Abstract
Peripheral intravenous therapy is frequently used in routine hospital practice and, due to various factors, its most common side effect is phlebitis. The infusion of vancomycin is particularly associated with phlebitis despite its widespread use. French guidelines recommend central intravenous infusion for high concentrations of vancomycin, but peripheral intravenous therapy is often preferred in intensive care units. Methods of vancomycin infusion are either intermittent infusion or continuous infusion. A comparison of these methods under in vitro conditions simulating clinical use could result in better infusion efficacy. Human umbilical vein endothelial cells (HUVECs) were therefore challenged with clinical doses of vancomycin over a 24- to 72-h period using these infusion methods. Cell death was measured with the alamarBlue test. Concentration-dependent and time-dependent vancomycin toxicity on HUVECs was noted with a 50% lethal dose at 5 mg/ml after 24 h, reaching 2.5 mg/ml after 72 h of infusion, simulating long-term infusion. This toxicity does not seem to be induced by acidic pH. In comparing infusion methods, we observed that continuous infusion induced greater cell toxicity than intermittent infusion at doses higher than 1 g/day. The increasing use of vancomycin means that new guidelines are required to avoid phlebitis. If peripheral intravenous therapy is used to reduce infusion time, along with intermittent infusion, vein irritation and localized phlebitis may be reduced. Further studies have to be carried out to explore the causes of vancomycin endothelial toxicity.
INTRODUCTION
Intravenous (i.v.) therapy is one of the most common routine medical practices performed in hospitals, due to the increasing complexity of disease processes. Numerous complications, including phlebitis, thrombophlebitis, extravasations and infections are however associated with peripheral i.v. (p.i.v.) therapy. Phlebitis is the most common side effect and may occur at rates ranging from 30 to 70% (1–4), depending on various factors. Infusion phlebitis is an inflammation of the vein endothelium without infection and is characterized by pain, redness, swelling, and warmth at the i.v. site. Phlebitis is a real public health problem, due to the risk of thrombophlebitis, which increases pain and prolongs hospital stays. A large number of risk factors have been implicated in the onset of phlebitis: gender, site of catheter insertion, catheter materials, infusion rates, place of treatment (emergency or hospital), frequency of infusion, and the drug infused (2–5). Studies have revealed the potential endothelial toxicity of some i.v. antibiotics, such as amoxicillin and aminoglycosides (5), erythromycin and dicloxacillin (6), fusidic acid (7), and vancomycin (8, 9).
Vancomycin is an old but powerful antibiotic widely used in industrial countries. With the increasing prevalence of methicillin-resistant Staphylococcus aureus, Clostridium difficile, and nosocomial infections due to the Staphylococcus species, more patients require vancomycin therapy. With this increased use of vancomycin, nurses report a higher incidence of p.i.v. complications (8, 9), especially as vancomycin requires long-term therapy. The general toxicity (e.g., nephrotoxicity and ototoxicity) of vancomycin is well known, as well as the “red man syndrome,” generally associated with the rapid administration of vancomycin (<30 min) and characterized by pruritus, erythema of the face, neck, and upper torso and in severe cases, angioedema and cardiovascular collapse (10). Nevertheless, local vancomycin toxicity has not yet really been studied. The acidity of a vancomycin solution (pH < 4) is often accepted as a cause of complications, but other causes may exist. Indeed, Roszell and Jones (8) have shown that the administration of vancomycin involves more complications (i.e., due to the number of venipunctures, infiltrations, etc.) than that of other antibiotics with a low pH.
The methods for vancomycin infusion vary worldwide. Intermittent infusion is still the reference method, while continuous infusion is increasingly used, especially in France. Both of these methods are effective. Studies have analyzed the continuous i.v. infusion method, which is increasingly used to obtain a higher vancomycin serum level and steadier target concentration (11–13) compared to the conventional method, i.e., intermittent infusion. However, no improved drug activity and no change in the course of the disease has been demonstrated with either method (14, 15). Indeed, Wysocki et al. found that the microbiological and clinical results of the two methods were similar (16). French guidelines also recommend central i.v. infusion for high concentrations of vancomycin, but p.i.v. infusion is often preferred in intensive care units because of user-facility and vancomycin incompatibility with certain drugs.
