Abstract
Patients with advanced hepatic fibrosis or cirrhosis with chronic hepatitis C virus (HCV) infection represent an unmet need. The HCV NS3/4A inhibitor, faldaprevir, was evaluated in combination with the nonnucleoside NS5B inhibitor, deleobuvir, with or without ribavirin in treatment-naive patients with HCV genotype 1 infection in the SOUND-C2 study. Here, the efficacy and safety of this interferon-free regimen in a subset of patients with advanced liver fibrosis, including those with compensated cirrhosis, were assessed. Patients (n = 362) were randomized to once-daily faldaprevir with either twice-daily (BID) or three-times-daily (TID) deleobuvir for 16 (TID16W), 28 (TID28W and BID28W), or 40 (TID40W) weeks with or without ribavirin (TID28W-NR). Patients were classified according to fibrosis stage (F0 to F2 versus F3 to F4) and the presence of cirrhosis (yes/no). In total, 85 (24%) patients had advanced fibrosis/cirrhosis (F3 to F4) and 33 (9%) had cirrhosis. Within each treatment arm, differences in rates of sustained virologic response 12 weeks after completion of treatment (SVR12) between patients with mild to moderate fibrosis (F0 to F2) versus F3 to F4 did not show a consistent pattern and were not statistically significant (63% versus 47% for TID16W, 53% versus 76% for TID28W, 48% versus 67% for TID40W, 70% versus 67% for BID28W, and 40% versus 36% for TID28W-NR, respectively; P > 0.05 for each arm). The most frequent adverse events in patients with/without cirrhosis were gastrointestinal and skin events, which were mostly mild or moderate in intensity. The degree of liver fibrosis did not appear to affect the probability of achieving SVR12 following treatment with the interferon-free regimen of faldaprevir, deleobuvir, and ribavirin. (This study has been registered at ClinicalTrials.gov under registration no. NCT01132313.)
INTRODUCTION
Patients chronically infected with the hepatitis C virus (HCV) are at increased risk of developing advanced liver disease, including cirrhosis, and hepatocellular carcinoma (1–4). Treatment with an effective antiviral regimen and the achievement of a sustained virologic response (SVR) can significantly reduce these risks (5–7). Since the rate of liver-related complications and mortality is particularly high in patients with advanced fibrosis or cirrhosis, they are a priority group for treatment. Treatment success has been hampered by low response rates to pegylated interferon alpha and ribavirin (PegIFN/RBV), as well as high rates of serious adverse events (AEs) (8–10). While the addition of the protease inhibitors telaprevir and boceprevir improved response rates, they were still inferior to those observed in patients with less-advanced fibrosis (11–18). In addition, increases in the numbers of serious AEs, in rates of discontinuation, and in complications have been reported in clinical trials and real-life settings in patients with cirrhosis, highlighting the need for more-effective and less toxic treatment options for this patient population (14, 18). More recently, sofosbuvir and simeprevir have provided new options for patients infected with HCV genotype 1 (GT-1) (19–23). Reduced response rates have been reported for both agents in combination with PegIFN/RBV in patients with cirrhosis (21, 24).
Interferon-free treatment regimens have demonstrated high antiviral efficacy combined with improved safety, offering great promise for patients with advanced liver disease. In the phase 2b SOUND-C2 (registration no. NCT01132313) study, the efficacy and safety of the NS3/4A protease inhibitor faldaprevir (25, 26) were evaluated in an interferon-free combination with the nonnucleoside NS5B polymerase inhibitor deleobuvir, with or without ribavirin, in 362 treatment-naive patients with chronic HCV GT-1 infection, including those with cirrhosis (27). Treatment with faldaprevir, deleobuvir, and ribavirin for 16, 28, or 40 weeks resulted in rates of sustained virologic response 12 weeks after completion of treatment (SVR12) of up to 47% and 85% in GT-1a- and GT-1b-infected patients, respectively. Here, we report an analysis of the efficacy and safety of faldaprevir plus deleobuvir with or without ribavirin in patients with advanced fibrosis or liver cirrhosis enrolled in the SOUND-C2 study.
MATERIALS AND METHODS
Patients and study design.
SOUND-C2 was a multicenter, open-label, randomized phase 2b study that enrolled patients from 48 sites in Europe, Australia, and New Zealand (27). Eligible patients were 18 to 75 years of age, were treatment naive, had chronic HCV GT-1 infection (HCV RNA levels of ≥10,000 IU/ml), and had compensated liver disease.
Patients were randomized 1:1:1:1:1 to one of five treatment groups: faldaprevir 120 mg once daily (QD) and deleobuvir 600 mg three times daily (TID; 6-h–6-h–12-h dosing schedule) plus ribavirin for 16 weeks (TID16W), 28 weeks (TID28W), or 40 weeks (TID40W); faldaprevir 120 mg QD and deleobuvir 600 mg twice daily (BID) plus ribavirin for 28 weeks (BID28W); and faldaprevir 120 mg QD and deleobuvir 600 mg TID without ribavirin for 28 weeks (TID28W-NR). Patients were stratified according to viral subtype (GT-1a or GT-1b; determined by the Trugene HCV genotyping assay [Bayer, Tarrytown, NY, USA] or the Versant HCV Genotype 2.0 assay [Siemens AG, Tarrytown, NY, USA] if the Trugene result was inconclusive) and IL28B rs12979860 genotype (CC or non-CC; assessed using TaqMan PCR allelic discrimination assays [Applied Biosystems, Foster City, CA, USA]). For the first dose of the study drugs, patients received an additional 600-mg dose of deleobuvir and 120-mg dose of faldaprevir. Ribavirin was dosed at 1,000 mg/day (body weight, <75 kg) or 1,200 mg/day (≥75 kg). Patients who experienced virologic breakthrough or detectable HCV RNA at weeks 6 and 8 were switched to PegIFN/RBV and counted as treatment failures (futility rules). Breakthrough was defined as a confirmed increase in HCV RNA levels in two consecutive measurements of ≥25 IU/ml in those with HCV RNA levels of <25 IU/ml previously or an increase of ≥1 log10 IU/ml over their HCV RNA nadir in those with HCV RNA levels of ≥25 IU/ml previously. Relapse was defined as an HCV RNA level of >25 IU/ml after undetectable HCV RNA at the end of planned treatment.
Patients were classified according to fibrosis stage (F0 to F2 versus F3 to F4; by biopsy or Fibroscan) and the presence of cirrhosis (yes/no; by biopsy, Fibroscan, or other parameters as explained below). Biopsy or transient elastography (Fibroscan) was performed within 3 years or 6 months of randomization, respectively. For fibrosis stage, patients were classified as having mild to moderate fibrosis (Metavir score of F0 to F2 or Fibroscan score of <9.5 kPa) or advanced fibrosis to cirrhosis (Metavir score of F3 to F4 or Fibroscan score of ≥9.5 kPa). The presence of cirrhosis was determined by the investigator based on liver biopsy result (Metavir score of F4), Fibroscan (score of ≥13 kPa), or other clinical parameters (aspartate aminotransferase [AST]-to-platelet ratio index [APRI] score of ≥2 and Fibrosure [Fibrotest] score of ≥0.73). Where patients had both biopsy results and Fibroscan scores at baseline, then the biopsy result was used. If neither assessment was performed and the patient was indicated to have cirrhosis based on investigator clinical and laboratory assessments, then a Metavir score of F4 was recorded.
