Individual toxins were detected by immediately adding 50 μl of HRP-conjugated specific anti-toxin A or anti-toxin B antibodies. Coated plates containing specimens plus conjugate were incubated for 60 min at 37°C before washing three times with wash buffer. Subsequently, 100 μl of substrate (tetramethylbenzidine) was added, and the samples were incubated for 20 min at room temperature. The reactions were stopped by the addition of 50 μl of H2SO4, and signals were detected by spectrophotometry (SpectraMax), followed by subtracting the background (A620) from the signal (A450). Purified toxins A and B (tgcBiomics) were used as controls. The lower limits of detection in this assay were 1.25 ng/ml of purified toxin A and 0.6 ng/ml of purified toxin B.
RESULTS
Characterization of antibiotic MIC profiles and toxin A and B production patterns of five C. difficile strains.
Table 1 lists the in vitro antibiotic activities and toxin expression phenotypes of five strains of C. difficile, including three clinical isolates. The antibacterial concentrations of the test agents used in this study were determined under the TY broth growth conditions used for toxin analysis, as well as by CLSI-recommended agar dilution methods. The MIC results obtained were comparable under these two conditions.
To measure the combined levels of toxins A and B in supernatants from these strains, we used a commercially available ELISA kit (Wampole). Using this assay, we confirmed the previously reported toxigenic phenotypes of C. difficile strains NB95013 (14), NB95016 (D. Low, personal communication), NB95029 (D. Low, personal communication), and NB95031 (D. Low, personal communication) (Table 1). NB95009 (ATCC 700057), a nontoxigenic Clinical and Laboratory Standards Institute (CLSI) quality control strain (15), was confirmed to be negative for toxin A and B expression (data not shown) and was used as a negative control in all the toxin detection experiments. Using the tcgBiomics ELISA, which detects toxins A and B independently, we verified that the clinical strain NB95029 expresses only toxin B (Table 1).
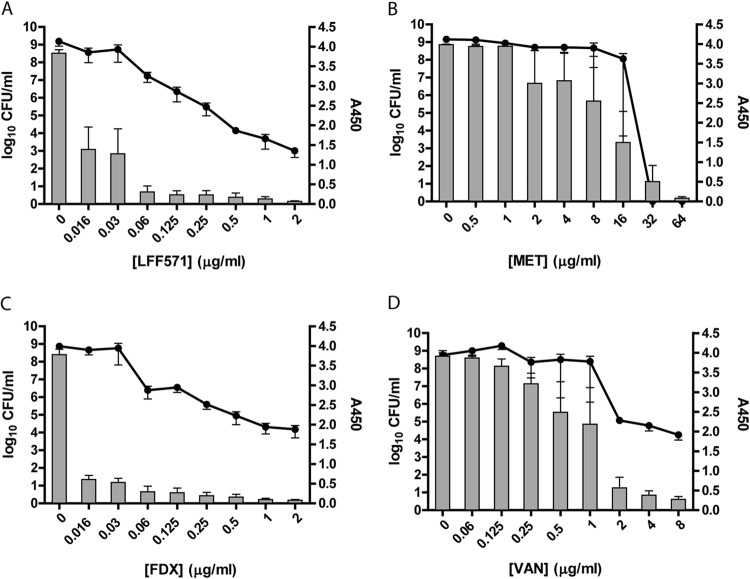
Decrease in toxin levels in supernatants of C. difficile NB95016 cultures precedes bacterial growth inhibition by LFF571 or FDX.
Strain NB95016 is a clinical isolate of the B1/NAP1/027 type, which has been associated with severe disease in humans. Using the Wampole ELISA, high levels of toxins A and B were detectable in the absence of antibiotic treatment (Fig. 1). The total toxin A and B levels decreased in cultures treated with sub-growth-inhibitory concentrations of LFF571 or fidaxomicin. In contrast, toxin A and B levels declined only in the presence of growth-inhibitory concentrations of vancomycin or metronidazole (Fig. 1). Similar results were observed when toxins A and B were quantified separately (data not shown). These results indicate that, for this toxigenic C. difficile strain, the toxin inhibition conferred by vancomycin or metronidazole is the result of growth inhibition, which contrasts sharply with the reduction of toxin levels conferred by sub-growth-inhibitory concentrations of LFF571 and FDX.
FIG 1.
Toxin production and cell viability after antibiotic treatment of strain NB95016. Strain NB95016 was grown for 24 h in the presence of the indicated antibiotics. Remaining viable cells (log10 CFU/ml, lines) and toxin A and B production (A450, bars) were measured. FDX, fidaxomicin; VAN, vancomycin; MET, metronidazole. Data are means and standard errors of the mean (SEM) of three independent experiments, each with duplicate antibiotic treatments.
