Abstract
Acinetobacter baumannii is a globally important nosocomial pathogen characterized by an evolving multidrug resistance. A total of 35 representative clinical A. baumannii strains isolated from 13 hospitals in nine cities in China from 1999 to 2011, including 32 carbapenem-resistant and 3 carbapenem-susceptible A. baumannii strains, were selected for whole-genome sequencing and comparative genomic analysis. Phylogenetic analysis revealed that the earliest strain, strain 1999BJAB11, and two strains isolated in Zhejiang Province in 2004 were the founder strains of carbapenem-resistant A. baumannii. Ten types of AbaR resistance islands were identified, and a previously unreported AbaR island, which comprised a two-component response regulator, resistance-related proteins, and RND efflux system proteins, was identified in two strains isolated in Zhejiang in 2004. Multiple transposons or insertion sequences (ISs) existed in each strain, and these gradually tended to diversify with evolution. Some of these IS elements or transposons were the first to be reported, and most of them were mainly found in strains from two provinces. Genome feature analysis illustrated diversified resistance genes, surface polysaccharides, and a restriction-modification system, even in strains that were phylogenetically and epidemiologically very closely related. IS-mediated deletions were identified in the type VI secretion system region, the csuE region, and core lipooligosaccharide (LOS) loci. Recombination occurred in the heme utilization region, and intrinsic resistance genes (blaADC and blaOXA-51-like variants) and three novel blaOXA-51-like variants (blaOXA-424, blaOXA-425, and blaOXA-426) were identified. Our results could improve the understanding of the evolutionary processes that contribute to the emergence of carbapenem-resistant A. baumannii strains and help elucidate the molecular evolutionary mechanism in A. baumannii.
INTRODUCTION
Acinetobacter baumannii is an important opportunistic pathogen that has caused severe nosocomial infections worldwide (1). Multidrug-resistant A. baumannii strains resistant to carbapenems have been increasingly reported worldwide, which raises serious concerns about the limited antimicrobial treatment options available (2). Our previous study indicated that the percentage of imipenem- and meropenem-resistant A. baumannii strains in China increased from 4.5% in 2003 to 61.7% in 2010 and from 4.5% in 2003 to 62.8% in 2010, respectively. In 2012, according to the Chinese Meropenem Surveillance Study (CMSS), the rates of A. baumannii susceptibility to imipenem and meropenem were 37.8% and 36.0%, respectively (3).
A. baumannii rapidly develops multidrug resistance due to the presence of mobile genetic elements (MGEs), such as insertion sequences (ISs), transposons, and resistance islands (4). Many recent studies have highlighted the diversity in the genomic location, architecture, and content of resistance islands, demonstrating the dynamic nature of A. baumannii antibiotic resistance mechanisms and the adaptive significance of these elements (5–7). However, the evolution of antibiotic resistance over time in relation to changes in the major clones in China has not yet been investigated. Moreover, only a few studies have focused on the genetic background differences among A. baumannii isolates collected from different locations at different times.
Comparative genomics studies help in the evaluation of the resistance mechanisms, pathogenicity, and evolution of bacterial pathogens at the genome-wide level (8). Despite the increased amount of research on A. baumannii epidemiology and evolution that has been performed (9–12), large gaps remain in our understanding of the evolutionary processes that contribute to multidrug resistance and genomic diversification of A. baumannii, especially in China. In the present study, a total of 35 representative A. baumannii strains isolated from 13 hospitals in nine cities in China from 1999 to 2011 were selected for comparative whole-genome sequencing to examine strain-level genetic diversity and gene content variation. Through the analysis of these strains with different backgrounds, we aimed to gain a better understanding of the evolution of A. baumannii resistance in China.
MATERIALS AND METHODS
Strain isolation and genotypic and phenotypic characterization.
The A. baumannii strains were collected from 13 hospitals in nine cities in China from 1999 to 2011. The isolates from 1999 to 2005 were selected from the 221 carbapenem-resistant A. baumannii (CRAB) strains evaluated in our previous study (13), and the isolates from 2011 were selected from 283 A. baumannii strains in the Chinese Antimicrobial Resistance Surveillance of Nosocomial Infections (CARES) collection (14). The isolates were subjected to additional analysis to characterize the antibiotic resistance phenotypes (see Table S1 in the supplemental material) and genotypes via multilocus sequence typing (MLST) (http://pubmlst.org/abaumannii/) (15). From this collection of isolates, 35 representative isolates consisting of the predominant clone in different locations and from different dates and sources were selected. To put the sequenced strains in a phylogenetic context and assess the shared and clade-specific gene contents, the genomes were compared with those of 17 reference Acinetobacter genomes available in NCBI as of January 2014.
DNA preparation, library construction, sequencing, and assembly.
DNA was isolated with a DNA purification kit (Qiagen). Illumina sequencing libraries were prepared by using Nextera kits with indexed-encoded adapters from Illumina, according to the manufacturer's instructions. The libraries were pooled for sequencing on a MiSeq sequencer, and paired-end sequence reads representing 50- to 200-fold genome coverage were obtained. The Illumina sequence data were assembled using the SOAPdenovo (version 2.04) package. PCR amplification and Sanger sequencing were used to solve the ambiguity of the order and orientation of scaffolds.
Genome annotation.
The assembled genome sequence was annotated by the NCBI Prokaryotic Genome Automatic Annotation Pipeline (PGAAP), using the programs Glimmer (version 3.0) for the identification of protein-coding genes (16), tRNAscan-SE for the identification of tRNA genes (17), and RNAmmer for the identification of rRNA genes (18). The ISs were identified using the IS Finder database (www-is.biotoul.fr) (19). The origin of replication (oriC) and putative DnaA boxes were identified using the Ori-Finder system (20).
Whole-genome phylogeny construction.
Multiple-sequence alignments of 52 genomes were performed using the Mugsy program (21). The tree was constructed on the basis of single nucleotide polymorphisms (SNPs) from the whole-genome alignment. This alignment included SNPs from 17 reference genomes and 35 genomes sequenced in this study.
eBURST analysis.
eBURST analysis was employed to investigate the evolutionary relationships and clonal complexes (CCs) within the isolates, using the software on the eBURST website (http://eburst.mlst.net/v3/enter_data/single/), with statistical support for the complexes being assessed via the bootstrap resampling method with 1,000 resamplings (22). The analysis was performed using both stringent (a minimum of six shared alleles) and relaxed (a minimum of five shared alleles) grouping parameters.