The study protocol was approved by the appropriate institutional review boards for each participating site, according to national and international regulations, and the study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation guidelines. All patients provided written informed consent before enrollment.
Efficacy assessments.
The primary efficacy endpoint was SVR12, defined as an HCV RNA level of <25 IU/ml target not detected 12 weeks after completion of therapy. Plasma HCV RNA levels were measured using the quantitative Roche COBAS TaqMan HCV/HPS assay version 2 (lower limit of quantification, 25 IU/ml; lower limit of detection, 17 IU/ml; Roche Diagnostics AG, Rotkreuz, Switzerland). HCV RNA level was measured on days 1 and 4; at weeks 1, 2, 4, 6, 8, 12, and 16 and every 4 weeks thereafter during treatment; and at 4, 8, 12, and 24 weeks after the end of treatment.
Pharmacokinetic assessments.
One blood sample per patient was collected approximately 10 min prior to the morning dose of study drugs at week 8 for measurement of plasma faldaprevir and deleobuvir concentrations. A validated high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) assay was used to analyze the plasma samples (faldaprevir, Tandem Labs, Salt Lake City, UT, USA; deleobuvir, Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA). The faldaprevir and deleobuvir methods were validated for a range of 20.0 to 10,000 ng/ml and 15.0 to 15,000 ng/ml, respectively; analyte quantitation in both methods was performed using a weighted (1/x2) linear least-squares regression analysis generated from calibration standards.
Safety assessments.
AEs, physical examinations, and biochemical/hematologic assessments were performed at the screening visit, at the same time points as the efficacy assessments during the treatment period, 4 weeks after the administration of the last dose of study drug, and as needed during treatment visits. An independent data and safety monitoring committee conducted regular planned reviews of the safety data.
Statistical analysis.
Calculations of SVR12 rates were based on the intent-to-treat population (all randomized patients who received at least one dose of study drug) and compared between fibrosis-stage categories using the chi-squared test post hoc. When comparing subgroups of patients with and without cirrhosis, patients receiving deleobuvir 600 mg TID were pooled (TID16W, TID28W, and TID40W) due to the small number of patients with cirrhosis in these arms. Multiple logistic regression analysis was used to assess the effect of the presence or absence of cirrhosis and other covariates on SVR12. This analysis was based on the per-protocol population, which excludes patients who prematurely discontinued study therapy for reasons other than per-protocol futility rules, such as AEs, loss to follow-up, or withdrawal of consent.
Baseline liver elasticity and baseline APRIs were compared between responders and nonresponders with respect to SVR12 using the Wilcoxon-Mann-Whitney test. Multiple logistic regression analysis was used to assess the influence of baseline liver elasticity and APRI on SVR12 as a continuous variable, adjusted by treatment regimen and genotype. The per-protocol patient population was used in this analysis.
RESULTS
Patient population.
Patient disposition and baseline characteristics for the overall patient population have been previously described (27). A total of 362 patients received at least one dose of study medication (see Fig. S1 in the supplemental material). Liver biopsy results were available and used to determine fibrosis stage in 124 (34%) patients, including 22 patients who had both biopsy and Fibroscan results available. Fibroscan results were available for the remaining 236 patients. Two patients were classified as missing for stage of fibrosis. In total, 85/360 (24%) patients were diagnosed with advanced fibrosis/cirrhosis (F3 to F4) as evaluated by liver biopsy (Metavir, ≥F3, n = 22) or Fibroscan (≥9.5 kPa, n = 63). Information on the presence/absence of cirrhosis was available for all patients. Cirrhosis was diagnosed in 33/362 (9%) patients as evaluated by liver biopsy (Metavir, F4, n = 5), Fibroscan (≥13 kPa, n = 21), or other parameters (n = 7). Baseline demographics and disease characteristics were comparable across treatment arms (Table 1).
TABLE 1.
Baseline characteristics by fibrosis stageg
| Characteristic | Value by treatment group and fibrosis stagec,d: |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TID16Wa |
TID28Wa |
TID40W |
Pooled TID,b cirrhosis (n = 21) | BID28W |
TID28W-NR |
||||||||
| F0–F2 (n = 63) | F3–F4 (n = 17) | F0–F2 (n = 58) | F3–F4 (n = 21) | F0–F2 (n = 62) | F3–F4 (n = 15) | F0–F2 (n = 57) | F3–F4 (n = 21) | Cirrhosis (n = 9) | F0–F2 (n = 35) | F3–F4 (n = 11) | Cirrhosis (n = 3) | ||
| Male, n (%) | 34 (54) | 10 (59) | 29 (50) | 12 (57) | 28 (45) | 8 (53) | 12 (57) | 26 (46) | 15 (71) | 7 (78) | 20 (57) | 4 (36) | 2 (67) |
| Median age, yr (IQR) | 49 (39–55) | 56 (48–64) | 45 (38–53) | 52 (46–58) | 49 (43–56) | 45 (42–62) | 53 (45–57) | 47 (40–56) | 49 (48–54) | 49 (47–54) | 44 (34–56) | 51 (41–57) | 51 (41–57) |
| White, n (%) | 62 (98) | 16 (94) | 57 (98) | 21 (100) | 61 (98) | 15 (100) | 20 (95) | 56 (98) | 21 (100) | 9 (100) | 35 (100) | 11 (100) | 3 (100) |
| IL28B non-CC, n (%) | 44 (70) | 15 (88) | 43 (74) | 14 (67) | 44 (71) | 14 (93) | 17 (81) | 41 (72) | 18 (86) | 7 (78) | 24 (69) | 9 (82) | 2 (66) |
| HCV genotype 1a, n (%) | 27 (43) | 7 (41) | 25 (43) | 6 (29) | 29 (47) | 5 (33) | 7 (33) | 25 (44) | 5 (24) | 4 (44) | 16 (46) | 2 (18) | 0 (0) |
| Mean HCV RNA, log10 IU/ml (SD) | 6.5 (0.69) | 6.7 (0.49) | 6.4 (0.60) | 6.8 (0.69) | 6.7 (0.53) | 6.5 (0.50) | 6.6 (0.74) | 6.6 (0.67) | 6.6 (0.50) | 6.4 (0.66) | 6.5 (0.61) | 6.8 (0.29) | 6.8 (0.24) |
| HCV RNA level of ≥800,000 IU/ml, n (%) | 54 (86) | 15 (88) | 46 (79) | 19 (90) | 54 (87) | 13 (87) | 17 (81) | 47 (82) | 19 (90) | 7 (78) | 25 (71) | 11 (100) | 3 (100) |
| Mean elastography, kPa (SD)e | 6.2 (3.10) | 14.9 (6.87) | 5.8 (1.45) | 15.8 (12.35) | 6.4 (1.76) | 12.3 (2.64) | 20.1 (11.84) | 5.7 (1.52) | 14.1 (4.33) | 17.3 (3.89) | 6.3 (1.65) | 14.6 (6.02) | 20.2 (8.20) |
| Mean APRI score (SD)f | 0.6 (0.58) | 1.1 (0.82) | 0.6 (0.39) | 0.9 (0.42) | 0.6 (0.40) | 1.0 (0.80) | NA | 0.6 (0.53) | 1.4 (1.12) | NA | 0.6 (0.32) | 1.1 (0.74) | NA |
Information on the stage of fibrosis was classified as “missing” for two patients (one in TID16W and one in TID28W).
The TID16W, TID28W, and TID40W treatment arms were pooled because of the small number of patients with cirrhosis in these treatment arms.