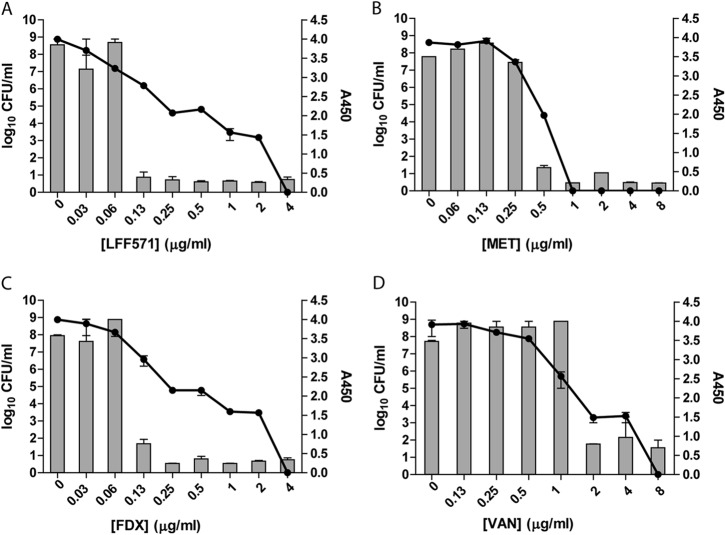
Toxin levels in supernatants of C. difficile NB95013 treated with LFF571 or FDX drop more sharply than reduction in CFU.
Strain NB95013 is a clinical isolate that produces high levels of detectable toxins A and B in culture (14). The treatment of NB95013 with increasing concentrations of LFF571 or fidaxomicin led to a dose-dependent decrease in viable cells; however, toxin titers dropped much more sharply (Fig. 2). Treatment with vancomycin or metronidazole resulted in a loss of culture viability that largely paralleled the decrease in toxin levels in culture supernatants.
FIG 2.
Toxin production and cell viability after antibiotic treatment of strain NB95013. Strain NB95013 was grown for 48 h in the presence of the indicated antibiotics. Remaining viable cells (log10 CFU/ml, lines) and toxin A and B production (A450, bars) were measured. FDX, fidaxomicin; VAN, vancomycin; MET, metronidazole. Data are means and SEM of duplicate antibiotic treatments.
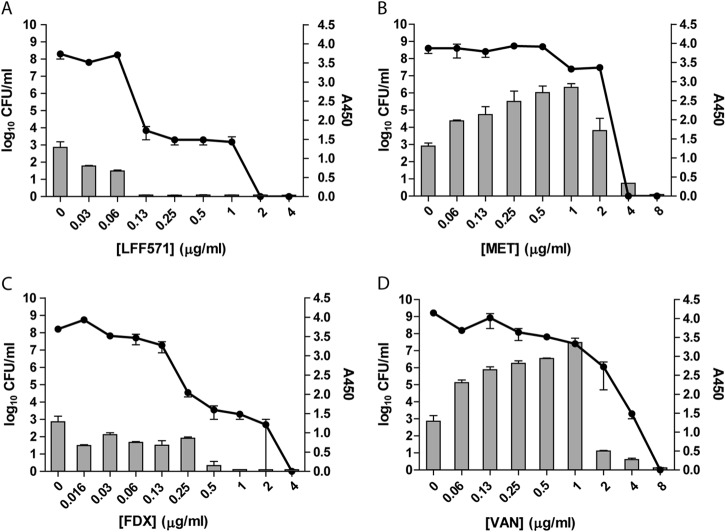
Subinhibitory concentrations of metronidazole and vancomycin confer increased toxin levels in supernatants of C. difficile NB95031 cultures.
Strain NB95031 is a clinical isolate of C. difficile. Toxins A and B were not detectable in supernatants from untreated cultures of NB95031 incubated for 24 h (data not shown). This strain was therefore incubated in the presence of antibiotics for 72 h prior to analysis. Viable cell counts declined with increasing concentrations of LFF571 or FDX (Fig. 3). Toxin A and B levels were steady until the culture densities dropped by at least 5 logs, at which point the toxin titers were near the lower limit of detection. In contrast, the treatment of cultures with metronidazole or vancomycin led to an increase of toxin A and B levels at subinhibitory concentrations of the drugs, followed by a sharp decline at growth-inhibitory concentrations. These results suggest that these antibiotics enhance toxin production and/or release from some strains of C. difficile.