The rate of vancomycin-induced phlebitis through intermittent infusion varies from 0 to 18% for a known concentration of 4 to 5 mg/ml over 1 h (17, 18). As for continuous infusion, Farber and Moellering, using an infusion of 4 mg/ml over 24 h, reported a phlebitis rate of 13% (19). Clinical studies comparing continuous and intermittent vancomycin infusion offer very limited data on the rate of vancomycin-induced phlebitis (11, 16, 20). Since vancomycin is being used increasingly, it is essential to assess the impact of administration methods and the mechanism of its local toxicity in order to avoid associated complications. A comparison of different infusion methods should result in an improvement in vancomycin infusion, as well as a reduction in endothelial vancomycin toxicity.
The aim of the present study was to assess vancomycin endothelial cell toxicity under in vitro conditions simulating clinical use to determine the factors implicated in local vancomycin toxicity.
MATERIALS AND METHODS
Drugs.
Vancomycin (500 mg/10 ml; Mylan, France) was reconstituted in saline solution (0.9% NaCl; Viaflo, Baxter, France) and diluted in culture medium (Promocell GmbH, Heidelberg, Germany) at 50/50 (vol/vol) to reach the final concentration.
Cell culture.
Tests were performed with proliferating human umbilical vein endothelial cells (HUVECs; Promocell GmbH, Heidelberg, Germany) cultured in endothelial cell growth medium (Promocell GmbH) enriched with endothelial cell growth supplement mix (Promocell GmbH), streptomycin (0.1 g/liter) and penicillin (100 IU/ml), at 37°C in a CO2 incubator (CB 150/APT line/binder; LabExchange, Paris, France) with 5% CO2–95% atmosphere and 100% relative humidity. One day before adding the antibiotic solution, the cells were placed in a 96-well plate at a density of 3 × 103 cells/well to establish an 80% confluent monolayer the next day. The culture medium in each well was then replaced by the tested drug solution prepared at different concentrations. The culture plate was further incubated for 24 h. A 100% cellular viability control was made with 0.9% NaCl. Each test was performed in triplicate.
Cell test protocols.
To assess toxicity related to concentration and exposure time (i.e., contact time between vancomycin and cells), as well as simulating average adult doses, we tested vancomycin doses from 1 to 10 mg/ml over 24 h and from 0.5 to 7.5 mg/ml over 24, 48, and 72 h. To assess the absence of any impact of pH variations on cell toxicity, the pH of vancomycin solutions at 5 and 7.5 mg/ml diluted in culture medium at 50/50 (vol/vol) was measured (SympHony Meter, VWR, France), and the 0.9% NaCl solution in culture medium 50/50 (vol/vol) was acidified with hydrochloric acid to reach the same pH. The test was carried out over 24 h. To compare continuous infusion and intermittent infusion from 1 to 2.75 g/day, we used a vancomycin solution at fixed concentrations ranging from 1.5 to 5 mg/ml for continuous infusion, and from 4 to 11.5 mg/ml in two 1-h periods for intermittent infusion.
Cell vitality assay.
After a 24-h culture, the cell reaction to different antibiotic solutions was evaluated by fluorometric assay with nontoxic alamarBlue dye (Interchim Montluçon, France), a redox indicator; this is a very simple and versatile means for measuring cell proliferation and cytotoxicity (21). Briefly, the culture medium in each well was replaced by 200 μl of culture medium supplemented with 10% (vol/vol) alamarBlue dye. After a 2-h incubation, 150 μl of reacted dye from each well was transferred into a 96-well Fluoro-LumiNunc plate (Polylabo, Strasbourg, France), and the fluorescence intensity was measured by a Twinkle LB970 fluorometer (Berthold Technology, GmbH & Co. KG, Bad Wildbad, Germany) with an excitation at 560 nm and an emission at 590 nm. The results were expressed as a percentage of viable cells compared to 100% control made with 0.9% NaCl.
Statistical tests.
Nonparametric tests were used to compare the percentages of surviving HUVECs with the null hypothesis that there is no difference between the experimental conditions assessed. The Mann-Whitney U test was used to assess concentration-dependent toxicity and the influence of pH. The Kruskal-Wallis test was used to assess time-dependent toxicity and to compare continuous and intermittent infusion. In the presence of a significant P value (P < 0.05), an analysis using the Conover and Iman method was made to detect significant differences between couples of contact time. Each of these tests was performed with XLSTAT software version 2012.2.01 (Addinsoft, Paris, France). The significance level was 0.05.