Estimation of core genome and pan-genome size.
A BLASTP search between every pair of protein sequences from each strain was performed. The PanOCT program was used to identify the orthologs with the BLASTP output (23).
Nucleotide sequence accession numbers.
The data from this whole-genome shotgun project have been deposited at DDBJ/EMBL/GenBank under accession number PRJNA261018. The three novel blaOXA types were designated blaOXA-424 (GenBank accession number KM588352), blaOXA-425 (GenBank accession number KM588353), and blaOXA-426 (GenBank accession number KM588354) (http://www.lahey.org/Studies/).
RESULTS
A total of 35 representative A. baumannii strains, including 32 carbapenem-resistant A. baumannii (CRAB) strains and 3 carbapenem-susceptible A. baumannii strains, isolated from patients with hospital-acquired infections in 13 hospitals in nine cities in China from 1999 to 2011 were selected for whole-genome sequencing. The 32 CRAB isolates consisted of 1 isolate from 1999, 8 isolates from 2003 to 2005, and 23 isolates from 2011. Most of the isolates were collected in Beijing, China (14/35, 40%). The sources of these 35 isolates were sputum (20/35, 57.1%), blood (10/35, 28.6%), and abdominal fluid (5/35, 14.3%). The general characteristics of the 35 A. baumannii genomes are listed in Table S2 in the supplemental material.
Whole-genome phylogeny of the genus Acinetobacter.
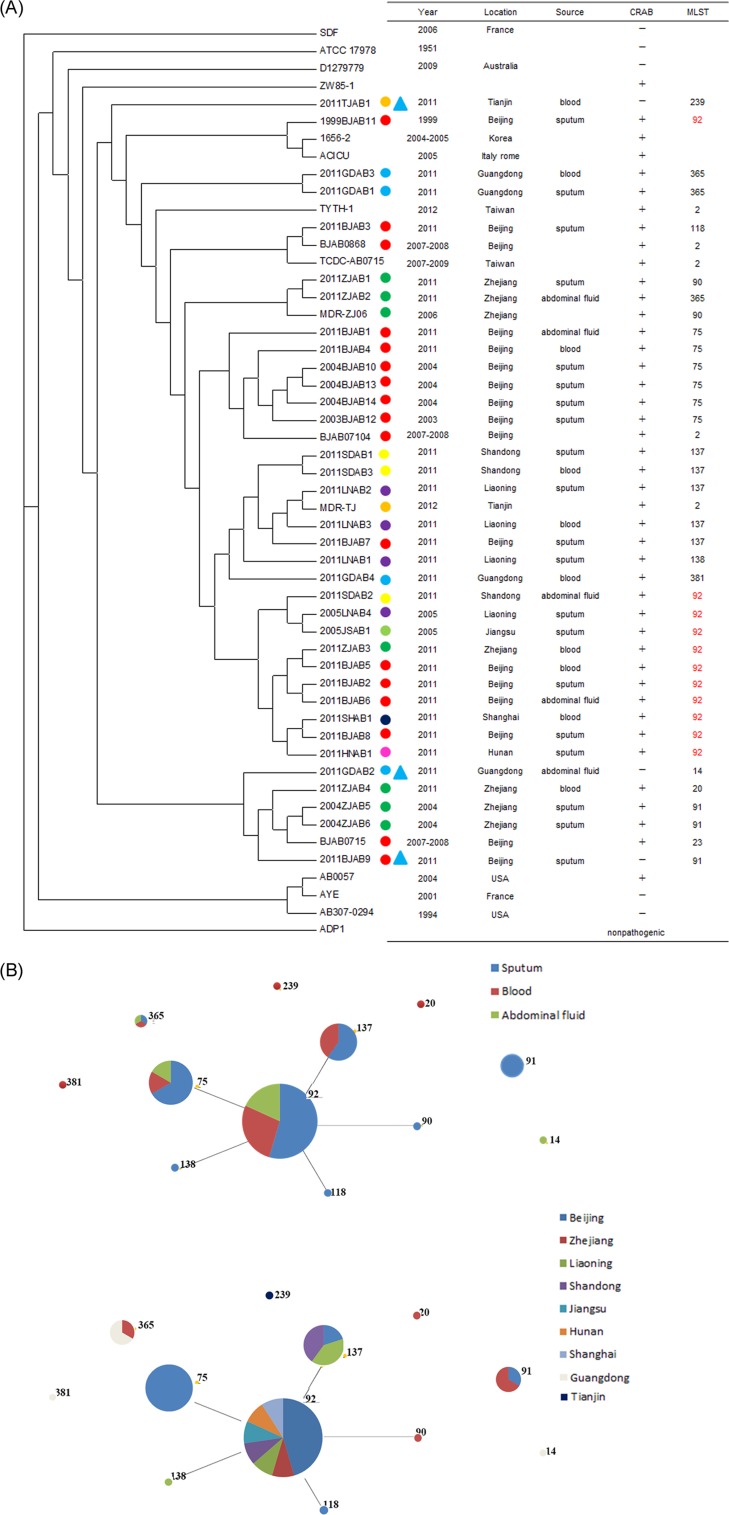
To facilitate detailed analysis of strain relationships, we developed a phylogeny based on single nucleotide polymorphisms (SNPs) from the whole-genome alignment to represent the ancestral relationships among 35 strains and 16 other A. baumannii strains (Fig. 1A). These included 12 multidrug-resistant A. baumannii strains (AYE, AB0057, ACICU, 1656-2, TYTH-1, TCDC-AB0715, BJAB07104, BJAB0715, BJAB0868, ZW85-1, MDR-TJ, and MDR-ZJ06), 2 susceptible strains (ATCC 17978 and AB307-0294), 1 community-acquired strain (D1279779), and 1 nonclinical strain (SDF) isolated from a human body louse. ADP1, a soil-living Acinetobacter baylyi strain, was used as the outgroup for comparison.
FIG 1.
(A) Whole-genome phylogeny of the genomes of the 35 A. baumannii strains evaluated in this study and 17 sequenced A. baumannii genomes. The phylogeny tree was constructed on the basis of SNPs and was rooted with A. baylyi ADP1. Colored circles, the different locations of the strains isolated; blue triangles, the strains were susceptible to carbapenems. The predominant MLST, ST92, is highlighted in red. (B) Results of eBURST analysis conducted to assign CCs to the 35 A. baumannii strains utilizing seven loci. The CCs are indicated by circles, and the predicted clonal ancestors are shown by the central circles. Numbers indicate the MLST type. The sizes of the points are proportional to the number of isolates assigned to each MLST type.