FIG 3.
NB95031 demonstrates increased toxin levels at subinhibitory concentrations of metronidazole and vancomycin. Strain NB95031 was grown for 72 h in the presence of the indicated antibiotics. Remaining viable cells (log10 CFU/ml, lines) and toxin A and B production (A450, bars) were measured. FDX, fidaxomicin; VAN, vancomycin; MET, metronidazole. Data are means and SEM of duplicate antibiotic treatments.
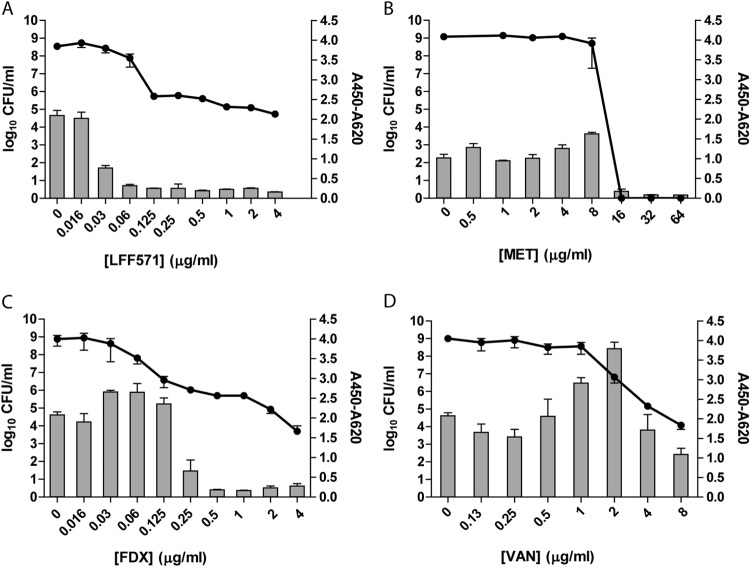
Toxin B levels in the supernatants of C. difficile strain NB95029 increase in the presence of vancomycin.
Strain NB95029 is a clinical isolate that produces toxin B but not toxin A. Therefore, the tcgBiomics ELISA, which is more sensitive for the detection of toxin B than the Wampole assay, was used to assay this strain. In cultures treated with vancomycin, levels of toxin B in the supernatants increased before ultimately decreasing with a decline in viable cell counts (Fig. 4). This was similar to the pattern observed after vancomycin treatment of strain NB95031 (Fig. 4). In contrast, toxin levels declined in cultures treated with increasing concentrations of LFF571 or FDX. The toxin levels in supernatants of cultures treated with increasing concentrations of metronidazole were steady until the antibacterial concentration was reached, after which the toxin levels approached the lower limit of detection.
FIG 4.
NB95029 demonstrates increased toxin B levels at subinhibitory concentrations of vancomycin. Strain NB95029 was grown for 24 h in the presence of the indicated antibiotics. Remaining viable cells (log10 CFU/ml, lines) and toxin B production (A450–620, bars) were measured. FDX, fidaxomicin; VAN, vancomycin; MET, metronidazole. Data are mean and SEM of duplicate antibiotic treatments; toxin B values for samples treated with metronidazole represent a single experiment.
DISCUSSION
LFF571 is a novel EF-Tu inhibitor that has been shown to be noninferior to vancomycin in patients with moderate C. difficile infections and is safe and well tolerated after multiple daily doses (24). As part of the microbiological profiling of LFF571, we investigated the effects of a range of concentrations of the compound on toxin production by C. difficile. A variety of C. difficile strains were used for analysis, including three recent clinical isolates. Two commercially available ELISAs were used to monitor steady-state C. difficile toxin levels in the culture supernatant. Since we were unable to confirm that the polyclonal antibodies used in these assays recognized all toxin A and B proteins equally, no attempts were made to quantitatively compare the toxin production between strains. Instead, toxin A and B production and cell growth in the presence of increasing antibiotic concentrations were assessed for each C. difficile isolate independently.
Previous studies have shown that subinhibitory concentrations of antibiotics can have a variety of effects on bacterial species, including modulating the expression of genes involved in stress, metabolism, and pathogenesis (reviewed in reference 16).