Fibroscan results were used to determine stage of fibrosis for patients without a liver biopsy result (<F3 = <9.5 kPa, ≥F3 = ≥9.5 kPa).
Cirrhosis was determined by the investigator based on Fibroscan, biopsy, and/or other clinical parameters.
Among patients with baseline liver elasticity data (n = 258).
Among patients with a baseline APRI score (n = 360).
Abbreviations: APRI, aspartate aminotransferase/platelet ratio index; BID, twice daily; HCV, hepatitis C virus; IQR, interquartile range; NA, not available; NR, no ribavirin; SD, standard deviation; TID, three times daily.
Efficacy.
Within each treatment arm, differences in SVR12 rates between patients with mild to moderate fibrosis (F0 to F2) and those with advanced fibrosis/cirrhosis (F3 to F4) did not show a consistent pattern and were not statistically significant (63% versus 47% in the TID16W arm, 53% versus 76% in the TID28W arm, 48% versus 67% in the TID40W arm, 70% versus 67% in the BID28W arm, and 40% versus 36% in the TID28W-NR arm, respectively; P > 0.05 for each arm; Fig. 1 and Table 2). Pooled SVR12 rates were 56% for F0 to F2 and 61% for F3 to F4, P = 0.2409 (Cochran-Mantel-Haenszel test adjusted for treatment). Similarly, SVR12 rates in patients with cirrhosis seemed to be comparable to those achieved by patients without cirrhosis (Table 2). In a univariate regression analysis, the presence of cirrhosis did not significantly influence the achievement of SVR12 (odds ratio, 0.91; 95% confidence interval [CI], 0.41 to 2.06; P = 0.83).
FIG 1.
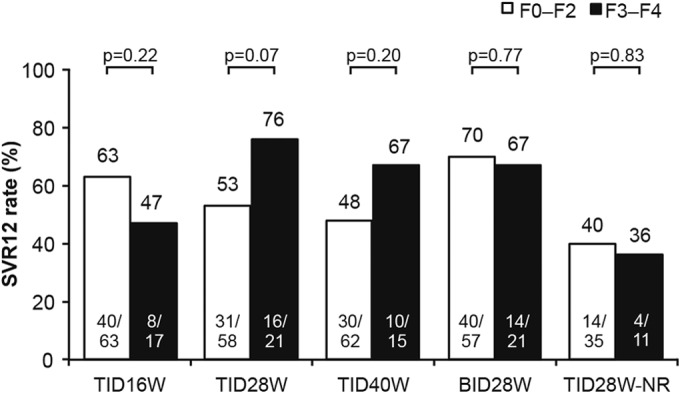
SVR12 in patients according to fibrosis stage. SVR12 was defined as undetectable HCV RNA 12 weeks after completion of treatment. P values were determined using the chi-squared test. BID, twice daily; HCV, hepatitis C virus; NR, no ribavirin; SVR12, sustained virologic response 12 weeks after completion of treatment; TID, three times daily. Numbers at the bottom of bars are number of patients with SVR12/total number of patients. Numbers above the bars are percentages.
TABLE 2.
Rates of virologic response, virologic breakthrough, and relapse by treatment group and fibrosis stagee
| Response | No. positive/total no. (%) by treatment group and fibrosis stagec,d: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TID16Wa |
TID28Wa |
TID40W |
Pooled TIDb |
BID28W |
TID28W-NR |
|||||||||||
| F0–F2 | F3–F4 | F0–F2 | F3–F4 | F0–F2 | F3–F4 | No cirrhosis | Cirrhosis | F0–F2 | F3–F4 | No cirrhosis | Cirrhosis | F0–F2 | F3–F4 | No cirrhosis | Cirrhosis | |
| SVR12 | ||||||||||||||||
| Overall | 40/63 (63) | 8/17 (47) | 31/58 (53) | 16/21 (76) | 30/62 (48) | 10/15 (67) | 124/217 (57) | 11/21 (52) | 40/57 (70) | 14/21 (67) | 48/69 (70) | 6/9 (67) | 14/35 (40) | 4/11 (36) | 17/43 (40) | 1/3 (33) |
| Genotype 1a | 12/27 (44) | 1/7 (14) | 10/25 (40) | 4/6 (67) | 13/29 (45) | 3/5 (60) | 40/93 (43) | 3/7 (43) | 11/25 (44) | 2/5 (40) | 11/26 (42) | 2/4 (50) | 2/16 (13) | 0/2 (0) | 2/18 (11) | 0/0 (0) |
| Genotype 1b | 28/36 (78) | 7/10 (70) | 21/33 (64) | 12/15 (80) | 17/33 (52) | 7/10 (70) | 84/124 (68) | 8/14 (57) | 29/32 (91) | 12/16 (75) | 37/43 (86) | 4/5 (80) | 12/19 (63) | 4/9 (44) | 15/25 (60) | 1/3 (33) |
| Virologic breakthrough | 6/63 (10) | 3/17 (18) | 13/58 (22) | 0/21 (0) | 12/62 (19) | 3/15 (20) | 35/217 (16) | 3/21 (14) | 13/57 (23) | 5/21 (24) | 16/69 (23) | 2/9 (22) | 13/35 (37) | 6/11 (55) | 17/43 (40) | 2/3 (67) |
| Relapse | 9/51 (18) | 1/12 (8) | 1/40 (3) | 0/17 (0) | 1/40 (3) | 0/11 (0) | 12/156 (8) | 1/16 (6) | 0/41 (0) | 0/14 (0) | 0/49 (0) | 0/6 (0) | 2/17 (12) | 0/4 (0) | 2/20 (10) | 0/1 (0) |
Information on the stage of fibrosis was classified as “missing” for two patients (one in the TID16W arm and one in the TID28W arm).
The TID16W, TID28W, and TID40W treatment arms were pooled because of the small number of patients with cirrhosis in these treatment arms.
Fibroscan results were used to determine stage of fibrosis for patients without a liver biopsy result (<F3 = <9.5 kPa, ≥F3 = ≥9.5 kPa).
Cirrhosis was determined by the investigator based on Fibroscan, biopsy, and/or other clinical parameters.
Abbreviations: BID, twice daily; NR, no ribavirin; SVR12, sustained virologic response 12 weeks after completion of treatment; TID, three times daily.
Across all stages of fibrosis, SVR12 rates were higher among patients who received faldaprevir and deleobuvir in combination with ribavirin than among patients in the ribavirin-free arm (Fig. 1 and Table 2). In the BID28W arm, 67% of patients with cirrhosis achieved SVR12, compared with 52% in the pooled TID arms and 33% in the ribavirin-free arm. Patients with HCV GT-1b infection achieved higher SVR12 rates than did patients with HCV GT-1a infection, regardless of the stage of liver fibrosis or the presence of cirrhosis (Table 2).
In general, rates of virologic breakthrough and relapse were similar within each treatment arm for patients with mild to moderate fibrosis (F0 to F2) and those with advanced fibrosis/cirrhosis (F3 to F4), as well as among patients with and without cirrhosis (Table 2).