On the basis of the phylogenetic data, all the strains, along with nine previously reported Asian strains (including MDR-ZJ06, MDR-TJ, ZW85-1, BJAB07104, BJAB0715, and BJAB0868 from mainland China; TCDC-AB0715 and TYTH-1 from Taiwan, China; and 1656-2 from South Korea) were grouped together with ACICU, a strain of the global clone II (GC II) group. The susceptible strains were clustered on the edge of the evolutionary tree. Two groups of founder strains were identified among the CRAB strains on the basis of the phylogenetic tree. These included the earliest isolated strain (strain 1999BJAB11) and two strains from Zhejiang Province, China, isolated in 2004. Interestingly, the earliest isolated strain, 1999BJAB11, was separated from all of the other sequence type 92 (ST92) strains, suggesting that it may have a genome content different from that of the other ST92 strains.
The eBURST algorithm revealed one clonal complex (CC), CC1, and six singletons (Fig. 1B). CC1 contained ST92, which was identified to be a potential founder, with ST75, ST137, ST90, ST118, and ST138 radiating from it. All the isolates in CC1 were CRAB, and the three susceptible strains were clustered into singletons. The location and source of these isolates showed diversity in different STs.
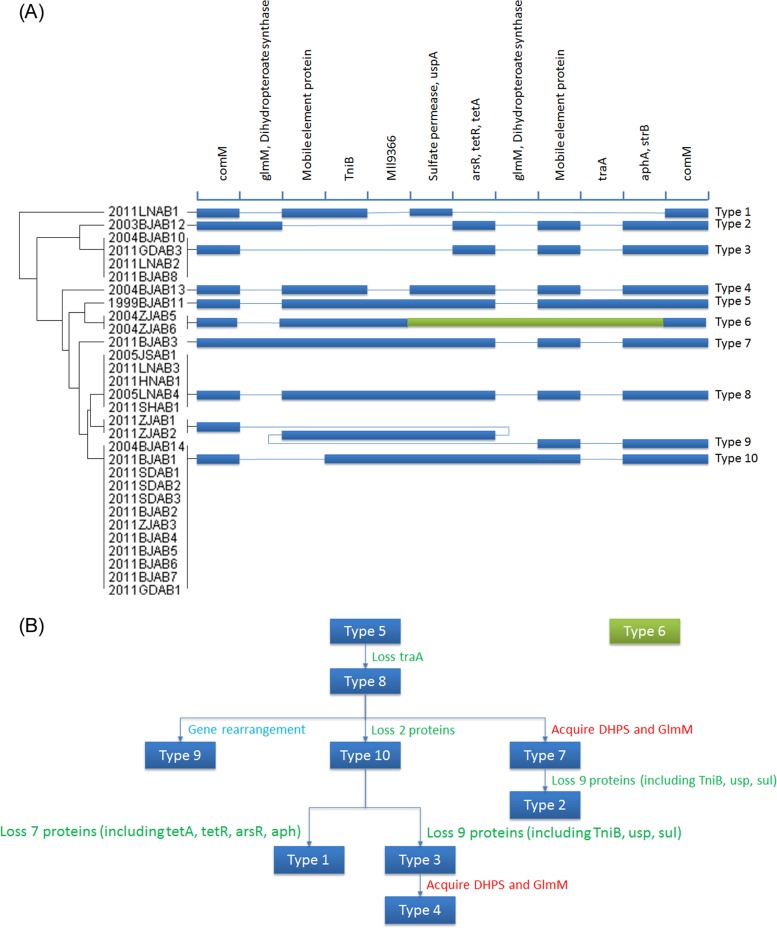
Evolution of AbaR resistance islands.
Ten types of AbaR resistance islands were identified in the 32 CRAB strains (Fig. 2A). Except for the type 6 AbaR island, the other types shared high homology. The type 10 AbaR island was the predominant type and was identified in 12 strains (12/32, 37.5%); it was 19.123 kb in length and contained the highest number of resistance genes, including tniB, usp, sul, tetA, tetR, arsR, and aph, similar to those noted in a strain from Japan with one different nucleotide (24). The type 5 AbaR island in 1999BJAB11 contained the largest number of genes, and the conjugal transfer gene traA was identified only in this isolate. The resistance genes tetA, tetR, arsR, aphA, and strB were not found in the type 1 AbaR island, while the sulfonamide resistance proteins dihydropteroate synthase and phosphoglucosamine mutase (GlmM) were detected in the type 2, 7, and 10 AbaR islands. GlmM can catalyze the conversion of glucosamine-6-phosphate to glucosamine-1-phosphate, which is an essential step in the formation of the cell wall precursor UDP-N-acetylglucosamine (25). In Streptococcus gordonii, mutations in GlmM appear to influence bacterial cell growth and morphology, biofilm formation, and sensitivity to penicillins (26). The function of GlmM in A. baumannii is currently not well-known. The mobile element protein was not observed in type 3 and 10 AbaR islands. Two strains isolated in Zhejiang Province in 2011 contained the type 9 AbaR island, which showed a gene rearrangement different from that of the type 8 AbaR island. Two Zhejiang strains collected in 2004 had the type 6 AbaR island, which included the two-component response regulator, the osmosensitive K+ channel histidine kinase KdpD, methyl viologen resistance protein SmvA, an RND multidrug efflux transporter acriflavine resistance protein, and RND efflux system membrane fusion protein CmeA. These genes were not observed in the other nine types of AbaR islands. The evolutionary ideograph of the AbaR islands according to time is shown in Fig. 2B. The type 5 AbaR island in the earliest strain (1999BJAB11) contained the largest number of genes, and with genome evolution, gene acquisition, gene loss, and gene rearrangement were detected in strains isolated from 2003 to 2011. The type 6 AbaR island in two Zhejiang strains was relatively independent of the AbaR island types found in other strains during AbaR island evolution.
FIG 2.
(A) Structure and distribution of the AbaR resistance islands. (B) Evolutionary ideograph of the AbaR islands in the evolution of CRAB. DHPS, dihydropteroate synthase.