Subinhibitory concentrations of vancomycin and metronidazole, the standards of care for the treatment of CDI, have previously been reported to increase toxin production in C. difficile cultures (17, 18). We recapitulated these results in two of four toxigenic strains treated with vancomycin and one of the strains treated with metronidazole, suggesting variability in the effects of subinhibitory drug concentrations between bacterial isolates. Vancomycin is an inhibitor of cell wall synthesis. Cell wall disruption may cause the release of intracellular toxins into the environment. Indeed, another cell wall biogenesis inhibitor, penicillin, has been shown to increase C. difficile toxin release (18). Alternatively, vancomycin might induce gene expression or otherwise promote toxin production. Consistent with this, increased transcription of toxin A and B genes has been reported in exponentially growing C. difficile cultures treated with these drugs (17). The mode of increased toxin levels in supernatants after metronidazole treatment is not clear from what is known about the antibacterial mechanism of action. The sharp declines in viable cell counts in cultures treated with inhibitory versus subinhibitory concentrations, however, suggest that a sublethal lytic effect may lead to the release of toxin from some strains.
Because LFF571 inhibits translation, we hypothesized that this antibiotic might decrease, rather than increase, toxin levels in C. difficile cultures. We observed that LFF571 led to decreases in toxin production at or below the growth-inhibitory concentrations of the compound in all four of the strains tested. While this is the first study to test the effects of LFF571 on toxin synthesis, other protein translation inhibitors, including REP3123 (19), RBx 11760 (20), and cadazolid (21), have been shown to reduce C. difficile toxin A and B levels in vitro. Interestingly, the protein synthesis inhibitor linezolid has been reported to increase toxin A and B transcription (17). In contrast, the transcription inhibitor fidaxomicin and its metabolite, OPT-1118, have been shown to inhibit toxin gene transcription and toxin production from strains B1/NAP1/027 and UK1 (22, 23). In our experiments, sub-growth-inhibitory to inhibitory concentrations of fidaxomicin reduced toxin levels in a manner similar to LFF571.
In summary, in vitro treatment with the novel semisynthetic thiopeptide LFF571 led to a reduction in toxin A and B production from various C. difficile strains. This differentiates the microbiological profile of LFF571 from that of metronidazole and vancomycin. The relevance of in vitro toxin inhibition to clinical infection, however, remains to be determined.
ACKNOWLEDGMENTS
Financial support for the conduct of this study and preparation of the manuscript was provided by Novartis.
We thank Catherine Jones for editorial support.
REFERENCES
- 1.Rupnik M, Wilcox MH, Gerding DN. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol 7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 2.Viswanathan VK, Mallozzi MJ, Vedantam G. 2010. Clostridium difficile infection: an overview of the disease and its pathogenesis, epidemiology and interventions. Gut Microbes 1:234–242. doi: 10.4161/gmic.1.4.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananthakrishnan AN. 2011. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol 8:17–26. doi: 10.1038/nrgastro.2010.190. [DOI] [PubMed] [Google Scholar]
- 4.Gerding DN, Johnson S. 2011. Clostridium difficile infection in 2010: advances in pathogenesis, diagnosis and management of CDI. Nat Rev Gastroenterol Hepatol 8:67–68. doi: 10.1038/nrgastro.2010.215. [DOI] [PubMed] [Google Scholar]
- 5.Kuehne SA, Cartman ST, Minton NP. 2011. Both, toxin A and toxin B, are important in Clostridium difficile infection. Gut Microbes 2:252–255. doi: 10.4161/gmic.2.4.16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies AH, Roberts AK, Shone CC, Acharya KR. 2011. Super toxins from a super bug: structure and function of Clostridium difficile toxins. Biochem J 436:517–526. doi: 10.1042/BJ20110106. [DOI] [PubMed] [Google Scholar]