RESULTS
Endothelial cell toxicity of vancomycin infusion at a clinical dose.
HUVECs were challenged with clinical doses of vancomycin and cell death was measured. We observed the concentration-dependent toxicity of vancomycin on HUVECs. The results showed a significant increase in HUVEC death from a vancomycin concentration of 2.5 mg/ml on. Indeed, the 50% lethal dose (LD50) of vancomycin on HUVECs reached 5 mg/ml after 24 h of treatment (Fig. 1).
FIG 1.
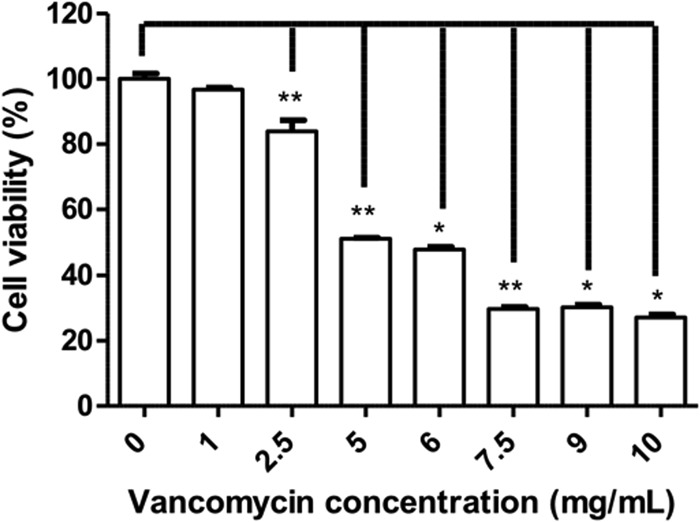
Viability of HUVECs after 24 h of contact with vancomycin. The percentages of HUVECs surviving a 24-h contact with vancomycin solution at fixed concentrations ranging from 1 to 10 mg/ml are shown. *, P < 0.05; **, P < 0.01.
Endothelial toxicity of long-term vancomycin infusion at a clinical dose.
We observed local toxicity to be also time dependent with significantly higher cell death after 48 and 72 h of treatment than after 24 h (Fig. 2). From 2.5 mg/ml on, vancomycin significantly increased HUVEC death after 48 h and even further after 72 h of treatment. Up to 5 mg/ml, cell death was close to 100%, and there was no difference between 48 h and 72 h of contact.
FIG 2.
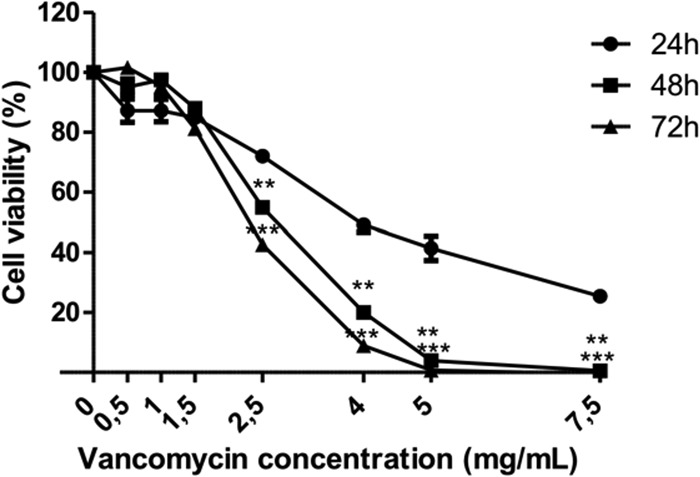
Viability of HUVECs after 24, 48, and 72 h of contact with vancomycin. The percentages of HUVECs surviving 24, 48, or 72 h of contact with vancomycin solution at fixed concentrations ranging from 0.5 to 7.5 mg/ml are shown. **, P < 0.01; ***, P < 0.001 (versus 24 h).
Influence of vancomycin pH on endothelial toxicity.
As shown in Fig. 3, low-pH 0.9% NaCl solution did not induce cell toxicity compared to vancomycin at the same pH. Vancomycin toxicity does not seem to be induced by acidic pH.
FIG 3.