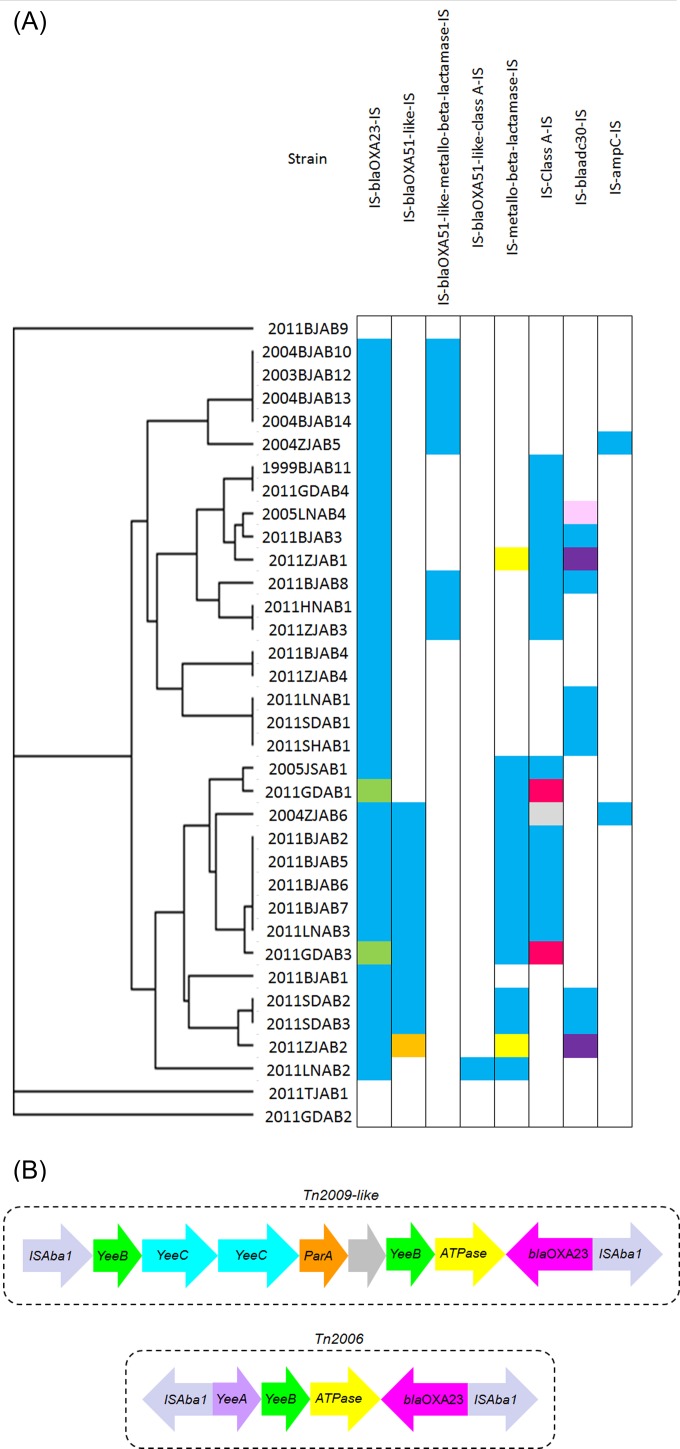
Variations in transposon-associated antibiotic resistance genes.
The transposons containing drug resistance genes varied in the different A. baumannii strains (Fig. 3A). The blaOXA-51-like gene was located in three types of transposons. Eleven strains possessed an IS–blaOXA-51-like–IS transposon, and 10 of them were flanked by two ISAba1 elements. One strain, 2011ZJAB2, was flanked by ISAba1 and ISAba16, and eight strains possessed ISAba1–blaOXA-51-like–metallo-beta-lactamase–ISAba1 transposons. Only one strain, 2011LNAB2, possessed an ISAba1–blaOXA-51-like–class A beta-lactamase–ISAba1 transposon. ISAba1-metallo-beta-lactamase-ISAba1 was detected in 11 stains, but in 2 strains from Zhejiang isolated in 2011, metallo-beta-lactamase was flanked by ISAba14 and ISAba16. ISAba1–class A beta-lactamase–ISAba1 was identified in 13 strains. In one strain from Zhejiang isolated in 2004, ISAba20 and ISAba1 were detected on both sides of a class A beta-lactamase, and in two strains from Guangdong Province, China, isolated in 2011, ISAba1 and ISAba13 were detected. In seven strains isolated in 2011, blaADC-30 was located in ISAba1–blaADC-30–ISAba1, but in 2005LNAB4, blaADC-30 was flanked by ISAba1 and ISAba18, and in two strains from Zhejiang isolated in 2011, blaADC-30 was flanked by ISAba1 and ISAba14. Furthermore, in two strains from Zhejiang isolated in 2004, ISAba1-ampC-ISAba1 was identified.
FIG 3.
Transposons containing drug resistance gened in different A. baumannii strains. (A) Blue and green, ISAba1; orange, ISAba1 and ISAba16; yellow, ISAba14 and ISAba16; gray, ISAba20 and ISAba1; rose red, ISAba1 and ISAba13; pink, ISAba1 and ISAba18; purple, ISAba1 and ISAba14; blank areas, the absence of transposons containing drug resistance genes. (B) Structures of transposons containing blaOXA23, Tn2009-like (blue in panel A) and Tn2006 (green in panel A).
The blaOXA-23-containing resistance island was identified in all 32 CRAB strains (Fig. 3B). In most of the sequenced strains (30/32, 93.8%), blaOXA-23 was located in a transposon, which showed 99% identity to Tn2009. Tn2009 was initially identified on the chromosome of A. baumannii MDR-ZJ06 from China, had a length of 8,421 bp, and was flanked by two ISAba1 elements. Tn2009 was noted to carry the blaOXA-23 gene, the truncated DEAD/DEAH box helicase gene, the ATPase gene, the yeeC gene, and the yeeB gene. However, in two isolates from Guangzhou, China, obtained in 2011, blaOXA-23 was found in transposon Tn2006, which was shorter than Tn2009. Tn2006 contained the ATPase gene, the yeeA gene, and the yeeB gene and was flanked by two ISAba1 elements.