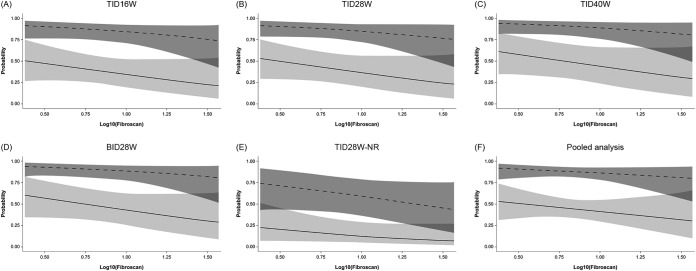
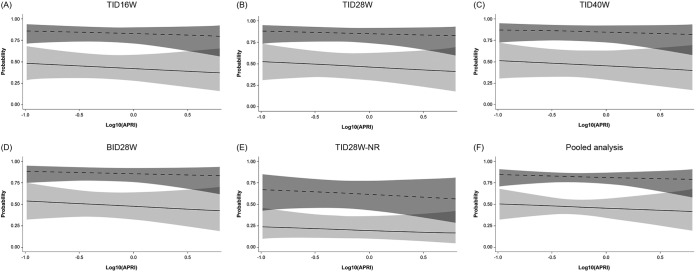
Differences in the degree of baseline fibrosis, as estimated by liver elasticity or by APRI score, between patients who achieved SVR12 and those who did not achieve SVR12 seemed to be comparable and were not statistically significant (see Fig. S2 in the supplemental material). The effect of degree of fibrosis on the probability of achieving SVR12 as assessed in multiple regression analyses was small and not statistically significant, regardless of whether liver elasticity or APRI score was used to estimate liver fibrosis (Fig. 2 and 3). When analyzed by GT-1 subtype, there were no signs of different effects of fibrosis within genotypes 1a and 1b (Fig. 4).
FIG 2.
Probability of SVR12, with 95% confidence limits, in patients with HCV GT-1a (solid lines) or GT-1b (dashed lines) infection according to liver elasticity. For the pooled analysis, the plot is based on a multiple logistic regression model of SVR12 versus log10(Fibroscan) and genotype, with data restricted to ribavirin-containing groups. Odds ratios (ORs), 95% confidence intervals (CIs), and P values are outlined below. BID, twice daily; GT, genotype; NR, no ribavirin; SVR12, sustained virologic response 12 weeks after completion of treatment; TID, three times daily. For the multiple regression model detailed in panels A to E, OR (95% CI) and P value for increasing Fibroscan by 1 log were 0.33 (0.06, 1.80) and 0.2008, respectively. The ORs (95% CIs) and P values for GT-1a versus GT-1b were 0.10 (0.05, 0.20) and <0.0001 and for treatment TID16W versus TID28W were 0.91 (0.36, 2.31) and 0.8489, respectively; those for TID40W versus TID28W were 1.37 (0.48, 3.89) and 0.5550, respectively; those for BID28W versus TID28W were 1.31 (0.50, 3.44) and 0.5782, respectively; those for TID28W-NR versus TID28W were 0.25 (0.08, 0.78) and 0.0173, respectively. For the multiple regression model detailed in panel F, pooled data (excluding the no-RBV arm) are used. OR (95% CI) and P value for increasing Fibroscan by 1 log were 0.47 (0.08, 2.74) and 0.3986, respectively. The OR (95% CI) and P value for GT-1a versus GT-1b in this model were 0.11 (0.05, 0.22) and <0.0001, respectively.
FIG 3.
Probability of SVR12, with 95% confidence limits, in patients with HCV GT-1a (solid lines) or GT-1b (dashed lines) infection according to APRI score. For the pooled analysis, the plot is based on a multiple logistic regression model of SVR12 versus log10(APRI) and genotype, with data restricted to ribavirin-containing groups. Odds ratios (ORs), 95% confidence intervals (CIs), and P values are outlined below. APRI, aspartate aminotransferase/platelet ratio index; BID, twice daily; GT, genotype; NR, no ribavirin; SVR12, sustained virologic response 12 weeks after completion of treatment; TID, three times daily. For the multiple regression model detailed in panels A to E, OR (95% CI) and P value for increasing APRI by 1 log were 0.77 (0.31, 1.95) and 0.5852, respectively. The ORs (95% CIs) and P value for GT-1a versus GT-1b in this model were 0.15 (0.09, 0.26) and < 0.0001 and for treatment TID16W versus TID28W were 0.85 (0.39, 1.86) and 0.6916, respectively; those for TID40W versus TID28W were 0.97 (0.42, 2.23) and 0.9469, respectively; those for BID28W versus TID28W were 1.07 (0.48, 2.35) and 0.8750, respectively; those for TID28W-NR versus TID28W were 0.28 (0.11, 0.71) and 0.0071, respectively. For the multiple regression models detailed in panel F, pooled data (excluding the no-RBV arm) are used. OR (95% CI) and P value for increasing APRI by 1 log were 0.81 (0.30, 2.20) and 0.6805, respectively. The OR (95% CI) and P value for GT-1a versus GT-1b in this model were 0.16 (0.09, 0.29) and <0.0001, respectively.
FIG 4.
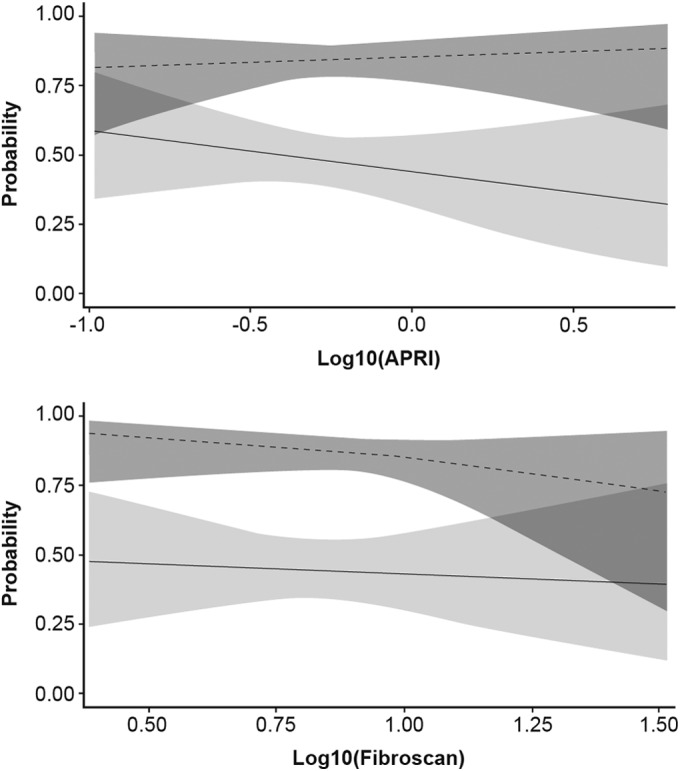
Probability of SVR12, with 95% confidence limits, in patients with HCV GT-1a (solid lines) or GT-1b (dashed lines) infection according to APRI score (top) or liver elasticity (bottom). The plots are based on a multiple logistic regression model of interaction of SVR12 versus log10(APRI score or Fibroscan), genotype, and genotype by log10(APRI score or Fibroscan) and are limited to ribavirin-containing groups. Genotype by log10(APRI), P = 0.3867; genotype by log10(Fibroscan), P = 0.5041. APRI, aspartate aminotransferase/platelet ratio index; GT, genotype; SVR12, sustained virologic response 12 weeks after completion of treatment.
Pharmacokinetics.