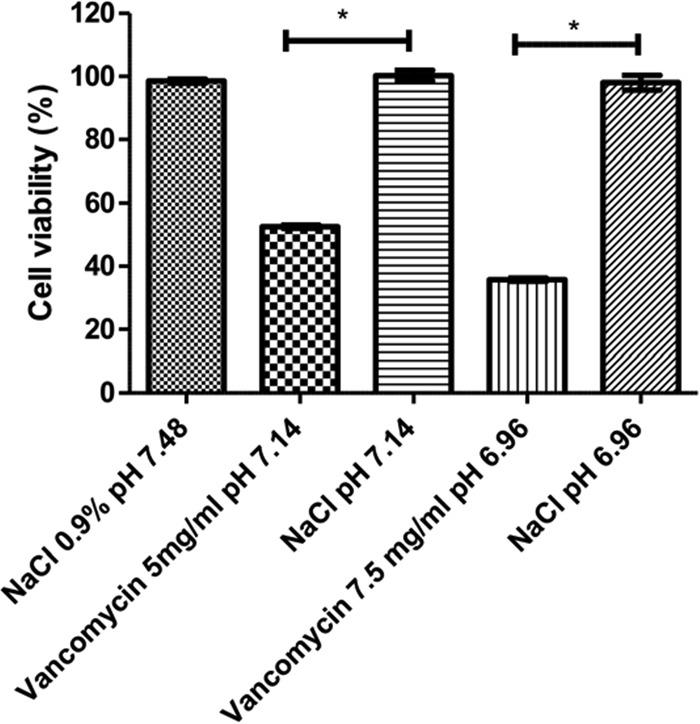
Cell viability of HUVECs after 24 h of contact with low-pH solution. Percentage of HUVECs surviving a 24 h contact with vancomycin solution at 5 mg/ml (pH 7.15) or 7.5 mg/ml (pH 6.96), compared to 0.9% NaCl solution at the same pH. *, P < 0.05.
Comparison between continuous infusion and intermittent infusion of vancomycin.
With the same daily dose of vancomycin, the results showed that continuous infusion induced more cell toxicity than intermittent infusion at doses higher than 1 g/day (Fig. 4). Moreover, intermittent infusion induced lower dose-dependent vancomycin toxicity than continuous infusion.
FIG 4.
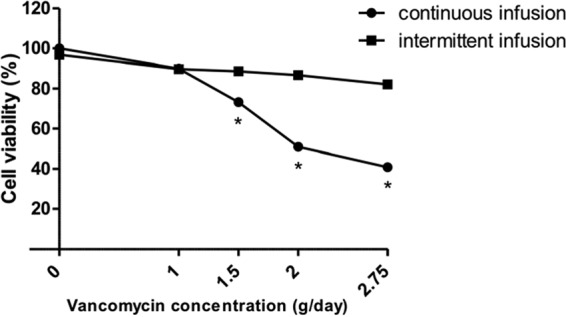
Viability of HUVECs after 24 h of contact with vancomycin at fixed concentrations ranging from 1.5 to 5 mg/ml for continuous infusion and with two 1-h infusions of vancomycin solution at fixed concentrations ranging from 4 to 11.5 mg/ml for intermittent infusion corresponding to daily doses from 1 to 2.75 g. *, P < 0.05.
DISCUSSION
Our results confirm the local toxicity of vancomycin under clinical conditions with an LD50 of about 5 mg/ml for a 24-h continuous infusion, as described in previous studies (22, 23). To discern cell toxicity induced by low pH, an acidified mixture of NaCl solution (0.9%) was prepared in culture medium. It was observed that pH was not responsible for vancomycin toxicity under test conditions, in conformity with a previous study (8). Since vancomycin is infused over a long period postoperatively, we tested vancomycin toxicity on HUVECs for 48 and 72 h. The results showed that the local toxicity of vancomycin was not only concentration dependent but also time dependent. To be more precise, the LD50 was reduced by half after 48 and 72 h compared to 24 h. In our study, the clinical use of vancomycin was simulated at different concentrations under various conditions. We decided to test intermittent infusion and continuous infusion to compare their toxicity on HUVECs. The results showed, with the same daily dose of vancomycin, that continuous infusion induced greater endothelial toxicity than intermittent infusion did.