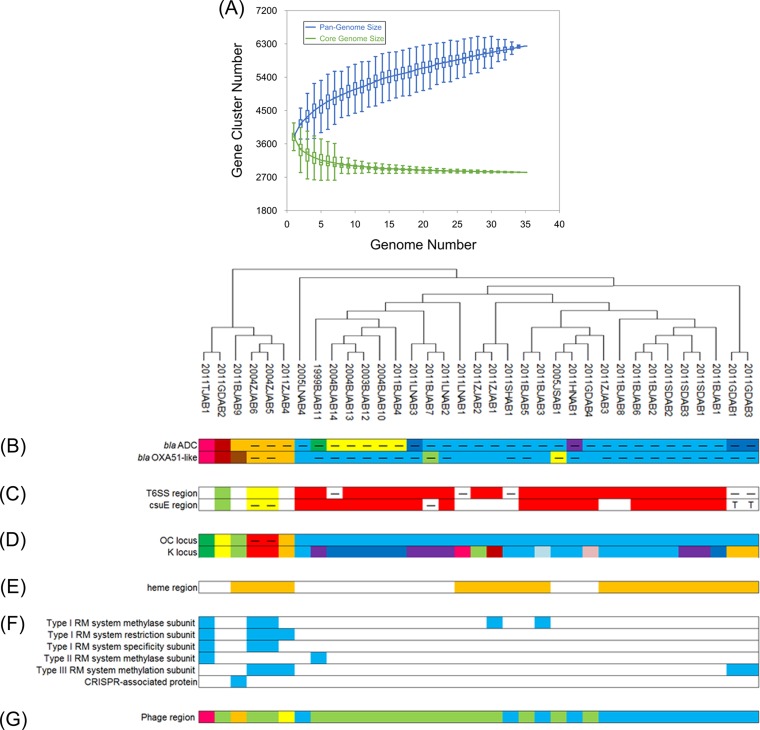
Pan-genome: core and accessory genes.
All the sequenced A. baumannii strains contained a genetically highly homogeneous core genome that encodes proteins involved in DNA replication, transcription, and translation as well as many metabolic pathways. By using the best reciprocal BLAST matches, we identified 2,830 core genes/proteins and 1,206 accessory genes/proteins among all the 35 A. baumannii isolates. Graphing of the numbers of core genome and pan-genome genes as a function of the number of strains sequenced revealed that the slope for the core gene number was approaching an asymptote, whereas the pan-genome gene number continued to expand even after the compilation of 35 genomes (Fig. 4A).
FIG 4.
Genome features of the 35 A. baumannii strains. (A) Number of total features in the core genome and pan-genome as a function of the number of strains sequenced. An average of 500 random permutations of the genome order is presented for the pan-genome and core genome content; the error bars represent the standard deviations of these results. (B) Intrinsic chromosomal β-lactamase alleles. Different colors, the different types; blue, blaADC is an ADC30 variant and a blaOXA66 variant; horizontal bars, the presence of an upstream ISAba1. (C) Variation in the presence of a T6SS gene cluster and a csuE gene cluster. Different colors, the different types; blank areas, the region is absent; horizontal bars, ISAba1 or ISAba13 insertion locations within each locus; T, transposase insertion locations within each locus. (D) Surface polysaccharide variants for the OC and capsular polysaccharide (K) loci. Different colors, the different types; horizontal bars, the ISAba1 insertion locations within each locus. (E) Variant region showing recombination around the heme utilization region. Orange, the heme region is present; blank areas, the heme region is absent. (F) RM system. Blue, the subunit is present; blank areas, the subunit is absent. (G) Phage content refers to the phage-related region located in strain TYTH-1. Blue, both the phages are present; green, the first phage is present; other colors, different phages are present (refer to the text for details).
Variation in intrinsic chromosomal resistance genes related to carbapenem resistance.
The phylogenetic distribution of different alleles of blaOXA-51-like and blaADC showed evidence of recombination and mutation. The presence of an upstream insertion element, ISAba1, suggested that the entire region was replaced by homologous recombination. The blaADC gene was always associated with an upstream ISAba1 oriented to allow overexpression of the gene, except in the three susceptible strains (Fig. 4B). The sequences of 19 strains were identical to the sequence of the extended-spectrum blaADC-30 variant (blue), and the sequences of 3 strains showed 99% identity to the blaADC-30 sequence (dark blue). Five strains contained blaADC-25 (yellow), and one strain carried blaADC-56 (purple). The sequences of one susceptible strain, 2011BJAB9, and three resistant strains showed 99% identity to the blaADC-53 sequence (orange), while the sequence of blaADC in 1999BJAB1 showed 99% identity to that of blaADC-29. The sequences of the susceptible strains 2011GDAB2 and 2011TJAB1 showed 98% and 99% identity to those of blaADC-52 and blaADC-63, respectively.
The blaOXA-51-like variants were different in the resistant strains and susceptible strains. The most common blaOXA-51-like variant in resistant strains was blaOXA-66, which was detected in 27 resistant strains (27/35, 77.1%), and blaOXA-68 was detected in 3 resistant strains from Zhejiang. One unusual variant, blaOXA-234, was detected in a resistant strain from Jiangsu Province, China, in 2005. Furthermore, three novel variants were identified, including blaOXA-425 in the resistant strain 2011BJAB7 and blaOXA-424 and blaOXA-426 in the susceptible strains 2011GDAB2 and 2011BJAB9, respectively. blaOXA-69 was detected in the susceptible strain 2011TJAB1. In most CRAB strains (25/32, 78%), blaOXA-51-like variants were associated with an upstream ISAba1 element, but no upstream ISAba1 element was detected in any of the three susceptible strains.
IS-mediated T6SS and csuE region deletions.
There were multiple instances of chromosomal gene loss that might have possibly been mediated by an IS adjacent to the deletion (Fig. 4C). Two large deletions adjacent to ISAba1 elements have been previously described (10), including a 40-kbp region encoding the entire type VI secretion system (T6SS) and an ∼20-kbp region of adhesion genes (csuE) involved in aspartate metabolism. In the present study, three different types of T6SS regions were noted, and eight strains lacked a T6SS region. Among these eight strains, in five strains, deletion was mediated by an IS insertion. It is worth noting that, except for the ISAba1 insertion in three strains isolated in 2011, an ISAba13 insertion was detected in two strains from Guangdong isolated in 2011. Similarly, three different types of csuE regions were also noted, and 12 strains lacked this region. Among these 12 strains, the deletion was mediated by an IS insertion in 3 strains and a transposase insertion in 2 strains. Except for an ISAba1-mediated deletion in 2011BJAB7, this study is the first to identify an ISAba125 insertion, which was found in two strains from Zhejiang isolated in 2004, and a transposase insertion, which was found in the csuE region in two strains from Guangdong isolated in 2011.