- 7.LaMarche MJ, Leeds JA, Brewer J, Bushell S, Dewhurst J, Ding J, Dzink-Fox J, Gamber G, Jain A, Lee K, Lister T, Mullin S, Osborne C, Palestrant D, Patane M, Rann E, Sachdeva M, Shao J, Tiamfook S, Whitehead L, Yan W, Yifru A, Yu D, Zhu Q. 2011. Lead optimization of thiopeptide GE2270 A: identification of clinical compound LFF571, abstr B-1196 Abstr 51st Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 8.Citron DE, Goldstein EJC. 2011. Comparative in vitro activity of LFF571 and lipiarmycin against Clostridium difficile and 641 other intestinal strains of aerobic and anaerobic bacteria, abstr E-110 Abstr 51st Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 9.Dzink-Fox J, LaMarche M, Bradford P, Brewer J, Bushell S, Dewhurst J, Ding J, Gamber G, Palestrant D, Rann E, Shao J, Tiamfook S, Whitehead L, Yan W, Zhu Q, Leeds JA. 2011. Antimicrobial activity of the novel elongation factor Tu inhibitor, LFF571, abstr F1-1346 Abstr 51st Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 10.Trzasko A, Leeds JA, Praestgaard J, Lamarche MJ, McKenney D. 2012. Efficacy of LFF571 in a hamster model of Clostridium difficile infection. Antimicrob Agents Chemother 56:4459–4462. doi: 10.1128/AAC.06355-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng G, Lee LV, Palestrant D, Whitehead L, Sachdeva M, LaMarche MJ, Leeds JA. 2011. Investigation of mode of binding of elongation factor Tu inhibitor LFF571, abstr F1-1859 Abstr 51st Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 12.Leeds JA, Sachdeva M, Mullin S, Dzink-Fox J, LaMarche MJ. 2012. Mechanism of action of and mechanism of reduced susceptibility to the novel anti-Clostridium difficile compound LFF571. Antimicrob Agents Chemother 56:4463–4465. doi: 10.1128/AAC.06354-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecht DW, Citron DM, Cox M, Jacobus N, Jenkins SG, Onderdonk A, Roe-Carpenter D, Rosenblatt JE, Wexler HM. 2010. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 7th ed. Approved standard M11-7 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Lyerly DM, Barroso LA, Wilkins TD, Depitre C, Corthier G. 1992. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect Immun 60:4633–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moncrief JS, Zheng L, Neville LM, Lyerly DM. 2000. Genetic characterization of toxin A-negative, toxin B-positive Clostridium difficile isolates by PCR. J Clin Microbiol 38:3072–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies J, Spiegelman GB, Yim G. 2006. The world of subinhibitory antibiotic concentrations. Curr Opin Microbiol 9:445–453. doi: 10.1016/j.mib.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Gerber M, Walch C, Loffler B, Tischendorf K, Reischl U, Ackermann G. 2008. Effect of sub-MIC concentrations of metronidazole, vancomycin, clindamycin and linezolid on toxin gene transcription and production in Clostridium difficile. J Med Microbiol 57:776–783. doi: 10.1099/jmm.0.47739-0. [DOI] [PubMed] [Google Scholar]
- 18.Onderdonk AB, Lowe BR, Bartlett JG. 1979. Effect of environmental stress on Clostridium difficile toxin levels during continuous cultivation. Appl Environ Microbiol 38:637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochsner UA, Bell SJ, O'Leary AL, Hoang T, Stone KC, Young CL, Critchley IA, Janjic N. 2009. Inhibitory effect of REP3123 on toxin and spore formation in Clostridium difficile, and in vivo efficacy in a hamster gastrointestinal infection model. J Antimicrob Chemother 63:964–971. doi: 10.1093/jac/dkp042. [DOI] [PubMed] [Google Scholar]
- 20.Mathur T, Kumar M, Barman TK, Kumar GR, Kalia V, Singhal S, Raj VS, Upadhyay DJ, Das B, Bhatnagar PK. 2011. Activity of RBx 11760, a novel biaryl oxazolidinone, against Clostridium difficile. J Antimicrob Chemother 66:1087–1095. doi: 10.1093/jac/dkr033. [DOI] [PubMed] [Google Scholar]
- 21.Locher HH, Seiler P, Chen X, Schroeder S, Pfaff P, Enderlin M, Klenk A, Fournier E, Hubschwerlen C, Ritz D, Kelly CP, Keck W. 2014. In vitro and in vivo antibacterial evaluation of cadazolid, a new antibiotic for treatment of Clostridium difficile infections. Antimicrob Agents Chemother 58:892–900. doi: 10.1128/AAC.01830-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouillaut L, Babakhani F, Sonenshein AL. 2011. Inhibition of Clostridium difficile toxin synthesis and sporulation by fidaxomicin, abstr C1-635/101 Abstr 51st Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 23.Babakhani F, Bouillaut L, Sears P, Sims C, Gomez A, Sonenshein AL. 2013. Fidaxomicin inhibits toxin production in Clostridium difficile. J Antimicrob Chemother 68:515–522. doi: 10.1093/jac/dks450. [DOI] [PubMed] [Google Scholar]
- 24.Mullane K, Lee C, Bressler A, Buitrago M, Weiss K, Dabovic K, Praestgaard J, Leeds JA, Blais J, Pertel P. 22 December 2014. Multicenter, randomized clinical trial to compare the safety and efficacy of LFF571 and vancomycin for Clostridium difficile infections. Antimicrob Agents Chemother. doi: 10.1128/AAC.04251-14. [DOI] [PMC free article] [PubMed] [Google Scholar]