The availability of HUVECs to test drug solutions for i.v. compatibility is a valuable alternative to animal models, as demonstrated by some studies that have analyzed antibiotic compatibility and inflammatory processes on HUVECs (24, 25). In particular, Robibaro et al. used HUVECs to mimic a clinical dose of vancomycin at the infusion site in an intermittent infusion model and obtained results similar to those obtained with an animal model (22). Some hypotheses remain regarding vancomycin toxicity. In postsurgical intensive care units, vancomycin is frequently infused along with other i.v. antibiotics from the same Y-site infusion device. In our laboratory, flow variations in p.i.v. infusion during multi-infusion therapy through a single i.v. access have induced variations in drug concentration (26, 27). Disturbances in infusion flow by hydration or other i.v. medication could also influence vancomycin exposure to endothelial cells and so modulate toxicity. A limitation to our method is that it does not reproduce fluid dynamics during infusion. A dynamic cell test mimicking flow variations over 24 h of vancomycin infusion should be performed to connect flow variation and cell toxicity.
Other antibiotics, such as erythromycin or clarithromycin, have been associated with the appearance of proinflammatory cytokines (e.g. interleukin-8 [IL-8] or IL-6) and the upregulation of endothelial receptors involved in inflammatory response (25). It might be interesting to test whether vancomycin could induce such expressions of inflammation markers on HUVECs in long-term infusion and multidrug infusion conditions. An inflammatory response could also be induced by reactive oxygen species (ROS), as indeed vancomycin has already been associated with ROS production in renal cells, inducing nephrotoxicity (28, 29). ROS-induced necrosis could therefore be associated with vancomycin-induced endothelial cell toxicity. The production of vancomycin-induced ROS and/or proinflammatory cytokines by HUVECs will have to be evaluated with the intermittent and/or continuous methods to validate this hypothesis.
Moreover, vancomycin is incompatible with numerous other drugs, especially through Y-site administration. The use of saline flush before and after each dose of incompatible medicines is required to avoid contact with vancomycin. The cell toxicity of vancomycin coinfused with other drugs has to be analyzed to determine the phlebitis risk of such associations. Drug incompatibility is a physical or chemical phenomenon resulting in a concentration-dependent precipitation or a pH alteration. The acidic pH of vancomycin might be modified during the blending process, altering the stability of the drug and possibly causing precipitation (30). Studies have shown that i.v. antibiotics, such as vancomycin, amphotericin B, and especially β-lactam have been associated with a 2-fold increase in the risk of phlebitis, which may be attributed to the presence of microparticles in the antibiotic solution (31, 32) capable of inducing an inflammatory response in HUVECs and inducing cell apoptosis. A study has already associated the systemic inflammatory response syndrome (SIRS) with the presence of microparticles (33). The use of a filter for long-term vancomycin infusion could reduce this potential risk, although it may be the catheter material itself that contributes to the risk of phlebitis. Indeed, polyurethane catheters seem to be responsible for a 30 to nearly 50% reduction in the incidence of phlebitis compared to those made of tetrafluoroethylene-hexafluoropropylene (Teflon) (34).
Blood dilutes and neutralizes vancomycin, so reducing vein irritation. Since the blood flow of a central vein is more powerful, the Infusion Nursing Standards of Practice has recommended using a central catheter for vancomycin infusion at concentrations higher than 5 mg/ml to avoid phlebitis or other complications (35). However, a p.i.v. catheter is more frequently used in clinics for vancomycin infusion because of its practicality. We suggest that new guidelines for the p.i.v. infusion of vancomycin should be published to help nurses make an informed choice. Indeed, current recommendations fail to include long-term vancomycin infusion therapy, and studies have shown that phlebitis usually occurs after 24 h of treatment (3).
In conclusion, the increasing use of vancomycin makes new recommendations essential to prevent phlebitis. If a peripheral vein is used to reduce infusion time and limit coinfusion on the same line, the choice of intermittent infusion may reduce vein irritation and localized phlebitis. Nevertheless, this model is an in vitro model and more studies have to be carried out to gain insight into vancomycin toxicity, such as clinical trials comparing intermittent and continuous methods of vancomycin infusion.