Variation in surface polysaccharide synthesis and heme utilization region.
The 35 strains showed substantial variation in the content and organization of loci involved in surface polysaccharide synthesis (Fig. 4D). The core lipooligosaccharide (LOS) loci (outer core [OC] locus) (27) consisted of one predominant type (blue), which was present in most of the strains (29/35, 82.9%); however, we also identified several variations in the three susceptible strains and one resistant strain. In two strains from Zhejiang isolated in 2004, ISAba20 insertions were detected. With regard to capsular (K) loci, 12 variations were identified. One predominant type (blue) existed in 10 strains (28.6%), whose sequences were most closely related to the ACICU sequence (99.5% identity at the nucleotide sequence level).
The heme utilization region, containing several genes involved in heme utilization, was more common in strains collected in 2011. However, this region was absent in 1999BJAB1 and most of the strains collected from 2003 to 2005, except for two strains from Zhejiang isolated in 2004. Among the 23 strains collected in 2011, the heme utilization region was noted in 18 strains, and their sequences were 96% identical to the sequence of the ACICU heme utilization region (Fig. 4E).
Variation in the RM system.
The restriction-modification (RM) system is a major participant in the coevolutionary interaction between mobile genetic elements (MGEs) and their hosts through the regulation of horizontal gene transfer (HGT). In the present study, six strains that clustered with two strains from Zhejiang isolated in 2004 contained more RM system genes (Fig. 4F). In the susceptible strain 2011TJAB1 and two resistant strains from Zhejiang isolated in 2004, we identified a type I RM system methylase subunit, a restriction subunit, and a specificity subunit. Furthermore, strains 2011ZJAB1 and 2011BJAB3 had only a type I RM system methylase subunit, and strain 2011ZJAB4 had only a type I RM system restriction subunit. A type II RM system methylase subunit was detected in strain 1999BJAB11 and susceptible strain 2011TJAB1, whereas a type III RM system methylation subunit was found in three Zhejiang strains and two Guangdong strains. A CRISPR-associated protein was detected in only one susceptible strain, 2011BJAB9.
Variation in phage-related regions.
Phage-related regions are another source of variability contributing to the difference among A. baumannii strains. Two predominant types of phage-related regions were located within the same chromosomal locations, corresponding to 1.11 to 1.16 Mbp in strain ACICU (ACICU_00997 to ACICU_01077) and 1.45 to 1.53 Mbp in strain TYTH-1 (M3Q_1334 to M3Q_1458) (Fig. 4G). One primary variant typified by the completed reference genome of strain TYTH-1 consisted of two probable phage insertion events flanked by phage integrases (blue), whereas another variant possessed only the second of the two phage elements (green). Two susceptible strains (2011TJAB1 and 2011BJAB9) and strain 2011ZJAB4 had phage-related regions different from those of the two main variants, and all these strains were susceptible to tigecycline.
DISCUSSION
Despite increased research on A. baumannii evolution, the development of multidrug resistance and the genomic diversification of A. baumannii are not yet clear, especially in China. In the present study, we have reported the whole-genome sequences and on the comparative genomic analysis of 35 representative A. baumannii strains isolated from 1999 to 2011 in 13 hospitals in nine cities in China, including 32 CRAB and 3 carbapenem-susceptible A. baumannii strains with different genotypes and phenotypes. Data for these genomes represent a significant addition of genomic data from the genus and could serve as a valuable resource for future genomic studies.
It has been indicated that the AbaR resistance islands have evolved into diverse types through multiple events of insertion, deletion, and recombination (6, 28). The predominance of AbaR4-type resistance islands among the CRAB isolates has been reported in South Korea (29) and Taiwan (30). However, the evolution of AbaR resistance islands has not yet been investigated, especially in China. In the present study, it was found that the type 6 AbaR island in two Zhejiang strains collected in 2004 is different from the previously identified AbaR islands and may thus be a novel AbaR island. The type 6 AbaR island was noted to comprise the two-component response regulator, osmosensitive K+ channel histidine kinase KdpD, methyl viologen resistance protein SmvA, an RND multidrug efflux transporter acriflavine resistance protein, and RND efflux system membrane fusion protein CmeA. These regulators and resistance-related proteins in the type 6 AbaR island may enhance the strain's adaptability and antibiotic resistance, which should be further studied.
The frequent detection of transposons and ISs carrying resistance genes in bacteria shows that recombination flexibility exists during the acquisition of resistance (4). In the present study, each strain contained multiple transposons or ISs, which gradually tended to diversify in the process of evolution. It must be noted that some of these ISs or transposons are novel and that most of the novel IS element insertions or transposons were mainly found in strains from Zhejiang or Guangdong Province of China. The resistance genes or elements may spread through a transposon- or IS-mediated mechanism, and the diversification of transposons or ISs in the process of evolution increases the possibility of acquisition of novel resistance genes or elements.
Recombination has been reported to contribute to the change in the A. baumannii genome (28). Furthermore, the heme utilization region was also highly variable among the A. baumannii strains, and it was more common in strains collected in 2011 in the present study, thus making it difficult to assess whether this region was present in a common ancestor and lost by some strains or was gained and subsequently transferred via recombination to different lineages, as initially observed. In addition, evidence of recombination events in the allelic distribution of blaADC and blaOXA-51-like variants was also noted. In all CRAB strains, the blaADC gene was associated with an upstream ISAba1 oriented to allow overexpression of the blaADC genes. However, in all three susceptible strains, no upstream ISAba1 was detected. In addition, the blaOXA-51-like variants were different in the resistant strains and susceptible strains. Four uncommon blaOXA-51-like genes were identified, including blaOXA-234 and a novel blaOXA-type, blaOXA-425, in CRAB strains and novel blaOXA types blaOXA-424 and blaOXA-426 in two susceptible strains. In most CRAB strains (25/32, 78%), blaOXA-51-like variants were associated with an upstream ISAba1, but no upstream ISAba1 was detected in any of the three susceptible strains. Furthermore, the blaOXA-23-containing resistance island was identified in all 32 CRAB strains and absent in all susceptible strains. It is very likely that blaOXA-234 or blaOXA-425 contributes to carbapenem resistance in A. baumannii, as demonstrated previously with blaOXA-23, blaOXA-24, and blaOXA-58, and blaOXA-234 or blaOXA-425 was also associated with an upstream ISAba1, which has been shown to promote overexpression of OXA (31).