Blood samples collected 10 to 14 h relative to the most recent intake of deleobuvir (22 to 26 h relative to the last intake of faldaprevir) were considered representative of plasma trough drug concentrations. In the TID arms, plasma trough concentrations of faldaprevir and deleobuvir at week 8 were higher in patients with cirrhosis (geometric mean, 5,650 ng/ml and 11,900 nmol/liter, respectively) than in patients without cirrhosis (geometric mean, 2,060 ng/ml and 2,410 nmol/liter, respectively) (see Table S1 in the supplemental material). In the BID arm, the difference in plasma trough concentrations for faldaprevir and deleobuvir was less apparent in patients with cirrhosis (geometric mean, 2,940 ng/ml and 2,500 nmol/liter, respectively) than in patients without cirrhosis (geometric mean, 1,810 ng/ml and 2,000 nmol/liter, respectively).
Safety.
AEs were mostly mild or moderate in intensity (Table 3). Severe AEs were reported by 3 (14%), 1 (11%), and 1 (33%) patient with cirrhosis in the pooled TID, BID28W, and TID28W-NR arms, respectively, compared with 18 (8%), 8 (12%), and 3 (7%) patients without cirrhosis in those arms, respectively. The most common AEs across all stages of liver fibrosis were gastrointestinal and skin events, which were, in most cases, more frequently observed in the TID arms than the BID28W arm. In the BID28W arm, all rash and photosensitivity events were classified as mild and no severe cases of diarrhea, nausea, and vomiting were reported regardless of the degree of fibrosis (Tables 3 and 4).
TABLE 3.
Adverse events and laboratory abnormalities by treatment group in patients with and without cirrhosish
| AE or laboratory abnormality | No. (%) by treatment group and cirrhosis status: |
|||||
|---|---|---|---|---|---|---|
| Pooled TIDa |
BID28W |
TID28W-NR |
||||
| Cirrhosisb (n = 21) | No cirrhosis (n = 217) | Cirrhosisb (n = 9) | No cirrhosis (n = 69) | Cirrhosisb (n = 3) | No cirrhosis (n = 43) | |
| Patients with any AEc | 20 (95) | 203 (94) | 9 (100) | 64 (93) | 2 (67) | 42 (98) |
| Severe AEs | 3 (14) | 18 (8) | 1 (11) | 8 (12) | 1 (33) | 3 (7) |
| Serious AEs | 4 (19)d | 12 (6) | 1 (11)e | 7 (10) | 0 (0) | 3 (7) |
| Treatment discontinuation due to AEs | 6 (29)f | 27 (12) | 1 (11)e | 5 (7) | 0 (0) | 5 (12) |
| Rate of AE by preferred term | ||||||
| Rash | 8 (38) | 53 (24) | 3 (33) | 12 (17) | 2 (67) | 11 (26) |
| Photosensitivity reaction | 6 (29) | 63 (29) | 1 (11) | 19 (28) | 0 (0) | 11 (26) |
| Diarrhea | 11 (52) | 93 (43) | 1 (11) | 28 (41) | 1 (33) | 11 (26) |
| Nausea | 8 (38) | 117 (54) | 5 (56) | 34 (49) | 1 (33) | 25 (58) |
| Vomiting | 8 (38) | 68 (31) | 3 (33) | 17 (25) | 1 (33) | 12 (28) |
| Jaundice | 7 (33) | 55 (25) | 2 (22) | 14 (20) | 0 (0) | 2 (5) |
| Changes in laboratory values (grade 3–4)g | ||||||
| Hemoglobin (g/dl) | ||||||
| 6.5–6.9 | 1 (5) | 4 (2) | 1 (11) | 0 (0) | 0 (0) | 0 (0) |
| <6.5 | 0 (0) | 0 (0) | 1 (11) | 0 (0) | 0 (0) | 0 (0) |
| White blood cells (no./mm3) | ||||||
| 1,000–1,499 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| <1,000 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Platelets (no./mm3) | ||||||
| 25,000–49,499 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| <25,000 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ALT (× ULN) | ||||||
| 5.1–10 | 0 (0) | 1 (<1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| >10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total bilirubin (× ULN) | ||||||
| 2.6–5 | 11 (52) | 58 (27) | 4 (44) | 16 (23) | 0 (0) | 6 (14) |
| >5 | 0 (0) | 14 (6) | 2 (22) | 8 (12) | 0 (0) | 0 (0) |
The TID16W, TID28W, and TID40W treatment arms were pooled because of the small number of patients with cirrhosis in these treatment arms.
Cirrhosis was determined by the investigator based on Fibroscan, biopsy, and/or other clinical parameters.
Adverse events were reported according to MedDRA (Medical Dictionary for Regulatory Activities; http://www.meddra.org/) definitions (version 15) and were defined as mild (awareness of sign[s] or symptom[s] which is/are easily tolerated), moderate (enough discomfort to cause interference with usual activity), or severe (incapacitating or causing inability to work or to perform usual activities).
One case each of postnarcotic psychotic ideation, pulmonary embolism (underlying thrombophilia), nausea and vomiting, and rash and photosensitivity.
Anemia.
Rash (n = 3, with no cases of erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrosis, or drug reaction with eosinophilia and systemic symptoms), photosensitivity (n = 2, one patient with photosensitivity and rash), vomiting (n = 1), and jaundice (n = 2, reported posttreatment, associated with vomiting in one case and decreased appetite in one case).
Laboratory abnormalities were reported according to the Division of AIDS grading system (grades 3 to 4 are presented).
AE, adverse event; ALT, alanine aminotransferase; BID, twice daily; NR, no ribavirin; TID, three times daily; ULN, upper limit of normal.
TABLE 4.
Adverse events and laboratory abnormalities by treatment group and fibrosis stage (F0 to F2 versus F3 to F4)d
| AE or laboratory abnormality | No. (%) by treatment group and fibrosis stagea: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TID16W |
TID28W |
TID40W |
BID28W |
TID28W-NR |
||||||
| F0–F2 (n = 63) | F3–F4 (n = 17) | F0–F2 (n = 58) | F3–F4 (n = 21) | F0–F2 (n = 62) | F3–F4 (n = 15) | F0–F2 (n = 57) | F3–F4 (n = 21) | F0–F2 (n = 35) | F3–F4 (n = 11) | |
| Patients with any AEb | 61 (97) | 16 (94) | 52 (90) | 18 (86) | 59 (95) | 15 (100) | 52 (91) | 21 (100) | 35 (100) | 9 (82) |
| Severe AEs | 0 (0) | 1 (6) | 6 (10) | 2 (10) | 10 (16) | 2 (13) | 8 (14) | 1 (5) | 1 (3) | 3 (27) |
| Serious AEs | 2 (3) | 1 (6) | 4 (7) | 4 (19) | 3 (5) | 2 (13) | 7 (12) | 1 (5) | 2 (6) | 1 (9) |
| Treatment discontinuation due to AEs | 1 (2) | 3 (18) | 5 (9) | 5 (24) | 16 (26) | 3 (20) | 4 (7) | 2 (10) | 3 (9) | 2 (18) |
| Rate of AE by preferred term | ||||||||||
| Rash | ||||||||||
| Moderate | 0 (0) | 2 (12) | 0 (0) | 1 (5) | 2 (3) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 3 (27) |
| Severe | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Photosensitivity reaction | ||||||||||
| Moderate | 1 (2) | 3 (18) | 3 (5) | 0 (0) | 4 (6) | 2 (13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | ||||||||||
| Moderate | 0 (0) | 1 (6) | 3 (5) | 0 (0) | 3 (5) | 0 (0) | 3 (5) | 1 (5) | 2 (6) | 0 (0) |
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | ||||||||||
| Moderate | 2 (3) | 0 (0) | 5 (9) | 4 (19) | 5 (8) | 0 (0) | 4 (7) | 2 (10) | 2 (6) | 0 (0) |
| Severe | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | ||||||||||
| Moderate | 4 (6) | 0 (0) | 4 (7) | 6 (29) | 3 (5) | 0 (0) | 0 (0) | 3 (14) | 2 (6) | 0 (0) |
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (5) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 1 (9) |
| Jaundice | ||||||||||
| Moderate | 0 (0) | 2 (12) | 4 (7) | 2 (10) | 2 (3) | 1 (7) | 0 (0) | 2 (10) | 0 (0) | 0 (0) |
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Changes in laboratory values (grade 3–4)c | ||||||||||
| Hemoglobin (g/dl) | ||||||||||
| 6.5–6.9 | 0 (0) | 0 (0) | 1 (2) | 1 (5) | 2 (3) | 1 (7) | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| <6.5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| White blood cells (no./mm3) | ||||||||||
| 1,000–1,499 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| <1,000 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Platelets (no./mm3) | ||||||||||
| 25,000–49,499 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| <25,000 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ALT (× ULN) | ||||||||||
| 5.1–10 | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (5) | 0 (0) | 0 (0) |
| >10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total bilirubin (× ULN) | ||||||||||
| 2.6–5 | 24 (38) | 9 (53) | 9 (16) | 6 (29) | 14 (23) | 6 (40) | 14 (25) | 6 (29) | 5 (15) | 1 (9) |
| >5 | 1 (2) | 2 (12) | 8 (14) | 2 (10) | 4 (7) | 1 (7) | 6 (11) | 4 (19) | 0 (0) | 0 (0) |
Fibroscan results were used to determine stage of fibrosis for patients without a liver biopsy result (<F3 = <9.5 kPa, ≥F3 = ≥9.5 kPa).