REFERENCES
- 1.Nassaji-Zavareh M, Ghorbani R. 2007. Peripheral intravenous catheter-related phlebitis and related risk factors. Singapore Med J 48:733–736. [PubMed] [Google Scholar]
- 2.Maki DG, Ringer M. 1991. Risk factors for infusion-related phlebitis with small peripheral venous catheters: a randomized controlled trial. Ann Intern Med 114:845–854. [DOI] [PubMed] [Google Scholar]
- 3.Uslusoy E, Mete S. 2008. Predisposing factors to phlebitis in patients with peripheral intravenous catheters: a descriptive study. J Am Acad Nurse Pract 20:172–180. doi: 10.1111/j.1745-7599.2008.00305.x. [DOI] [PubMed] [Google Scholar]
- 4.Karadeniz G, Kutlu N, Tatlisumak E, Özbakkaloğlu B. 2003. Nurses' knowledge regarding patients with intravenous catheters and phlebitis interventions. J Vasc Nurs 21:44–47. doi: 10.1016/S1062-0303(03)00034-7. [DOI] [PubMed] [Google Scholar]
- 5.Mestre Roca G, Berbel Bertolo C, Tortajada Lopez P, Gallemi Samaranch G, Aguilar Ramirez MC, Caylà Buqueras J, Rodríguez-Baño J, Martinez JA. 2012. Assessing the influence of risk factors on rates and dynamics of peripheral vein phlebitis: an observational cohort study. Med Clin 139:185–191. doi: 10.1016/j.medcli.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Lanbeck P. 2004. Dicloxacillin and erythromycin at high concentrations increase ICAM-1 expression by endothelial cells: a possible factor in the pathogenesis of infusion phlebitis. J Antimicrob Chemother 53:174–179. doi: 10.1093/jac/dkh056. [DOI] [PubMed] [Google Scholar]
- 7.Iwarson S, Fasth S, Olaison L, Hultén L. 1981. Adverse reactions to intravenous administration of fusidic acid. Scand J Infect Dis 13:65–67. [DOI] [PubMed] [Google Scholar]
- 8.Roszell S, Jones C. 2010. Intravenous administration issues: a comparison of intravenous insertions and complications in vancomycin versus other antibiotics. J Infus Nurs 33:112–118. doi: 10.1097/NAN.0b013e3181cfcee4. [DOI] [PubMed] [Google Scholar]
- 9.Hadaway L, Chamallas SN. 2003. Vancomycin: new perspectives on an old drug. J Infus Nurs 26:278–284. doi: 10.1097/00129804-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Healy DP, Sahai JV, Fuller SH, Polk RE. 1990. Vancomycin-induced histamine release and “red man syndrome”: comparison of 1- and 2-hour infusions. Antimicrob Agents Chemother 34:550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuagnat A, Stern R, Lotthe A, Schuhmacher H, Duong M, Hoffmeyer P, Bernard L. 2004. High-dose vancomycin for osteomyelitis: continuous versus intermittent infusion. J Clin Pharm Ther 29:351–357. doi: 10.1111/j.1365-2710.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 12.Kitzis MD, Goldstein FW. 2006. Monitoring of vancomycin serum levels for the treatment of staphylococcal infections. Clin Microbiol Infect 12:92–95. doi: 10.1111/j.1469-0691.2005.01306.x. [DOI] [PubMed] [Google Scholar]
- 13.Jelassi ML, Benlmouden A, Lefeuvre S, Mainardi J-L, Billaud EM. 2011. Niveau de preuve pour le suivi thérapeutique pharmacologique de la vancomycine. Thérapie 66:29–37. [DOI] [PubMed] [Google Scholar]
- 14.James JK, Palmer SM, Levine DP, Rybak MJ. 1996. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother 40:696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klepser ME, Patel KB, Nicolau DP, Quintiliani R, Nightingale CH. 1998. Comparison of bactericidal activities of intermittent and continuous infusion dosing of vancomycin against methicillin-resistant Staphylococcus aureus and Enterococcus faecalis. Pharmacother J Hum Pharmacol Drug Ther 18:1069–1074. [PubMed] [Google Scholar]
- 16.Wysocki M, Delatour F, Faurisson F, Rauss A, Pean Y, Misset B, Thomas F, Timsit J-F, Similowski T, Mentec H, Mier L, Dreyfuss D. 2001. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 45:2460–2467. doi: 10.1128/AAC.45.9.2460-2467.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caparas JV, Hu J-P. 2014. Safe administration of vancomycin through a novel midline catheter: a randomized, prospective clinical trial. J Vasc Access 15:251–256. doi: 10.5301/jva.5000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanbeck P, Odenholt I, Paulsen O. 2002. Antibiotics differ in their tendency to cause infusion phlebitis: a prospective observational study. Scand J Infect Dis 34:512–519. doi: 10.1080/00365540110080908. [DOI] [PubMed] [Google Scholar]