Despite extensive research on the virulence potential of A. baumannii, little is known about its true pathogenic potential or virulence repertoire. A. baumannii thrives in hospital settings largely due to its persistence on abiotic surfaces (32). One mechanism for A. baumannii persistence in hospital settings is the presence of a putative tip adhesion gene, csuE. The csuE gene is involved in pilus and biofilm formation (33), and its presence has been associated with the persistence of A. baumannii on abiotic surfaces, such as plastic and glass (33). The resistant strains were found to contain two different types of csuE. Most of the strains contained the shortest-type csuE, which was not observed in 10 resistant strains. The second type of csuE was detected only in two Zhejiang strains isolated in 2004 and comprised ISAba125 and a gene cluster containing 16 proteins that were not present in the shortest type. T6SS is widespread among Gram-negative bacteria and can be used for toxicity against other bacteria and eukaryotic cells. T6SS has been implicated in the interaction between bacteria as well as between bacteria and their hosts. In Vibrio cholerae, T6SS confers toxicity toward other bacteria, providing a means of interspecies competition to enhance environmental survival (34). Furthermore, Pseudomonas aeruginosa has been found to activate T6SS during infection in patients with cystic fibrosis (35) and also use the T6SS-delivered toxins to actively kill competing bacteria (36, 37). In A. baumannii strains, T6SS is conserved and plays a role in competition with other bacterial species (38). In the present study, three different types of T6SS regions were noted, with one type being predominantly detected. Two of the three susceptible strains did not exhibit this region, and only one strain presented a unique type of T6SS region which was different from the T6SS regions noted in the carbapenem-resistant strains. Two resistant strains collected in 2004 in Zhejiang exhibited the third type of T6SS region, which contained more genes than the predominant type, including genes for VgrG, ATP synthase, and transcriptional regulators.
A. baumannii showed a strong ability to acquire foreign DNA, such as DNA encoding drug resistance and pathogenicity, which allows the bacterium to acquire genetic diversity and overcome antibiotic selection pressure. The flow of genetic information between the bacterial cells by HGT drives bacterial evolution, and RM systems are the key moderators of this process (39). The presence of DNA for a RM system may act as a selfish genetic element to ensure its dissemination and/or may function in defense against bacteriophages and the hindrance of lateral gene transfer (40). In the present study, on the basis of the phylogenetic tree, two groups of strains with different founder strains were identified among the CRAB strains. Furthermore, six strains that clustered with two strains from Zhejiang isolated in 2004 contained more RM system genes. The transfer of resistance genes may facilitate the rapid spread of these genes among A. baumannii strains, so the antimicrobial susceptibility profile of this pathogen should be closely monitored. We speculate that new antibiotics with a strong ability to interfere with the horizontal transfer of resistance genes or elements carrying them may contribute to the effective treatment of Acinetobacter infections in the future.
In conclusion, in the present study, we analyzed the genomes of 35 representative A. baumannii isolates from China. Extensive variation in the gene content was found even among strains that were phylogenetically and epidemiologically very closely related. In the process of evolution, the genome of A. baumannii gradually tended to diversify. Several mechanisms contributed to this diversity, including IS-mediated deletions, genome-wide homologous recombination, transfer of mobile genetic elements, and mobilization of transposons or ISs. Thus, the present study improves our understanding of the evolutionary processes that contribute to the emergence of CRAB in China. Future large-scale sampling across different areas and time scales is still needed to fully understand the evolution of A. baumannii and its drug resistance development and trends.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (31170125), the Beijing Natural Science Foundation (5122041), the Specialized Research Fund for the Doctoral Program of Higher Education (20110001110043), and Key Projects in the National Science & Technology Pillar Program (2012EP001002).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04609-14.
REFERENCES
- 1.Howard A, O'Donoghue M, Feeney A, Sleator RD. 2012. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 3:243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pogue JM, Mann T, Barber KE, Kaye KS. 2013. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev Anti Infect Ther 11:383–393. doi: 10.1586/eri.13.14. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Zhao CJ, Wang H, Yu YS, Zhu ZH, Chu YZ, Sun ZY, Hu ZD, Xu XL, Liao K, Xu YC, Zhang LY, Mei YN, Yang B, Ni YX. 2013. Antimicrobial resistance of Gram-negative bacilli isolated from 13 teaching hospitals across China. Zhonghua Yi Xue Za Zhi 93:1388–1396. (In Chinese.) [PubMed] [Google Scholar]
- 4.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krizova L, Dijkshoorn L, Nemec A. 2011. Diversity and evolution of AbaR genomic resistance islands in Acinetobacter baumannii strains of European clone I. Antimicrob Agents Chemother 55:3201–3206. doi: 10.1128/AAC.00221-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Post V, White PA, Hall RM. 2010. Evolution of AbaR-type genomic resistance islands in multiply antibiotic-resistant Acinetobacter baumannii. J Antimicrob Chemother 65:1162–1170. doi: 10.1093/jac/dkq095. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez MS, Vilacoba E, Stietz MS, Merkier AK, Jeric P, Limansky AS, Marquez C, Bello H, Catalano M, Centron D. 2013. Spreading of AbaR-type genomic islands in multidrug resistance Acinetobacter baumannii strains belonging to different clonal complexes. Curr Microbiol 67:9–14. doi: 10.1007/s00284-013-0326-5. [DOI] [PubMed] [Google Scholar]