Adverse events were reported according to MedDRA (Medical Dictionary for Regulatory Activities; http://www.meddra.org/) definitions (version 15) and were defined as mild (awareness of sign[s] or symptom[s] which is/are easily tolerated), moderate (enough discomfort to cause interference with usual activity), or severe (incapacitating or causing inability to work or to perform usual activities).
Laboratory abnormalities were reported according to the Division of AIDS grading system (grades 3 to 4 are presented).
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; BID, twice daily; NR, no ribavirin; TID, three times daily; ULN, upper limit of normal.
Five patients with cirrhosis experienced serious AEs, four (19%) in the pooled TID arm—one case each of postnarcotic psychotic ideation, pulmonary embolism (underlying thrombophilia, factor V Leiden variant), nausea and vomiting, rash, and photosensitivity—and one (11%) in the BID28W arm (anemia), who discontinued treatment (Table 3). Among patients in the pooled TID arm, 6 (29%) patients with cirrhosis discontinued due to AEs, compared with 27 (12%) patients without cirrhosis. Four of those involved skin events (rash and/or photosensitivity), including one patient who discontinued due to rash and photosensitivity and another one who discontinued due to rash and jaundice. One (11%) patient with cirrhosis in the BID28W arm discontinued due to an AE (anemia), compared with 5 (7%) patients without cirrhosis.
Increases in total bilirubin levels, which were mainly driven by unconjugated bilirubin, were observed in all treatment groups, irrespective of liver fibrosis stage or presence of cirrhosis, except in the ribavirin-free arm (Tables 3 and 4). Division of AIDS (DAIDS) grade 3 to 4 elevations in alanine aminotransferase (ALT) levels were rare in the total population, with no patient experiencing a DAIDS grade 4 event. ALT level elevations were not associated with concomitant bilirubin level elevations and were not observed among patients with advanced fibrosis/cirrhosis (F3 to F4).
DISCUSSION
This analysis of a subset of patients with advanced fibrosis or cirrhosis enrolled into the SOUND-C2 study suggests that treatment with the interferon-free regimen of faldaprevir and deleobuvir in combination with ribavirin can result in response rates comparable to those observed in patients with mild to moderate fibrosis.
The main limitation of this subgroup analysis is its small sample size, in particular, the number of patients with cirrhosis, which limits the feasibility of statistical testing and the certainty by which conclusions can be drawn. In addition, post hoc statistical tests and analyses have to be interpreted with caution.
Overall, SVR12 rates did not seem to be affected by the degree of liver fibrosis, with response rates comparable between patients with advanced fibrosis/cirrhosis (F3 to F4) and those with mild to moderate fibrosis (F0 to F2) (67% and 70% in the BID28W arm and 61% and 56% in pooled arms, respectively). Similar results were observed when comparing patients with and without cirrhosis. In addition, baseline fibrosis stagings in those who achieved SVR12 and those who did not achieve SVR12 seemed to be similar within each treatment arm and were not statistically significant, regardless of the method used to assess fibrosis.
Higher response rates were observed in patients with GT-1b infection than in those with GT-1a infection regardless of fibrosis stage, with GT-1b patients with advanced fibrosis/cirrhosis in the BID28W arm achieving SVR12 rates of 75/80% compared with 40/50% in patients with GT-1a infection, respectively. This is consistent with results in the more recent SOUND-C3 study (28) and may be due to the lower potency of deleobuvir against GT-1a than GT-1b (29, 30). Higher response rates were observed among GT-1b-infected patients with less advanced liver disease than among those with more advanced liver disease in the BID28W arm (86% without cirrhosis versus 80% with cirrhosis; 91% for F0 to F2 versus 75% for F3 to F4), although this was not a pattern consistently observed across arms. In the SOUND-C3 study, 19/20 (95%) patients with GT-1b infection achieved SVR12, including all four patients with cirrhosis (28).
Overall, the BID28W arm had the most favorable safety and tolerability profile in patients with advanced fibrosis/cirrhosis. The frequencies of serious AEs and rates of discontinuation due to AEs were similar between patients with and without cirrhosis in the BID28W arm, while in the pooled TID arms, cirrhosis was associated with a higher incidence of serious AEs and higher rates of discontinuation due to AEs. This observation is likely due to the increased plasma exposure of faldaprevir and deleobuvir in these patients. As the number of cirrhotic patients in the BID28W arm was small, the comparison has to be interpreted with caution. The AE profile was characterized by gastrointestinal and skin events that were predominantly mild to moderate in intensity and manageable across all stages of liver fibrosis.
Bilirubin level elevations were the most common laboratory abnormality in all treatment groups. Despite the increases in bilirubin levels and known effects of the treatment regimen on the bilirubin conjugation enzyme UGT1A1 (31) and the bilirubin transporters OATP1B1 and MRP2 (32), concurrent elevations in ALT level were not observed, including in patients with advanced fibrosis or cirrhosis.
The findings reported here are encouraging as they suggest that the impact of liver disease on the efficacy of interferon-free regimens may be far less than it is on interferon-containing regimens. The SOUND-C2 study was the first interferon-free study to suggest similar efficacies in patients with and without cirrhosis (27), and these results have now been confirmed for other interferon-free regimens in larger phase 3 data sets (33, 34). SVR12 rates with ledipasvir/sofosbuvir with or without ribavirin ranged from 94% to 100% among patients with cirrhosis in the ION-1 study (33). Similarly, ABT-450/r-ombitasvir and dasabuvir with ribavirin achieved SVR12 rates of 92% in patients with cirrhosis (34). Strategies that do not rely on interferon will likely provide the best option from an efficacy and a safety perspective for patients with advanced fibrosis/cirrhosis.