- 19.Farber BF, Moellering RC. 1983. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother 23:138–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N. 2012. Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother 67:17–24. doi: 10.1093/jac/dkr442. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien J, Wilson I, Orton T, Pognan F. 2000. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 22.Robibaro B, Vorbach H, Weigel G, Weihs A, Hlousek M, Presterl E, Georgopoulos A, Griesmacher A, Graninger W. 1998. Influence of glycopeptide antibiotics on purine metabolism of endothelial cells. Adv Exp Med Biol 431:833–838. [DOI] [PubMed] [Google Scholar]
- 23.Yoeruek E, Spitzer MS, Saygili O, Tatar O, Biedermann T, Yoeruek E, Bartz-Schmidt KU, Szurman P. 2008. Comparison of in vitro safety profiles of vancomycin and cefuroxime on human corneal endothelial cells for intracameral use. J Cataract Refract Surg 34:2139–2145. doi: 10.1016/j.jcrs.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Vorbach H, Weigel G, Robibaro B, Armbruster C, Schaumann R, Hlousek M, Reiter M, Griesmacher A, Georgopoulos A. 1998. Endothelial cell compatibility of clarithromycin for intravenous use. Clin Biochem 31:653–656. [DOI] [PubMed] [Google Scholar]
- 25.Millrose M, Kruse M, Flick B, Stahlmann R. 2009. Effects of macrolides on proinflammatory epitopes on endothelial cells in vitro. Arch Toxicol 83:469–476. doi: 10.1007/s00204-008-0388-5. [DOI] [PubMed] [Google Scholar]
- 26.Décaudin B, Dewulf S, Lannoy D, Simon N, Secq A, Barthélémy C, Debaene B, Odou P. 2009. Impact of multi-access infusion devices on in vitro drug delivery during multi-infusion therapy. Anesth Analg 109:1147–1155. doi: 10.1213/ane.0b013e3181ae06e3. [DOI] [PubMed] [Google Scholar]
- 27.Lannoy D, Decaudin B, Simon N, Barthelemy C, Debaene B, Odou P. 2012. The impact on drug mass flow rate of interrupting and resuming carrier fluid flow: an in vitro study on a very low dead-space volume infusion set. Anesth Analg 114:328–332. doi: 10.1213/ANE.0b013e3182373a27. [DOI] [PubMed] [Google Scholar]
- 28.Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. 2012. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors, and special populations: a literature review. Eur J Clin Pharmacol 68:1243–1255. doi: 10.1007/s00228-012-1259-9. [DOI] [PubMed] [Google Scholar]
- 29.Öktem F, Arslan MK, Ozguner F, Candir Ö, Yilmaz HR, Ciris M, Uz E. 2005. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: protection by erdosteine. Toxicology 215:227–233. doi: 10.1016/j.tox.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Raverdy V, Ampe E, Hecq J-D, Tulkens PM. 2013. Stability and compatibility of vancomycin for administration by continuous infusion. J Antimicrob Chemother 68:1179–1182. doi: 10.1093/jac/dks510. [DOI] [PubMed] [Google Scholar]
- 31.Campbell L. 1998. I.v.-related phlebitis, complications, and length of hospital stay: 1. Br J Nurs 7:1304–1312. [DOI] [PubMed] [Google Scholar]
- 32.Roberts GW, Holmes MD, Staugas RE, Day RA, Finlay CF, Pitcher A. 1994. Peripheral intravenous line survival and phlebitis prevention in patients receiving intravenous antibiotics: heparin/hydrocortisone versus in-line filters. Ann Pharmacother 28:11–16. [DOI] [PubMed] [Google Scholar]
- 33.Jack T, Brent BE, Boehne M, Müller M, Sewald K, Braun A, Wessel A, Sasse M. 2010. Analysis of particulate contaminations of infusion solutions in a pediatric intensive care unit. Intensive Care Med 36:707–711. doi: 10.1007/s00134-010-1775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karadağ A, Görgülü S. 2000. Effect of two different short peripheral catheter materials on phlebitis development. J Intraven Nurs 23:158–166. [PubMed] [Google Scholar]
- 35.Infusion Nurses Society. 2006. Infusion nursing standards of practice. J Infus Nurs 29:S1–S92. doi: 10.1097/00129804-200601001-00001. [DOI] [PubMed] [Google Scholar]