- 8.Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, Richet H, Robert C, Mangenot S, Abergel C, Nordmann P, Weissenbach J, Raoult D, Claverie JM. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet 2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarrilli R, Pournaras S, Giannouli M, Tsakris A. 2013. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents 41:11–19. doi: 10.1016/j.ijantimicag.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Wright MS, Haft DH, Harkins DM, Perez F, Hujer KM, Bajaksouzian S, Benard MF, Jacobs MR, Bonomo RA, Adams MD. 2014. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. mBio 5(1):e00963-13. doi: 10.1128/mBio.00963-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan SY, Chua SL, Liu Y, Hoiby N, Andersen LP, Givskov M, Song Z, Yang L. 2013. Comparative genomic analysis of rapid evolution of an extreme-drug-resistant Acinetobacter baumannii clone. Genome Biol Evol 5:807–818. doi: 10.1093/gbe/evt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahl JW, Gillece JD, Schupp JM, Waddell VG, Driebe EM, Engelthaler DM, Keim P. 2013. Evolution of a pathogen: a comparative genomics analysis identifies a genetic pathway to pathogenesis in Acinetobacter. PLoS One 8:e54287. doi: 10.1371/journal.pone.0054287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Guo P, Sun H, Wang H, Yang Q, Chen M, Xu Y, Zhu Y. 2007. Molecular epidemiology of clinical isolates of carbapenem-resistant Acinetobacter spp. from Chinese hospitals. Antimicrob Agents Chemother 51:4022–4028. doi: 10.1128/AAC.01259-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen HB, Zhao CJ, Wang H, Cao B, Xu XL, Chu YZ, Hu ZD, Zhuo C, Hu BJ, Liu WE, Liao K, Zhang R, Zeng J, Wang Y, Luo YP, Wang ZW, Liu YM, Chen X, Tian B, Su DH, Zhou CM, Zou MX, Guo PH, Zhou HW, Jin Y. 2013. An analysis of resistance of nosocomial infection pathogens isolated from 13 teaching hospitals in 2011. Zhonghua Nei Ke Za Zhi 52:203–212. (In Chinese.). doi: 10.3760/cma.j.issn.0578-1426.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Bartual SG, Seifert H, Hippler C, Luzon MA, Wisplinghoff H, Rodriguez-Valera F. 2005. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol 43:4382–4390. doi: 10.1128/JCM.43.9.4382-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delcher AL, Bratke KA, Powers EC, Salzberg SL. 2007. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. 2007. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao F, Zhang CT. 2008. Ori-Finder: a web-based system for finding oriCs in unannotated bacterial genomes. BMC Bioinformatics 9:79. doi: 10.1186/1471-2105-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angiuoli SV, Salzberg SL. 2011. Mugsy: fast multiple alignment of closely related whole genomes. Bioinformatics 27:334–342. doi: 10.1093/bioinformatics/btq665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol 186:1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fouts DE, Brinkac L, Beck E, Inman J, Sutton G. 2012. PanOCT: automated clustering of orthologs using conserved gene neighborhood for pan-genomic analysis of bacterial strains and closely related species. Nucleic Acids Res 40:e172. doi: 10.1093/nar/gks757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tada T, Miyoshi-Akiyama T, Shimada K, Shimojima M, Kirikae T. 2014. Dissemination of 16S rRNA methylase ArmA-producing Acinetobacter baumannii and emergence of OXA-72 carbapenemase coproducers in Japan. Antimicrob Agents Chemother 58:2916–2920. doi: 10.1128/AAC.01212-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tavares IM, Jolly L, Pompeo F, Leitao JH, Fialho AM, Sa-Correia I, Mengin-Lecreulx D. 2000. Identification of the Pseudomonas aeruginosa glmM gene, encoding phosphoglucosamine mutase. J Bacteriol 182:4453–4457. doi: 10.1128/JB.182.16.4453-4457.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimazu K, Takahashi Y, Uchikawa Y, Shimazu Y, Yajima A, Takashima E, Aoba T, Konishi K. 2008. Identification of the Streptococcus gordonii glmM gene encoding phosphoglucosamine mutase and its role in bacterial cell morphology, biofilm formation, and sensitivity to antibiotics. FEMS Immunol Med Microbiol 53:166–177. doi: 10.1111/j.1574-695X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 27.Kenyon JJ, Hall RM. 2013. Variation in the complex carbohydrate biosynthesis loci of Acinetobacter baumannii genomes. PLoS One 8:e62160. doi: 10.1371/journal.pone.0062160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snitkin ES, Zelazny AM, Montero CI, Stock F, Mijares L, Murray PR, Segre JA. 2011. Genome-wide recombination drives diversification of epidemic strains of Acinetobacter baumannii. Proc Natl Acad Sci U S A 108:13758–13763. doi: 10.1073/pnas.1104404108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DH, Park YK, Ko KS. 2012. Variations of AbaR4-type resistance islands in Acinetobacter baumannii isolates from South Korea. Antimicrob Agents Chemother 56:4544–4547. doi: 10.1128/AAC.00880-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HY, Chang RC, Su LH, Liu SY, Wu SR, Chuang CH, Chen CL, Chiu CH. 2012. Wide spread of Tn2006 in an AbaR4-type resistance island among carbapenem-resistant Acinetobacter baumannii clinical isolates in Taiwan. Int J Antimicrob Agents 40:163–167. doi: 10.1016/j.ijantimicag.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, Livermore DM, Pitt TL. 2006. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett 258:72–77. doi: 10.1111/j.1574-6968.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 32.Brossard KA, Campagnari AA. 2012. The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect Immun 80:228–233. doi: 10.1128/IAI.05913-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. 2003. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology 149:3473–3484. doi: 10.1099/mic.0.26541-0. [DOI] [PubMed] [Google Scholar]
- 34.MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. 2010. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci U S A 107:19520–19524. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, Goodman AL, Joachimiak G, Ordonez CL, Lory S, Walz T, Joachimiak A, Mekalanos JJ. 2006. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312:1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hood RD, Singh P, Hsu F, Guvener T, Carl MA, Trinidad RR, Silverman JM, Ohlson BB, Hicks KG, Plemel RL, Li M, Schwarz S, Wang WY, Merz AJ, Goodlett DR, Mougous JD. 2010. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7:25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell AB, Hood RD, Bui NK, LeRoux M, Vollmer W, Mougous JD. 2011. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475:343–347. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carruthers MD, Nicholson PA, Tracy EN, Munson RJ. 2013. Acinetobacter baumannii utilizes a type VI secretion system for bacterial competition. PLoS One 8:e59388. doi: 10.1371/journal.pone.0059388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira PH, Touchon M, Rocha EP. 2014. The interplay of restriction-modification systems with mobile genetic elements and their prokaryotic hosts. Nucleic Acids Res 42:10618–10631. doi: 10.1093/nar/gku734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi I. 2001. Behavior of restriction-modification systems as selfish mobile elements and their impact on genome evolution. Nucleic Acids Res 29:3742–3756. doi: 10.1093/nar/29.18.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.