The results reported here support a growing body of evidence demonstrating that the degree of liver fibrosis has little effect on the efficacy and safety of interferon-free regimens. Here, the interferon-free oral combination of faldaprevir, deleobuvir, and ribavirin demonstrated efficacy and tolerability in patients receiving faldaprevir 120 mg QD, deleobuvir 600 mg BID, and ribavirin (BID28W arm) regardless of the presence of advanced liver disease. These results add to the growing body of knowledge on the use of interferon-free regimens in this high-unmet-need population.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by Boehringer Ingelheim Pharma GmbH & Co. KG. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Andrew Brooks and Katrin Gudmundsdottir of Choice Healthcare Solutions during the preparation of the manuscript.
S. Zeuzem has received consultancy fees from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Idenix, Janssen, Merck, Novartis, Presidio, Roche, Santaris, and Vertex. V. Soriano has received grant support from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Janssen, and Merck; has served on speakers' bureaus for Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Janssen, and Merck; and has received compensation for educational presentations from Gilead, Janssen, Merck, and ViiV. T. Asselah is a consultant for BMS, Boehringer Ingelheim, Roche, Merck-Schering Plough, Gilead, and Janssen. E. J. Gane is a member of advisory boards for Abbott, Gilead, Janssen, Roche, and Tibotec. He has received speaker fees for Gilead, Janssen, AbbVie, Novartis, and Roche. J.-P. Bronowicki has received consultancy fees from Boehringer Ingelheim, BMS, Gilead, Janssen, MSD, and Novartis and has received speaker fees from BMS, Gilead, Janssen, MSD, and Roche. P. Angus has received consultancy fees from Gilead and MSD. A. W. Lohse has received grant support from Boehringer Ingelheim, BMS, Falk, Gilead, MSD, and Roche. F. Stickel is a member of advisory boards of Roche, Novartis, Biotest, and Gilead; has received grant support from Novartis, Roche, MSD, and Echosens; has served as a paid speaker for Roche, Novartis, Biotest, MSD, and Janssen; and is a consultant for Janssen, Biotest, and MSD. B. Müllhaupt has received grant support from MSD and Roche and is an advisor for Gilead, Janssen, MSD, and Roche. S. Roberts is a consultant for BMS, Gilead, Janssen, and Roche. M. Schuchmann has received consulting fees and speaker honoraria from Roche, Gilead, BMS, Merck, Boehringer Ingelheim, Janssen, and Falk. M. Manns has received speaker fees from BMS, Gilead, GSK, Janssen, Merck, and Roche; has worked as a consultant for Achillion, BMS, Boehringer Ingelheim, Gilead, GSK, Idenix, Janssen, Merck, Novartis, Roche, and Vertex; and has received grant/research support from AbbVie, BMS, Boehringer Ingelheim, Gilead, Janssen, Merck, Novartis, and Roche. M. Bourlière has served on advisory boards for Boehringer Ingelheim, Merck, Vertex, Janssen, Gilead, Abbott, GSK, and Roche and has received lecture fees from Merck, Janssen, Boehringer Ingelheim, and Gilead. M. Buti has served as a consultant for Boehringer Ingelheim and has received payment for lectures from BMS, Gilead, Janssen, MSD, and Novartis. J. O. Stern, J.-P. Gallivan, F. Voss, J. P. Sabo, W. Böcher, and F. J. Mensa are employees of Boehringer Ingelheim.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04383-14.
REFERENCES
- 1.European Association of the Study of the Liver. 2012. 2011 European Association of the Study of the Liver hepatitis C virus clinical practice guidelines. Liver Int 32(Suppl 1):2–8. doi: 10.1111/j.1478-3231.2011.02703.x. [DOI] [PubMed] [Google Scholar]
- 2.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. 2011. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 54:1433–1444. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alazawi W, Cunningham M, Dearden J, Foster GR. 2010. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther 32:344–355. doi: 10.1111/j.1365-2036.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 4.Dienstag JL, Ghany MG, Morgan TR, Di Bisceglie AM, Bonkovsky HL, Kim HY, Seeff LB, Szabo G, Wright EC, Sterling RK, Everson GT, Lindsay KL, Lee WM, Lok AS, Morishima C, Stoddard AM, Everhart JE. 2011. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology 54:396–405. doi: 10.1002/hep.24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saab S, Hunt DR, Stone MA, McClune A, Tong MJ. 2010. Timing of hepatitis C antiviral therapy in patients with advanced liver disease: a decision analysis model. Liver Transpl 16:748–759. doi: 10.1002/lt.22072. [DOI] [PubMed] [Google Scholar]
- 6.Everson GT, Balart L, Lee SS, Reindollar RW, Shiffman ML, Minuk GY, Pockros PJ, Govindarajan S, Lentz E, Heathcote EJ. 2008. Histological benefits of virological response to peginterferon alfa-2a monotherapy in patients with hepatitis C and advanced fibrosis or compensated cirrhosis. Aliment Pharmacol Ther 27:542–551. doi: 10.1111/j.1365-2036.2008.03620.x. [DOI] [PubMed] [Google Scholar]
- 7.Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, Ascione A, Santantonio T, Piccinino F, Andreone P, Mangia A, Gaeta GB, Persico M, Fagiuoli S, Almasio PL. 2007. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology 45:579–587. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 8.Bruno S, Shiffman ML, Roberts SK, Gane EJ, Messinger D, Hadziyannis SJ, Marcellin P. 2010. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology 51:388–397. doi: 10.1002/hep.23340. [DOI] [PubMed] [Google Scholar]
- 9.Aghemo A, Rumi MG, Monico S, Prati GM, D'Ambrosio R, Donato MF, Colombo M. 2009. The pattern of pegylated interferon-alpha2b and ribavirin treatment failure in cirrhotic patients depends on hepatitis C virus genotype. Antivir Ther 14:577–584. [PubMed] [Google Scholar]
- 10.Ghany MG, Strader DB, Thomas DL, Seeff LB. 2009. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vertex Pharmaceuticals Inc. 2013. Incivek (telaprevir) prescribing information. Vertex Pharmaceuticals Inc, Cambridge, MA: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/201917s012lbl.pdf Accessed 15 July 2014. [Google Scholar]
- 12.Merck & Co., Inc. 2014. Victrelis (boceprevir) prescribing information. Merck Sharp & Dohme Corp., Whitehouse Station, NJ: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202258s014lbl.pdf. Accessed 15 July 2014. [Google Scholar]
- 13.Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP. 2011. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruno S, Vierling JM, Esteban R, Nyberg LM, Tanno H, Goodman Z, Poordad F, Bacon B, Gottesdiener K, Pedicone LD, Albrecht JK, Brass CA, Thompson S, Burroughs MH. 2013. Efficacy and safety of boceprevir plus peginterferon-ribavirin in patients with HCV G1 infection and advanced fibrosis/cirrhosis. J Hepatol 58:479–487. doi: 10.1016/j.jhep.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, Burroughs M, Brass CA, Albrecht JK, Esteban R. 2011. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vierling JM, Zeuzem S, Poordad F, Bronowicki JP, Manns MP, Bacon BR, Esteban R, Flamm SL, Kwo PY, Pedicone LD, Deng W, Dutko FJ, DiNubile MJ, Koury KJ, Helmond FA, Wahl J, Bruno S. 2013. Abstract 1430. Safety and efficacy of boceprevir/peginterferon/ribavirin (BOC/P/R) combination therapy for chronic HCV G1 patients with compensated cirrhosis: a meta-analysis of five phase 3 clinical trials. J Hepatol 58(Suppl 1):S576–S577. doi: 10.1016/S0168-8278(13)61429-4. [DOI] [Google Scholar]
- 17.Pol S, Roberts S, Andreone P, Younossi Z, Diago M, Lawitz EJ, Focaccia R, Foster GR, Horban A, Lonjon-Domanec I, DeMasi R, van Heeswijk R, De Meyer S, Picchio G, Witek J, Zeuzem S. 2011. Efficacy and safety of telaprevir-based regimens in cirrhotic patients with HCV genotype 1 and prior peginterferon/ribavirin treatment failure: subanalysis of the REALIZE phase 3 study, abstr 31. Hepatology 54(Suppl 1) :374A–375A. [Google Scholar]
- 18.Hézode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, de Ledinghen V, Poynard T, Samuel D, Bourlière M, Zarski JP, Raabe JJ, Alric L, Marcellin P, Riachi G, Bernard PH, Loustaud-Ratti V, Métivier S, Tran A, Serfaty L, Abergel A, Causse X, Di Martino V, Guyader D, Lucidarme D, Grando-Lemaire V, Hillon P, Feray C, Dao T, Cacoub P, Rosa I, Attali P, Petrov-Sanchez V, Barthe Y, Pawlotsky JM, Pol S, Carrat F, Bronowicki JP. 2013. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC)—NCT01514890. J Hepatol 59:434–441. doi: 10.1016/j.jhep.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Janssen Therapeutics. 2013. Olysio (simeprevir) prescribing information. Janssen Therapeutics, Titusville, NJ: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/205123s001lbl.pdf Accessed 15 July 2014. [Google Scholar]
- 20.Gilead Sciences Inc. 2013. Sovaldi (sofosbuvir) prescribing information. Gilead Sciences Inc, Foster City, CA: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204671s000lbl.pdf Accessed 15 July 2014. [Google Scholar]
- 21.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, Jacobson IM, Kowdley KV, Nyberg L, Subramanian GM, Hyland RH, Arterburn S, Jiang D, McNally J, Brainard D, Symonds WT, McHutchison JG, Sheikh AM, Younossi Z, Gane EJ. 2013. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 22.Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Scott J, Peeters M, Lenz O, Ouwerkerk-Mahadevan S, De La Rosa G, Kalmeijer R, Sinha R, Beumont-Mauviel M. 2014. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 384:414–426. doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, Craxi A, Peeters M, Lenz O, Ouwerkerk-Mahadevan S, De La Rosa G, Kalmeijer R, Scott J, Sinha R, Beumont-Mauviel M. 2014. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 384:403–413. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson IM, Dore GJ, Foster GR, Fried MW, Manns MP, Marcellin P, Poordad F, Araujo ES, Peeters M, Lenz O, Ouwerkerk-Mahadevan S, De La Rosa G, Kalmeijer R, Sinha R, Beumont-Mauviel M. 2013. Poster 1122. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype 1 infection in treatment-naïve patients: efficacy in difficult-to-treat patient sub-populations in the QUEST 1 and 2 phase III trials. Hepatology 58:730A–760A. doi: 10.1002/hep.26858. [DOI] [Google Scholar]
- 25.White PW, Llinas-Brunet M, Amad M, Bethell RC, Bolger G, Cordingley MG, Duan J, Garneau M, Lagace L, Thibeault D, Kukolj G. 2010. Preclinical characterization of BI 201335, a C-terminal carboxylic acid inhibitor of the hepatitis C virus NS3-NS4A protease. Antimicrob Agents Chemother 54:4611–4618. doi: 10.1128/AAC.00787-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manns MP, Bourliere M, Benhamou Y, Pol S, Bonacini M, Trepo C, Wright D, Berg T, Calleja JL, White PW, Stern JO, Steinmann G, Yong CL, Kukolj G, Scherer J, Boecher WO. 2011. Potency, safety, and pharmacokinetics of the NS3/4A protease inhibitor BI201335 in patients with chronic HCV genotype-1 infection. J Hepatol 54:1114–1122. doi: 10.1016/j.jhep.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 27.Zeuzem S, Soriano V, Asselah T, Bronowicki JP, Lohse AW, Mullhaupt B, Schuchmann M, Bourlière M, Buti M, Roberts SK, Gane EJ, Stern JO, Vinisko R, Kukolj G, Gallivan JP, Bocher WO, Mensa FJ. 2013. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med 369:630–639. doi: 10.1056/NEJMoa1213557. [DOI] [PubMed] [Google Scholar]
- 28.Zeuzem S, Dufour JF, Buti M, Soriano V, Buynak RJ, Mantry P, Taunk J, Stern JO, Vinisko R, Gallivan JP, Bocher W, Mensa FJ, SOUND-C3 Study Group. 16 October 2014. Interferon-free treatment of chronic hepatitis C with faldaprevir, deleobuvir and ribavirin: SOUND-C3, a phase 2b study. Liver Int doi: 10.1111/liv.12693. [DOI] [PubMed] [Google Scholar]
- 29.Larrey D, Benhamou Y, Lohse AW, Trepo C, Mölleken C, Bronowicki JP, Heim M, Arasteh K, Zarski JP, Bourlière M, Wiest R, Calleja JL, Enriquez J, Erhardt A, Wedemeyer H, Gerlach T, Berg T, Stern J, Wu K, Abdallah N, Nehmiz G, Boecher W, Berger F, Steffgen J. 2009. BI 207127 is a potent HCV RNA polymerase inhibitor during 5 days monotherapy in patients with chronic hepatitis C, poster 1599. Hepatology 50(Suppl 4):267A. [Google Scholar]
- 30.Beaulieu PL, Anderson PC, Brochu C, Bos M, Cordingley M, Duan J, Garneau M, Lagrace L, Marquis M, McKercher G, Poupart B, Rancourt J, Stammers T, Tsantrizos YS, Kukolj G. 2012. Abstract 822. Preclinical characterization of the hepatitis C virus NS5B polymerase non-nucleoside inhibitor BI 207127. J Hepatol 56(Suppl 2):S321. doi: 10.1016/S0168-8278(12)60834-4. [DOI] [Google Scholar]
- 31.Sane RS, Steinmann GG, Huang Q, Li Y, Podila L, Mease K, Olson S, Taub ME, Stern JO, Nehmiz G, Bocher WO, Asselah T, Tweedie D. 2014. Mechanisms underlying benign and reversible unconjugated hyperbilirubinemia observed with faldaprevir administration in hepatitis C virus patients. J Pharmacol Exp Ther 351:403–412. doi: 10.1124/jpet.114.218081. [DOI] [PubMed] [Google Scholar]
- 32.Cooper C, Conway B, Ghesquiere W, Lalonde R, Ramji A, Chandok N, Tam E, Sane R, Ting N, Mensa FJ, Elgadi M, Sabo JP. 2013. Poster 1083. Pharmacokinetic interactions of faldaprevir and deleobuvir (BI 207127) and their individual and combined effect on selected cytochrome P450 (CYP) probe substrates in genotype 1 hepatitis C infected patients. Hepatology 58:730A–760A. doi: 10.1002/hep.26858. [DOI] [Google Scholar]
- 33.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, Foster GR, Brau N, Buti M, Jacobson IM, Subramanian GM, Ding X, Mo H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Mangia A, Marcellin P. 2014. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 34.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM, Forns X, Lovell SS, Da Silva-Tillmann B, Collins CA, Campbell AL, Podsadecki T, Bernstein B. 2014. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 370:1273–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.